1. Introduction
Frequent outbreaks of new and unexpected infectious diseases have become important risk factors affecting human public health and wellness, and vaccination remains the most critical means of dealing with infectious diseases.
Since the cowpox vaccine appeared as the world's first smallpox vaccine, vaccines have gone through three major reforms, the first was at the end of the 19th century, Louis Pasteur developed live attenuated vaccines for the first time, and then German scientists Kolle made inactivated vaccines in 1896, which is the first generation of vaccines for the two types, to the 1980s, with the help of molecular biology, biochemistry and immunology and other disciplines development, vaccine research has entered the molecular level, with yeast prepared hepatitis B vaccine marked the arrival of the second vaccine revolution [1]. By the 1980s, with the development of molecular biology, biochemistry and immunology, the research of vaccine entered the molecular level, and the hepatitis B vaccine prepared by yeast marked the arrival of the second vaccine revolution, and a variety of genetically engineered subunit vaccines, such as peptide vaccines, proteoglycan vaccines, and genetically recombinant vaccines have appeared successively. And the emergence of nucleic acid vaccines including DNA vaccine and mRNA vaccine marked the beginning of the third revolution.
And nowadays, mRNA vaccines have attracted a great deal of attention in public health and disease prevention. The fact that mRNA vaccines are effective against the new coronavirus has caused strong reactions, and people have recognized mRNA vaccines more broadly and gained new insights into their mechanism of action and properties, mRNA vaccines are expected to gradually replace traditional inactivated attenuated vaccines and genetically engineered subunit vaccines because of their high efficiency, short development process, low manufacturing costs and relatively safe administration. Currently, the applications of mRNA vaccines cover a wide range of fields such as infectious diseases, cancer, immune disorders and rare disease treatment, demonstrating the potential of mRNA vaccines in different therapeutic areas, and promoting the development of them against a wide range of diseases. At the current stage, mRNA vaccines for many diseases are in clinical trials [2]. The goal of this paper is to provide an overview of the basic characteristics and mechanisms of mRNA vaccines and their applications at this stage, to discuss mRNA vaccines that are under investigation or in clinical trials, It also explores the directions in which mRNA vaccines may be feasible in the future.
2. Mechanism of action and characteristics of mRNA vaccines
2.1. Classification of mRNA vaccines
The mRNA vaccine is mainly divided into two types: self-amplified RNA (saRNA) and non-replicating mRNA. Traditional non-replicating mRNA contains only one open reading frame (ORF), and includes set of nucleotide sequences encoding virus antigen, while saRNA has a set of nucleotide sequences controlling RNA replication of viral antigen and a transgene encoding a therapeutic antigen [3]. There are three other branches of saRNA vaccines, DNA plasmid-based saRNAs, virus-like particle packages saRNAs and in vitro transcribed saRNAs, and a modified vaccine, trans-amplifying RNA (taRNA), which is based on the formal study of saRNA structures, has been added to the research cohort.
2.2. The structure of the mRNA vaccine and its modification
The structure of an mRNA molecule consists of five parts, including the ORF, the 5' cap, the 3' poly(A) tail, and the 5' and 3' UTRs [4]. In order to make the exogenous RNA sequence in the mRNA vaccine can produce the expected effect after entering the human body, modulation of the immunogenicity of the mRNA, to ensure the stability of its structure, the degree of translation accuracy and translation rate, as well as the half-life of the RNA are necessary, and for this purpose, we need to modify the mRNA in vitro. To date, the main methods of mRNA modification include, but are not limited to (1) Enhancing its stability by adding a cap at the 5' end to ensure proper initiation and preventing degradation of translation by 5' nucleic acid exonucleases. (2) Optimizing the UTR region by adding specific modifiable `sequences to the 3' and 5' UTR regions to avoid the use of unstable sequences in order to improve translation efficiency and extend the half-life of the mRNA. (3) Modify the 3'poly (A) tail to stabilize the structure of mRNA and improve translation efficiency. (4) Optimization of codons in the ORF region, using codons with higher t RNA abundance and or synonymous frequent codons instead of rare codons in the nucleotide sequence to improve translation rate [5,6].
2.3. Mechanisms of immune response induction by mRNA vaccines
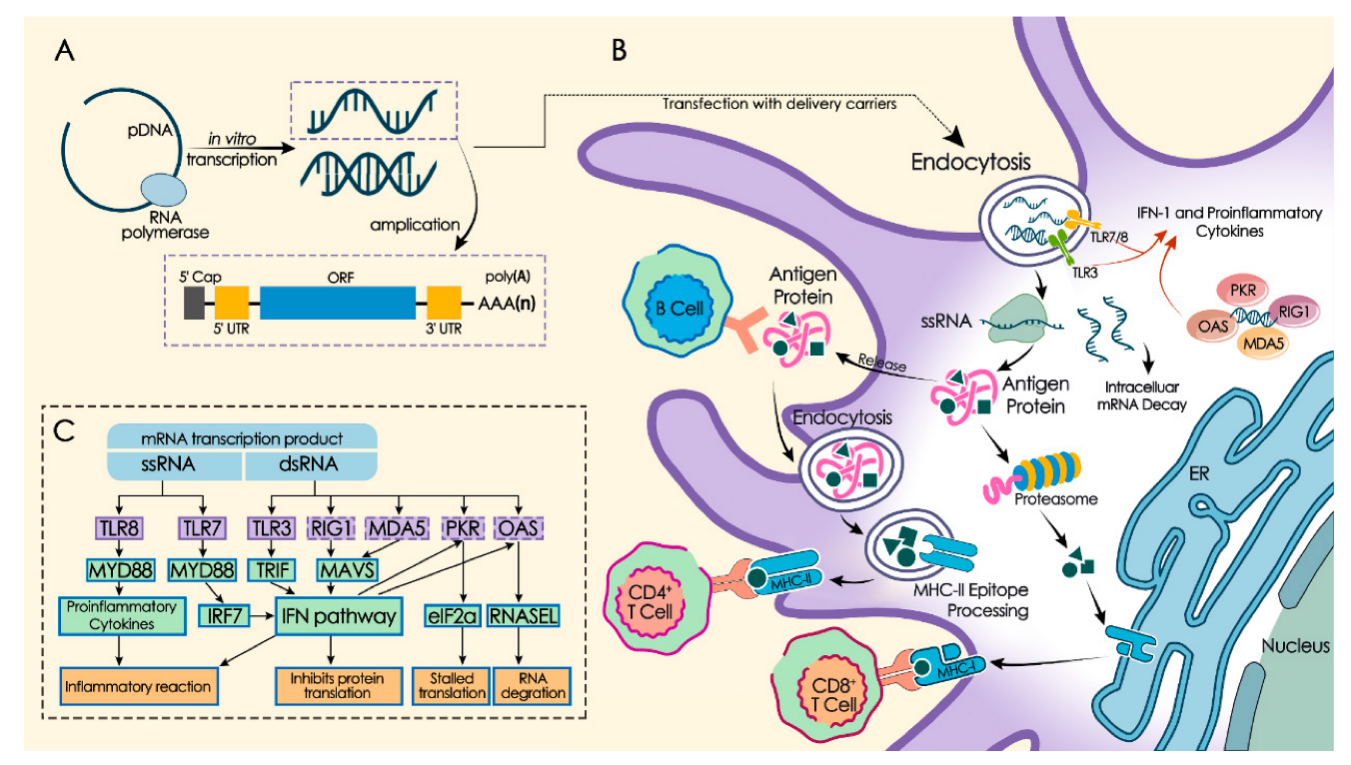
Conventional vaccinations, as seen in Figure 1, work by injecting genetically recombinant pathogen components or inactivated or attenuated pathogens to elicit immune responses. Upon inoculating host cells, mRNA vaccines are translated into matching antigens, which replicate viral infection and trigger both humoral and cellular immune responses. This antigen is presented to the T cell receptor (TCR) on CD8+ and CD4+ T cells after binding to major histocompatibility complex (MHC) I and II. This activation of T cells results in the induction of particular immunological responses.
After entering the human body as an exogenous immunogen, mRNA vaccines can be recognized by antigen presenting cells (APCs) such as macrophages and dendritic cells, activating a series of pattern recognition receptors (PRRs), such as Toll-like receptor 3 (TLR3), TLR7, TLR8, etc., and then activating these APCs, promoting the production of inflammatory cytokines and type I interferon (IFN), and ultimately inducing cellular and humoral immune responses [5,7]. However, when exogenous mRNA in the cytoplasm is recognized by PRR and causes an excessive immune response, the cell will secrete excessive IFN, which may inhibit the translation and post-translational modification of mRNA and promote mRNA degradation [8]. The research results on IFN are inconsistent. In the context of self-amplifying mRNA vaccines, it has been shown that blocking IFN signaling can enhance the expression of mRNA-targeted antigens and antigen-specific antibodies, as well as the immune response of CD8+T cells [9]. On the other hand, in the study of tumor RNA vaccines, it was found that IFN contributes to the anti-tumor effect of RNA-LNP vaccines [10].
Therefore, mRNA vaccines are translated into antigens in host cells, simulating viral infection and inducing humoral and cellular immunity. After entering the human body, they can activate antigen presenting cells and a series of pattern recognition receptors, inducing cellular and humoral immune responses. However, excessive immune responses may inhibit the translation of mRNA and promote its degradation. There is a duality in the role of IFN. Blocking its signaling in saRNA vaccines can enhance the immune response, while in tumor RNA vaccines it helps to enhance the anti-tumor effect. Subsequent studies can further analyze the specific mechanism of action of IFN in different vaccine types.
|
Figure 1. Immunity mechanism of mRNA vaccine [6].
2.4. Characteristics of mRNA vaccine
The first advantage of mRNA vaccines is that they are relatively simple and fast to manufacture. mRNA vaccines are based on the principle that mRNA sequences encoding viral target antigens or immunogens are delivered to the cytoplasm of the cell to trigger an immune response. Once the nucleic acid sequence used to encode the immunogen has been determined, RNA synthesis can be immediately initiated on the same platform, and the process can be cell-free and easily amplified. Thus the preparation and development of mRNA vaccines is platform-based and requires minimal changes. Secondly, mRNA vaccines can rapidly utilize cells to translate and express the target antigen after entering the cytoplasm. Compared to DNA vaccines, which are also nucleic acid vaccines, mRNA vaccines have a higher safety profile. Since the translation process of mRNA occurs in the cytoplasm of the cell and does not enter the nucleus of the cell, it is less likely to be integrated into the body's own genome. Thirdly, unlike attenuated inactivated vaccines and subunit vaccines, mRNA vaccines provide antigens synthesized by the body itself, so these antigens can mimic the structure of the viral genome to a certain extent, making the immune response triggered by them more targeted [11].
Overall, the advantages of mRNA vaccines over traditional vaccines are very obvious. Due to the platform development, the R&D and shortening cycle is greatly reduced, and due to its structural characteristics, it is possible to insert a variety of mRNA fragments encoding pathogens into a single vaccine; the probability of mutation is small after entry into the human body, which triggers a safer and more effective immune response. However, its shortcomings should not be ignored, in the regulation of immunogenicity, the safety and stability of the vaccine there is still some room for progress, while in the storage temperature mRNA vaccines generally need to be stored or transported at a lower temperature.
3. Current applications of mRNA vaccines
Community (BNT162b2) and Spikevax (mRNA-1273). Two of the first mRNA vaccines approved for use against COVID-19 demonstrated the potential of mRNA vaccines and ushered in another vaccine revolution. In the context of current research, the two main delivery agents for mRNA delivery are polymers and lipid nanoparticles (LNPs). Clinical studies on mRNA vaccines mainly focused on preventing infectious diseases and cancer (Table 1).
In the case of influenza, where the high degree of variability makes it difficult to treat and prevent, mRNA vaccines have been shown to be able to mimic the mRNA of the virus to a certain extent after entering the human body, and therefore have a natural advantage in preventing new subtypes of influenza viruses. Currently, mRNA vaccines against infectious diseases that have entered phase II of clinical trials include Moderna's mRNA-1647, targeting CMV, and mRNA-1010, targeting influenza A (H1N1, H3N2) and B (Yamagata lineage, Victoria lineage), with more mRNA vaccines in clinical trials. More mRNA vaccines are in clinical trials, including but not limited to: Zika virus, hMPV/PIV3, RSV, H7N9, H10N8, Chikungunya and Rabies.
The primary benefit of mRNA cancer vaccines is their potent immunogenicity, which can direct the body to generate potent cellular and humoral immune responses, hence exerting notable anti-tumor effects. In addition, the vaccine is highly successful in treating metastatic tumors because it elicits humoral and cellular immune responses that spread throughout the body. Moreover, mRNA cancer vaccines have the ability to halt tumor recurrence and develop enduring immunological memory. Currently, non-small cell lung cancer (NSCLC), melanoma, solid tumors, colorectal cancer, prostate cancer, head and neck squamous cell carcinoma, skin cancer, etc. are among the diseases for which mRNA tumor vaccines are being studied in Phase I and Phase II clinical studies [12].
Table 1. mRNA Cancer vaccines under clinical trials (phase Ⅰ, Ⅱ [12])
Vaccine type | Cancer type | Phase | Sponsor |
GRT-C901/GRT-R902 | NSCLC; Colorectal cancer; Gastroesophageal Adenocarcinoma; Urothelial Carcinoma | Ⅰ,Ⅱ | Gritstone bio |
mRNA-4157 | Melanoma | Ⅱ | Moderna |
mRNA-4157 | Unresectable solid tumors | Ⅰ | Moderna |
mRNA-4650 | Solid tumors | Ⅰ,Ⅱ | National cancer insitiute |
BNT-122 | Melanoma; NSCLC; Bladder; Colorectal; Triple negative; Renal; Head and neck | Ⅰ | Genentech |
RO7198457 | Colorectal cancer | Ⅱ | BioNTech |
RO7198457 | Pancreatic cancer | Ⅰ | BioNTech |
BNT-112 | Prostate cancer | Ⅰ,Ⅱ | BioNTech |
BNT-113 | HPV 16+ cancer | Ⅰ,Ⅱ | BioNTech |
BNT-115 | Ovarian cancer | Ⅰ | University Medical Center Groningen |
HBV mRNA vaccine | HBV-related Refractory Hepatocelluar | Ⅰ | West China Hospital |
EBV mRNA vaccine | EBV-positive advanced malignant Tumors | Ⅰ | West China Hospital |
4. Conclusion
After decades of research, the basic principles of mRNA vaccines have been basically understood, and since the outbreak of COVID-19 mRNA vaccines have made great progress, first from scratch to obtain FDA approval for marketing, and then for the mRNA vaccine research and development to achieve a spurt of growth, so far there are dozens of mRNA vaccines for different kinds of diseases are in clinical trials. To date, dozens of mRNA vaccines for different diseases are undergoing clinical trials. Through this paper, we have shown the core principles of mRNA vaccines, their current status, and their potential for future applications. However, there are still some limitations in this paper, and some problems and deficiencies still need to be explored in the current stage of mRNA vaccines; the delivery system, which is very important for mRNA vaccines, has not been mentioned in this paper. It is expected that readers can have a basic understanding of mRNA vaccines and learn the research results of It at this stage and their great potential in the future development, which will provide reference for the further development of subsequent vaccines. The author thinks that at this stage of research, compared with the traditional attenuated inactivated vaccines, subunit vaccines and DNA vaccines, the advantages and disadvantages of mRNA vaccines are very obvious, and the rapidity and convenience of its manufacturing and development, its high safety and its ability to carry a variety of encoded antigens at once have brought it a great potential for application. The further development of mRNA vaccines in the future is expected to be very fruitful. It is expected that we can find a perfect solution to regulate the immunogenicity of mRNA vaccines in order to obtain the best human immune response, solve the existing problems in structural stability, safety and transportation conditions, and make breakthroughs in the direction of targeted cancer therapy, so as to change the status quo of cancer treatment.
References
[1]. Negahdaripour M & Ghasemi Y 2022 Witnessing a revolution in the vaccinology field: A thought on its probable impact on future vaccines Trends Pharm Sci 8 2 67-68
[2]. Pardi N Hogan M J & Weissman D 2020 Recent advances in mRNA vaccine technology Curr Opin Immunol 65 14-20
[3]. Wang Y Zhang Z Luo J et al 2021 mRNA vaccine: A potential therapeutic strategy Mol Cancer 20 33
[4]. Wang Y S Kumari M Chen G H et al 2023 mRNA-based vaccines and therapeutics: An in-depth survey of current and upcoming clinical applications J Biomed Sci 30 84
[5]. Chen Y & Sun Y 2021 Progress in mRNA vaccine—The 2021 Lasker Clinical Medicine Research Award J Capital Med Univ 42 5 893-889
[6]. Xu S Yang K Li R & Zhang L 2020 mRNA vaccine era—Mechanisms drug platform and clinical prospection Int J Mol Sci 21 18 6582
[7]. Yang J Xiao L J & Fang T W 2024 Research strategies and prospects for tuberculosis mRNA vaccines Chin J Tuberc Dis 05 590-595
[8]. Linares-Fernández S Lacroix C Exposito J-Y & Verrier B 2019 Tailoring mRNA vaccine to balance innate/adaptive immune response Trends Mol Med
[9]. Pepini T Pulichino A-M Carsillo T Carlson A L Sari-Sarraf F Ramsauer K … Iavarone C 2017 Induction of an IFN-mediated antiviral response by a self-amplifying RNA vaccine: Implications for vaccine design J Immunol 198 10 4012-4024
[10]. Kranz L M Diken M Haas H et al 2016 Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy Nature 534 7607 396-401
[11]. Park J W Lagniton P N Liu Y & Xu R H 2021 mRNA vaccines for COVID-19: What why and how Int J Biol Sci 17 6 1446
[12]. Wang B Pei J Xu S Liu J & Yu J 2023 Recent advances in mRNA cancer vaccines: Meeting challenges and embracing opportunities Front Immunol 14 1246682
Cite this article
Wang,K. (2024). Mechanism of mRNA vaccine, current study and direction of development. Theoretical and Natural Science,48,28-33.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Environmental Geoscience and Earth Ecology
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Negahdaripour M & Ghasemi Y 2022 Witnessing a revolution in the vaccinology field: A thought on its probable impact on future vaccines Trends Pharm Sci 8 2 67-68
[2]. Pardi N Hogan M J & Weissman D 2020 Recent advances in mRNA vaccine technology Curr Opin Immunol 65 14-20
[3]. Wang Y Zhang Z Luo J et al 2021 mRNA vaccine: A potential therapeutic strategy Mol Cancer 20 33
[4]. Wang Y S Kumari M Chen G H et al 2023 mRNA-based vaccines and therapeutics: An in-depth survey of current and upcoming clinical applications J Biomed Sci 30 84
[5]. Chen Y & Sun Y 2021 Progress in mRNA vaccine—The 2021 Lasker Clinical Medicine Research Award J Capital Med Univ 42 5 893-889
[6]. Xu S Yang K Li R & Zhang L 2020 mRNA vaccine era—Mechanisms drug platform and clinical prospection Int J Mol Sci 21 18 6582
[7]. Yang J Xiao L J & Fang T W 2024 Research strategies and prospects for tuberculosis mRNA vaccines Chin J Tuberc Dis 05 590-595
[8]. Linares-Fernández S Lacroix C Exposito J-Y & Verrier B 2019 Tailoring mRNA vaccine to balance innate/adaptive immune response Trends Mol Med
[9]. Pepini T Pulichino A-M Carsillo T Carlson A L Sari-Sarraf F Ramsauer K … Iavarone C 2017 Induction of an IFN-mediated antiviral response by a self-amplifying RNA vaccine: Implications for vaccine design J Immunol 198 10 4012-4024
[10]. Kranz L M Diken M Haas H et al 2016 Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy Nature 534 7607 396-401
[11]. Park J W Lagniton P N Liu Y & Xu R H 2021 mRNA vaccines for COVID-19: What why and how Int J Biol Sci 17 6 1446
[12]. Wang B Pei J Xu S Liu J & Yu J 2023 Recent advances in mRNA cancer vaccines: Meeting challenges and embracing opportunities Front Immunol 14 1246682