1. Introduction
Siglec-15, officially known as Sialic Acid-Binding Immunoglobulin-Like Lectin 15, serves as a vital immunomodulatory protein. As an integral component of the Siglec family, characterized by its high degree of conservation, this molecule is detected in a range of immune system cells. These include, but are not limited to, osteoclasts, macrophages, and dendritic cells [1]. Siglec family is known for its unique ability to bind to sialylated glycans, contributing significantly to the regulation of the immune system. In normal non-immune organs, the expression of Siglec-15 is relatively low, but it exhibits an abnormally elevated expression pattern in a variety of cancers, and its expression level is closely connected to the survival of patients. Specifically, in malignant tumors such as pancreatic cancer, sarcoma, and clear cell renal carcinoma, an increase in the mRNA level of Siglec-15 often suggests a shorter survival period for patients. However, in cancers such as head and neck cancer, thyroid cancer, and endometrial cancer, the trend is reversed, where an increase in the expression of Siglec-15 appears to be associated with a more favorable prognosis [2]. This discrepancy may be related to the type of cancer, its biological characteristics, and individual patient differences, and further research will help to uncover the mechanisms behind it.
Siglec-15 is believed to be instrumental in tumor immune evasion [3]. Through its interaction with sialylated ligands, it contributes to the modulation of immune responses within the tumor microenvironment. Additionally, Siglec-15 is characterized by a diverse array of genetic mutations across various cancer types and interacts with numerous genes, potentially influencing the biological behavior and therapeutic response of tumors [2].
Given its complex role in tumor development, Siglec-15 has garnered attention as a promising target for cancer therapy. As a potential focus for immunotherapy, treatments targeting Siglec-15 could provide new avenues for cancer patients, particularly those who do not respond to PD-L1 immunotherapy [1]. Furthermore, the high expression levels or functional abnormalities of Siglec-15 may serve as valuable molecular markers for cancer treatment, facilitating the development of innovative diagnostic and therapeutic approaches. Siglec-15, as a promising target of cancer, has been proven in multiple studies, including lung adenocarcinoma, liver cancer, etc. In lung adenocarcinoma, the expression patterns of Siglec-15 suggest a potential association with prognostic outcomes [4]. In liver cancer, Siglec-15 has been found to enhance the migration of liver cancer cells by suppressing the lysosomal degradation of CD44 [5].
This review comprehensively examines the multifaceted role of Siglec-15 in the immune system and its significance in various types of cancer. It delves into the interplay between Siglec-15 and the immune system, as well as its regulatory mechanisms in immune function, with a focus on its impact on osteoclastogenesis and the tumor microenvironment. Additionally, the interactions of Siglec-15 with different cancer types are summarized, offering insights that could inform the development of innovative therapeutic strategies that harness the immunomodulatory characteristics of Siglec-15.
2. Role of Siglec-15 in immunology
Siglec-15, identified as a member of the Siglec family of type I transmembrane proteins, plays a sophisticated and multifaceted role in the modulation of the immune system [1]. This molecule interacts with a variety of ligands on immune cells, influencing the regulation of immune responses.
In the field of skeletal biology, Siglec-15 is crucial for osteoclastogenesis and the function of osteoclasts through its binding to DAP12 and the activation of the Syk signaling pathway [1]. Evidence suggests that the activation domain of Siglec-15 can transmit positive signals that promote the generation and bone resorptive activity of osteoclasts [1]. In mouse models with a genetic deletion of Siglec-15, an increase in bone density has been noted, further corroborating the molecule's key role in bone remodeling [3].
In tumor immunology, the role of Siglec-15 is equally significant. Its expression on tumor-associated macrophages (TAMs) enables it to regulate the tumor microenvironment via the DAP12-Syk signaling pathway, impacting tumor progression and metastasis [1]. Notably, the sequence homology between Siglec-15 and PD-L1 indicates a potential role in tumor immune evasion [3]. Indeed, Siglec-15 has been shown to suppress the activation and proliferation of T cells, thereby fostering an immunosuppressive environment in the tumor microenvironment [1].
Furthermore, the unique expression pattern and functionality of Siglec-15 make it an attractive therapeutic target, especially for cancer patients who do not respond to traditional PD-1/PD-L1 immunotherapies. Targeted therapeutic strategies against Siglec-15, such as the use of specific antibodies, may help restore normal immune responses within the tumor microenvironment, offering a novel approach to cancer treatment [1,3].
3. Targeting Siglec-15 on multiple cancers
Siglec-15 has been found upregulated in multiple cancers (Table 1). Targeting Siglec-15 has been shown therapeutic role in cancers.
3.1. Lung adenocarcinoma
In the field of lung adenocarcinoma (LUAD) research, the expression characteristics and prognostic role of the immune-suppressive molecule Siglec-15 have garnered widespread attention.
Utilizing bioinformatics methods, Sun et al. discovered that the expression of Siglec-15 is significantly upregulated in LUAD tissue across various types of human cancers [4]. Subsequent experiments using real-time quantitative PCR, Western blotting, and immunohistochemistry further confirmed the significantly higher expression of Siglec-15 at both the mRNA and protein levels compared to non-cancerous tissues. Additionally, the expression level of Siglec-15 was found to be closely correlated with the tumor's TNM staging, suggesting its potential key role in the progression of LUAD [4].
Researchers have meticulously described the prognostic parameters of LUAD patients using survival analysis and Kaplan-Meier curves. The analysis revealed that patients with high expression levels of Siglec-15, particularly those with lymph node metastasis (N-positive) and advanced TNM staging, have significantly poorer prognoses [4].
Synthesizing these findings, Sun and colleagues concluded that Siglec-15 is not only upregulated in LUAD but also that its high expression may predict advanced disease stages and unfavorable outcomes, making it a potential new prognostic biomarker [4]. Furthermore, therapeutic strategies targeting Siglec-15 could pave the way for new immunotherapies for LUAD, especially for patient populations that do not respond to traditional PD-1/PD-L1 treatments.
3.2. Liver cancer
Hepatocellular carcinoma (HCC) is a prevalent type of cancer that poses a serious threat to individuals' well-being and daily life [6]. Recent studies have indicated that Siglec-15 could emerge as a novel target for cancer immunotherapy. The research team led by Liu has conducted a series of in vitro experiments, encompassing cell culture, flow cytometry assays, pull-down experiments, wound healing assays, and Transwell migration assays, to elucidate the mechanisms by which Siglec-15 facilitates the migration of liver cancer cells [5]. Their findings reveal that Siglec-15 interacts with CD44 (a transmembrane glycoprotein known for its close association with tumor progression and metastasis) [7]. Notably, the α2,6-linked sialic acid on CD44 is crucial for its binding to Siglec-15 [5].
Further experimental results suggested that Siglec-15, through its interaction with CD44, enhanced the stability of the CD44 protein, specifically by inhibiting its lysosomal degradation [5]. The increased stability, in turn, promotes the migratory capacity of liver cancer cells (Figure 1). These insights provide a novel molecular mechanism for understanding how liver cancer cells acquire invasiveness and metastatic potential.
Additionally, the study leveraged public databases such as Oncomine, TIMER, and TCGA to analyze the expression patterns of Siglec-15 across various tumors, discovering its widespread overexpression in multiple solid tumors, which further underscores the potential of Siglec-15 as a target for cancer therapy [4].
The study concludes by highlighting a new mechanism by which Siglec-15, within the tumor microenvironment of liver cancer, promotes the migration of tumor cells through its interaction with CD44 [4]. These findings not only deepen our understanding of the molecular biology of liver cancer but may also contribute to the development of novel immunotherapeutic strategies. Targeting Siglec-15 or its interaction with CD44 could offer new therapeutic options for liver cancer patients.
3.3. Pancreatic ductal adenocarcinoma
Pancreatic Ductal Adenocarcinoma (PDAC) stands as an exceedingly malignant tumor with a survival outlook that is notably poor, with a 5-year survival rate that hovers below 8% and a suboptimal response to standard chemotherapeutic interventions [8]. There is an acute necessity for pioneering therapeutic methodologies. A comprehensive examination of 291 PDAC patient profiles was conducted to scrutinize the presence of Siglec-15 and its interplay with clinical and pathological traits, alongside its linkage to the programmed cell death protein 1 ligand (PD-L1), immune cell populations, and the DNA damage response (DDR) machinery [8].
The investigation yielded that Siglec-15 manifested a positive presence in 18.6% of the PDAC specimens, juxtaposed with a 30.3% positive rate for PD-L1. A particularly striking observation was that 6.1% of the specimens exhibited a concurrent presence of Siglec-15 and PD-L1, with an additional 18.0% of PD-L1-negative specimens demonstrating a positive indication for Siglec-15. The manifestation of Siglec-15 was notably more frequent in tumors exhibiting a moderate to robust differentiation grade and was correlated with a diminished concentration of T regulatory lymphocytes and CD45RO T lymphocytes, an elevated expression of the BRCA1 gene, and an enhanced survival prognosis [8].
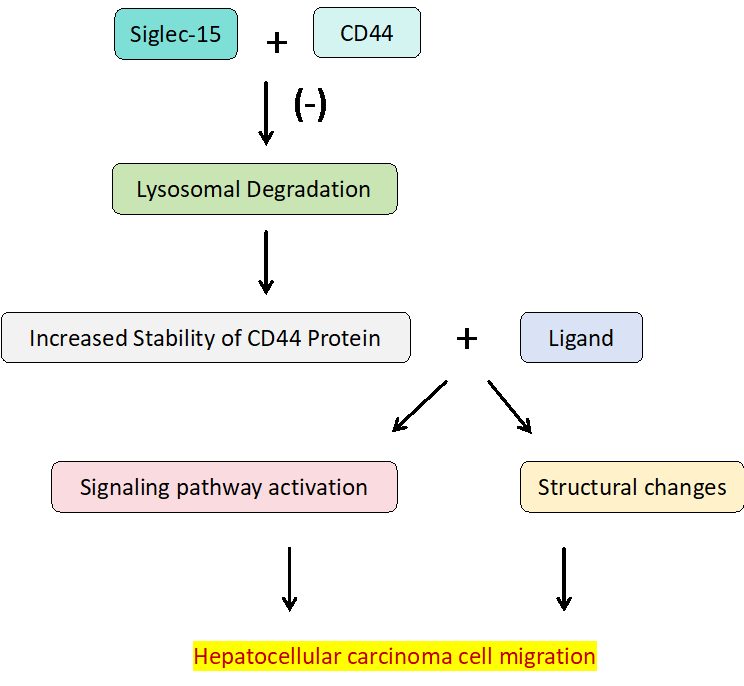
Figure 1. Mechanisms of siglec-15 promoting liver cancer cell migration. Siglec-15 binds to the sialic acid structures on CD44, inhibiting the lysosomal degradation pathway of CD44, thereby enhancing the stability of CD44. The stable CD44 protein interacts with its ligands to exert its effects, ultimately leading to the migration of liver cancer cells. Figure credit: original.
The research culminated in the assertion that Siglec-15 emerges as a prospective marker for the prognostication of PDAC patient outcomes. The presence of Siglec-15 was found to be in tandem with an augmented progression-free interval and disease-specific survival span, establishing it as a distinct prognostic determinant. Moreover, the study illuminated that the prognostic implications of Siglec-15 are subject to modulation by the status of lymph node involvement, the grade of the tumor, and the BRCA1/2 genetic milieu [8]. Specifically, the prognostic merit of Siglec-15 positivity is markedly pronounced in tumors that are free from lymph node involvement, exhibit heightened BRCA1 expression, or present with diminished BRCA2 expression [8].
3.4. Endometrial cancer
Endometrial cancer is one of the most prevalent malignancies within the female reproductive system, with an increasing incidence rate that is also trending towards younger demographics. In an effort to delineate the expression profiles of Siglec-15 within the spectrum of endometrial cancer variants and to scrutinize its clinical and pathological interconnections, a team of researchers spearheaded by Qiu, has conducted an in-depth analysis of medical records and tissue samples from 124 endometrial cancer patients who received surgical interventions at the First Hospital of Zhengzhou University from the onset of 2018 through the close of 2021 [9]. Employing immunohistochemical techniques, the team evaluated the presence of the Siglec-15 protein across the sampled tissues. The study uncovered a starkly low incidence of Siglec-15 in healthy endometrial tissues, contrasting with an escalating gradient of expression as the condition progresses from atypical hyperplasia to endometrioid and serous carcinomas [9]. Intriguingly, within the context of endometrioid adenocarcinoma, a pronounced correlation emerged between Siglec-15 expression and the depth of myometrial infiltration, as well as the hormonal status of the patients [9]. In the case of serous endometrial carcinoma, the research highlighted an intriguing phenomenon where the expression of Siglec-15 in metastatic lymph nodes was observed to surpass that of the primary lesion, indicating a potential role for Siglec-15 in the mechanisms of tumor invasiveness and metastatic spread [9].
Table 1. Targeting Siglec-15 in cancers.
Cancer type | Expression pattern | Prognosis correlation | Mechanism | |
Lung Adenocarcinoma (LUAD) | Upregulated in tumor tissues | Poor prognosis with high expression | Immunosuppression, T cell inhibition | [4] |
Liver Cancer (Hepatocellular Carcinoma, HCC) | Upregulated in tumor tissues | Associated with tumor progression and metastasis | Enhance the migration of liver cancer cells by interacting with CD44 | [5] |
Pancreatic Ductal Adenocarcinoma (PDAC) | Positive in tumor cells and macrophages | Associated with increased PFS and DSS | Inhibition of immune cell proliferation, interaction with DDR molecules | [8] |
Endometrial Cancer | Increased expression with disease progression | Related to myometrial invasion depth and patient hormonal status | Potential role in tumor invasion and metastasis in serous adenocarcinoma | [9] |
Abbreviations: PFS, Progression-Free Survival; DSS, Disease-Specific Survival; DDR, DNA Damage Response.
4. Conclusion
Siglec-15 plays a multifaceted role in the immune system. It has a unique high affinity for sialylated glycans on immune cells and cancer cells, modulating immune responses, promoting tumor progression and immune evasion, and is of significant importance in various cancers. In lung adenocarcinoma, the elevated expression of Siglec-15 is closely correlated with tumor TNM staging, indicating its role in tumor progression and serving as a potential prognostic biomarker for disease advancement and patient outcomes. Furthermore, in liver cancer, Siglec-15 enhances the migratory capacity of liver cancer cells by interacting with CD44, a mechanism that involves inhibiting the lysosomal degradation of CD44, thereby significantly enhancing the invasiveness and metastatic potential of the tumor. In pancreatic ductal adenocarcinoma, the expression of Siglec-15 correlates with improved survival outcomes, particularly in tumors without lymph node involvement and with increased BRCA1 or decreased BRCA2 expression, demonstrating its potential as an independent prognostic. Lastly, in endometrial cancer, the expression of Siglec-15 increases with the progression of the disease, especially in serous endometrial carcinoma, where its expression in metastatic lymph nodes exceeds that of the primary lesion, suggesting its role in tumor invasiveness and metastasis. Targeted therapeutic strategies for Siglec-15, such as specific antibodies, may help restore normal immune responses in the tumor microenvironment, providing new approaches for cancer treatment. Thus, it is necessary to further study the interaction mechanisms of Siglec-15 with other proteins, its role in tumor biology, and its potential as a diagnostic and therapeutic target. This could pave the way for innovative diagnostic tools and therapeutic strategies, ultimately improving the treatment outcomes for cancer patients.
References
[1]. Kang FB, Chen W, Wang L and Zhang YZ 2020 Pharmacol. Res. 155 104728
[2]. Li QT, Huang ZZ, Chen YB, Yao HY, Ke ZH, He XX, Qiu MJ, Wang MM, Xiong ZF and Yang SL 2020 J. Cancer. 11 2453-2464
[3]. Rashid S, Song D, Yuan J, Mullin BH, and Xu J 2022 J. Cell. Physiol. 237 1711-1719
[4]. Sun H, Du Q, Xu Y, Rao C, Xu L, Yang J, Mao Y and Wang L 2024 Clin. Respir. J. 18 e13772
[5]. Liu W, Ji Z, Wu B, Huang S, Chen Q, Chen X, Wei Y and Jiang J 2021 FEBS Lett. 595 2290-2302
[6]. Saeki I et al. 2018 World J. Hepatol. 10 571-584
[7]. Song Liao C, Wang Q, An J, Chen J, Li X, Long Q, Xiao L, Guan X and Liu J 2022 Front. Oncol. 12 883831
[8]. Chen X, Mo S, Zhang Y, Ma H, Lu Z, Yu S and Chen J. 2022 J. Pathol .Clin. Res. 8 268-278
[9]. Qiu HF, Wang M, Wang D, Yan SP, Jin YQ, Shi XJ and Han LP 2024 Journal of Basic and Clinical Oncology. 37 45-48
Cite this article
Wang,Z. (2024). The multifaceted role of SIGLIC-15 in the immune system and its implications in multiple cancers. Theoretical and Natural Science,61,122-127.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Kang FB, Chen W, Wang L and Zhang YZ 2020 Pharmacol. Res. 155 104728
[2]. Li QT, Huang ZZ, Chen YB, Yao HY, Ke ZH, He XX, Qiu MJ, Wang MM, Xiong ZF and Yang SL 2020 J. Cancer. 11 2453-2464
[3]. Rashid S, Song D, Yuan J, Mullin BH, and Xu J 2022 J. Cell. Physiol. 237 1711-1719
[4]. Sun H, Du Q, Xu Y, Rao C, Xu L, Yang J, Mao Y and Wang L 2024 Clin. Respir. J. 18 e13772
[5]. Liu W, Ji Z, Wu B, Huang S, Chen Q, Chen X, Wei Y and Jiang J 2021 FEBS Lett. 595 2290-2302
[6]. Saeki I et al. 2018 World J. Hepatol. 10 571-584
[7]. Song Liao C, Wang Q, An J, Chen J, Li X, Long Q, Xiao L, Guan X and Liu J 2022 Front. Oncol. 12 883831
[8]. Chen X, Mo S, Zhang Y, Ma H, Lu Z, Yu S and Chen J. 2022 J. Pathol .Clin. Res. 8 268-278
[9]. Qiu HF, Wang M, Wang D, Yan SP, Jin YQ, Shi XJ and Han LP 2024 Journal of Basic and Clinical Oncology. 37 45-48









