1. Introduction
Alzheimer's disease is a chronic neurodegenerative disorder that is characterized by a progressive deterioration in memory and cognitive abilities, accompanied by a range of behavioral abnormalities. As the global population continues to age, the prevalence of Alzheimer's disease has risen, becoming a significant public health and social care challenge on a global scale. In 2019, approximately 51.62 million individuals were living with AD worldwide, with an estimated growth to 140 million by 2050 [1]. In 2019, global spending on AD reached approximately US$2.8 trillion [3]. It is projected that the total expenditure on Alzheimer's disease will reach $17 trillion by the middle of the 21st century [2]. In China, the number of individuals diagnosed with Alzheimer's disease was approximately 10 million in 2019, with projections indicating a significant increase to over 30 million by 2050 [1,3].
Despite extensive research efforts by the scientific community on the pathogenesis of Alzheimer's disease, the precise mechanisms remain incompletely elucidated. The primary objective of therapeutic strategies is to facilitate the breakdown of β-amyloid (Aβ) and denatured aggregated tau proteins within the brain [4]. The existing AD therapeutic drugs, while capable of improving the cognitive function of early AD patients to a certain extent, are unable to effectively halt the continued progression of the disease. In recent years, several large-scale drug clinical trials on AD, including AN1792 (the first anti-Aβ monoclonal vaccine), monoclonal antibodies against Aβ and fibrous tangles, and Semagacestat (an inhibitor of γ-secretase), have been terminated due to adverse effects and substandard efficacy in mid-term evaluation [5]. In light of these developments, researchers have initiated investigations into novel therapeutic avenues with the objective of devising more efficacious interventions for individuals diagnosed with AD. In comparison to traditional pharmacological treatments, brain-computer interface technology, as an emerging intervention, has gradually attracted the attention of researchers, particularly in terms of its non-invasive, personalized treatment, and real-time feedback capabilities [6].
At present, there are over 20 companies worldwide employing BCI technology for the purpose of disease screening and intervention in cognitive disorders. Of these, 81% have adopted a non-invasive technology approach and are primarily engaged in research and development activities, as well as clinical trials. These companies are geographically dispersed across the United States, Canada, China, India, South Korea, and the United Kingdom. In 2021, a team from Zhejiang University in China developed a closed-loop neurostimulator based on BCI technology that is capable of recognizing epileptic seizures at an early stage and delivering therapeutic electrical stimulation. This technology has already yielded significant advances in clinical research, offering novel insights into the treatment of neurodegenerative disorders such as Alzheimer's disease.
2. Pathogenesis of Cognitive Impairment in Alzheimer's Disease
The pathogenesis of Alzheimer's disease remains incompletely understood. However, researchers have proposed a variety of hypotheses and theories to explain its occurrence. The current research indicates that the pathogenesis of AD is a highly complex process that involves multiple levels, including genetics, the environment, and molecular biology. These factors interact with one another to drive the pathogenesis of AD, which results in progressive cognitive decline in patients. As shown in figure 1, the following section presents a selection of the most prominent hypotheses regarding the pathogenesis of Alzheimer's disease.
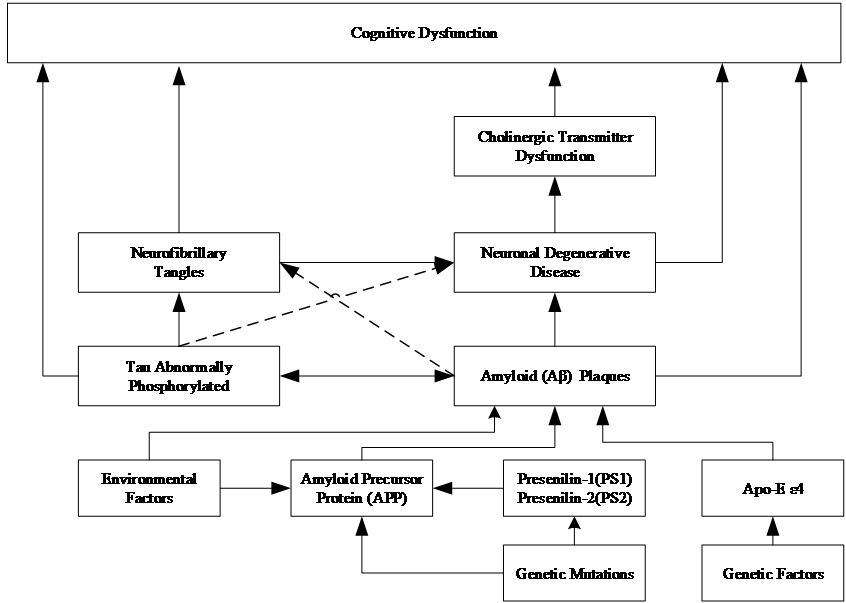
Figure 1. Pathogenesis of AD Cognitive Dysfunction.
2.1. Amyloid Plaques with Tau Protein Tangles
Abnormal accumulation of amyloid (Aβ) occurs in the brains of AD patients, leading to the formation of amyloid plaques. The formation of these plaques impedes the normal transmission of information between neurons. Concurrently, the tau protein undergoes excessive phosphorylation, resulting in the formation of neurofibrillary tangles. These tangles disrupt the cytoskeleton, influencing intracellular transport mechanisms and cellular function. This results in disruption to the transport of nutrients within neurons. These two processes interact in a manner that has a deleterious effect on brain function [7]. It has been demonstrated that inflammation plays a pivotal role in this process, intermingling with amyloid pathology and contributing to the proliferation of tau proteins, which ultimately results in extensive brain damage and cognitive impairment.
2.2. Neuronal damage and cholinergic transmitter dysfunction
As the disease progresses, neurons in the brains of AD patients gradually die and atrophy. This degenerative change is associated with a variety of factors, including the formation of Aβ plaques, and neurofibrillary tangles due to abnormal phosphorylation of tau protein, oxidative stress, neuroinflammation, and mitochondrial dysfunction. Concurrently, the cholinergic neurotransmitter system, which is responsible for memory and cognitive function, is damaged, and the reduction in cholinergic transmitters affects interneuronal communication, which in turn exacerbates degenerative changes. These lesions result in cognitive deficits, including memory loss, learning disabilities, poor concentration, executive dysfunction, decreased language and orientation, impaired judgment and social skills, and changes in mood and behavior.
2.3. Additional Considerations
The etiology of Alzheimer's disease is complex, with genetic factors, particularly the presence of the apolipoprotein E (Apo-E) ε4 allele, identified as a known genetic risk factor for AD. This increases the likelihood of an individual developing AD. Furthermore, pathological genetic mutations in the amyloid precursor protein (APP) gene, as well as progerins (PS1, PS2), have been linked to familial AD. These mutations directly impact the production of Aβ proteins and aberrant phosphorylation of tau proteins, leading to neuronal dysfunction and degeneration.
Environmental factors, including lifestyle, nutritional status, and occupational exposures, may also contribute to the development of AD. For example, poor dietary habits, a lack of physical activity, chronic stress, and sleep deprivation have been associated with an increased risk of developing AD. Furthermore, environmental factors may interact with genetic risk factors to jointly influence an individual's risk of developing AD.
Genetic mutations are a significant contributing factor in the early stages of Alzheimer's disease. Such mutations are frequently linked with familial AD and may precipitate disease onset at an earlier age. These mutations impact the production and processing of specific proteins within the brain, particularly those associated with Aβ production and aberrant phosphorylation of tau proteins.
3. Brain-computer interfaces and cognitive rehabilitation theory
3.1. Overview of brain-computer interface
A brain-computer interface is a technology that uses human or animal brainwave signals to communicate and control external devices, thereby enabling the realization of human intelligence, perception, language, behavior, and other aspects of control. BCI technology has made significant progress and breakthroughs in the fields of biomedicine, neurorehabilitation, and intelligent robotics.
BCI can be classified into two principal categories based on the methodology of signal acquisition and the intended application: invasive and non-invasive. Invasive BCI involves the direct implantation of electrodes into the brain in order to acquire signals. The advantage of BCI is that it can acquire high-quality neural signals; however, there is a risk of surgery and a potential immune response. Non-invasive BCI, which records brain activity by placing electrodes on the scalp, has the advantage of being relatively simple to perform and less risky, but the signal quality may be affected by noise. Systems such as electroencephalogram (EEG)-based systems represent a typical non-invasive BCI. Furthermore, BCIs can be categorized according to their function as motor or sensory, with the former being utilized to control external devices and the latter attempting to re-establish or enhance sensory function.
BCI's technology encompasses the entirety of the process, from signal acquisition to signal processing to control. Initially, electrophysiological signals emanating from the brain are captured by an array of sensors. These signals are then amplified and converted into a digital signal. Subsequently, the digital signal is filtered and the feature volume is extracted in order to obtain specialized information. Subsequently, pattern recognition algorithms are employed to transform these features into control commands that ultimately direct external devices to perform the requisite actions [8]. The ability to realize this process hinges on the accuracy and real-time nature of the modeling algorithms, as well as the accurate interpretation of the user's intent.
3.2. Neuroplasticity
Neuroplasticity is defined as the capacity of the brain to undergo structural and functional changes in response to external stimuli and to adapt accordingly. The brain's capacity for lifelong adaptation enables reorganization in response to learning, memory, environmental change, and injury. Neuroplasticity encompasses a range of processes, including synaptic plasticity, neurogenesis, synaptogenesis, and pruning. Neuroplasticity is a fundamental principle that underlies the entire process of functional rehabilitation following a nerve injury, and it plays a pivotal role in the rehabilitation process as a whole. The question of how to promote the plasticity and functional reorganization of the brain and spinal cord nerves is a topic of great interest in current research on functional rehabilitation after central nervous system (CNS) injury.
3.3. Cognitive Rehabilitation
Cognitive rehabilitation is a multidisciplinary treatment approach that aims to restore or optimize brain function. It is based on the theory of neuroplasticity. The theory of cognitive rehabilitation posits that the brain's intrinsic self-repairing and adaptive mechanisms can be activated through the implementation of a structured training program, thereby facilitating improvements in cognitive function. The training regimen encompasses the enhancement of memory, attention, executive functioning, and language skills. The practice of cognitive rehabilitation often necessitates the input of an interdisciplinary team, comprising neuropsychologists, speech therapists, occupational therapists, and other relevant professionals. This team works collectively to devise an individualized rehabilitation program, tailored to the specific needs of the patient and designed to facilitate their return to functioning in daily life.
4. Brain-computer interfaces in cognitive rehabilitation for Alzheimer's disease
4.1. Diagnosis of cognitive impairment
Despite the absence of a cure for cognitive disorders, early screening, diagnosis, and treatment are crucial for effective prevention and the slowing down of disease progression. At present, there are over 20 companies globally employing BCI technology for the purpose of screening and intervening in cases of cognitive impairment and decline. A total of 81% of the companies have adopted the non-implantable technology route, which is currently in the research and development phase and undergoing clinical trials.
The United States-based company CenSyn has developed a two-channel handheld device, PenEEG™, which is capable of recording electroencephalogram (EEG) activity in a mobile setting. Additionally, there are iPhone and iPad applications that are designed to view EEG data in a similar manner. Furthermore, machine learning algorithms have been developed which are able to detect brain health in less than two minutes. Looxid Labs, a company based in South Korea, employs virtual reality technology to integrate wearable electroencephalogram (EEG) sensors for the early detection of cognitive impairment. A research team led by George Stothart, a cognitive neuroscientist at the University of Bath, has developed the Fastball tool, which is based on EEG technology. The tool is capable of diagnosing early-stage dementia with mild cognitive impairment using the Fast Periodic Visual Stimulation (FPVS) method [9]. The Israeli company Quantalx employs transcranial magnetic stimulation (TMS) to induce neural activity in the brain, thereby enabling the mapping of brain network function in real-time based on neural features. This approach allows for the early detection, differential diagnosis, and prediction of neurodegenerative diseases in a non-invasive and radiation-free manner. The company's products have been recognized by the U.S. Food and Drug Administration as innovative and groundbreaking devices.
4.2. Monitoring and Evaluation
Alzheimer's disease is a degenerative disease of the central nervous system that results in a gradual decline in cognitive function. Brain-computer interface technology enables the real-time monitoring of alterations in brain activity, the assessment of the velocity and extent of disease progression, and the provision of a foundation for medical professionals to develop and modify treatment plans. By monitoring patients' EEG signals and other biomarkers over an extended period, it is possible to create personalized disease progression models that can predict future cognitive function status [10]. The 16-lead EEG head ring, developed by Cumulus Neuroscience in the UK, is a device that can be worn by patients at home. It allows them to view flashing images on tablets or cell phones in real-time, monitor changes in their EEG, and monitor, analyze, and assess their health in real-time through cloud platforms and artificial intelligence technology.
BCI technology offers a comprehensive assessment of patient cognitive function by capturing brain signals during cognitive tasks and analyzing performance in terms of attention, memory, and language ability. The reliability and validity of commonly used cognitive assessment tools, such as the Modified Mental State Examination (MMSE) and the Montreal Cognitive Assessment Scale (MoCA), are well established. However, these tools are susceptible to the influence of patients' subjective factors and the external environment. In comparison to the aforementioned cognitive assessment tools, brain-computer interface technology offers a more objective and accurate methodology for evaluation.
4.3. Personalized Cognitive Training and Rehabilitation
The use of brain-computer interface technology as an emerging means of neurofeedback has demonstrated advantages in cognitive disorders caused by Alzheimer's disease. BCI provides patients with cognitive training, life skills training, emotional management, and other aspects of cognitive training and rehabilitation programs through the real-time capture and analysis of brain activity and interaction with external devices.
In 2017, the deep brain stimulation technology developed by the Canadian company Functional Neuromodulation received CE marking for the treatment of Alzheimer's disease. The company's Vercise™ DBS system, developed in conjunction with Boston Scientific in the United States, also received United States Food and Drug Administration (FDA) breakthrough device approval in 2021, thereby providing a new treatment option for patients aged 65 and older with mild Alzheimer's disease.
A clinical trial of deep brain electrical stimulation (DBS) of the cerebral vault for the treatment of Alzheimer's disease, conducted by researchers at the Charité Medical School in Berlin, has demonstrated an improvement in cognitive function in a limited number of patients. It provides guidance for future research and treatment of neurodegenerative diseases [11].
Despite the nascent state of BCI technology in the context of Alzheimer's disease, preliminary studies and case reports have indicated the potential for this approach to enhance patient cognitive function and quality of life.
5. Challenges and Future Developments in Brain-Computer Interface Technology
5.1. Technical Challenges
Brain-computer interface technology faces numerous technical challenges in the field of assistive technology (AT) for cognitive rehabilitation. The initial challenge pertains to the accuracy of the signal. The intricate nature of brain signals and their vulnerability to external influences render the accurate interpretation and collection of signals related to cognitive functions a challenging endeavor. The quality of the signal may be affected by electromagnetic interference in the environment, individual physiological differences, and the patient's own emotional state, potentially leading to misjudgment or inaccurate guidance in rehabilitation training.
In regard to device comfort, existing BCI devices are typically bulky and uncomfortable to wear. Prolonged use may result in discomfort for patients, potentially impacting treatment compliance. Some invasive devices require surgical implantation, which carries the risk of postoperative infection. Non-invasive devices, such as electrode caps, may exert pressure on the head, thereby affecting the patient's experience. Moreover, improvements are required in terms of the stability and durability of the devices, in order to meet the needs of long-term treatment.
5.2. Ethical and Social Issues
The implementation of BCI in the context of AD treatment gives rise to a number of ethical and social concerns. From the perspective of personal privacy, the brain signals collected by BCI contain a substantial amount of personal information about the patient. Consequently, ensuring the safety and confidentiality of these sensitive data has become a critical issue. Additionally, there are debates surrounding the capacity of patients to make autonomous decisions, particularly in instances of diminished cognitive capacity. The question of how to ascertain the genuine desires of the patient and the potential for external influence must be addressed with careful scrutiny.
At the societal level, the advent of BCI technology may give rise to the emergence of novel social inequalities. The high cost of the necessary equipment may result in the exclusion of a significant proportion of patients, thereby exacerbating the existing disparities in the distribution of healthcare resources. Furthermore, the extensive implementation of BCI technology may alter the social perception of disease and health, potentially leading to discriminatory practices and other adverse outcomes. In order to address these ethical and social concerns, it is imperative that robust legislation, ethical guidelines, and regulatory frameworks be established to govern the application of this technology.
5.3. Future Development Trends
It is anticipated that BCI will make substantial advancements and gain broader acceptance in the domain of AD cognitive rehabilitation therapy in the future. As technology advances, signal acquisition and processing will become increasingly precise and efficient, enabling more accurate capture of subtle signal changes related to cognitive function and providing a more accurate basis for personalized treatment.
With regard to the equipment, it is anticipated that BCI equipment will evolve in a manner that renders it more compact, portable, and intelligent, thereby enhancing the convenience and comfort of patient use. Concurrently, the profound integration with artificial intelligence, big data, and other technologies will furnish more robust assistance for disease diagnosis, treatment, and rehabilitation, thereby enabling more precise prediction and intervention.
Furthermore, it is anticipated that BCI technology will be employed in the domain of AD prevention, with the objective of reducing the prevalence of the disease through the implementation of early monitoring and intervention strategies. Concurrently, there will be a greater emphasis on multidisciplinary collaboration, with the objective of integrating the strengths of multiple fields, including neuroscience, cognitive science, and engineering. This will facilitate the advancement of BCI technology in the treatment of AD, thereby enhancing the quality of life for patients.
6. Conclusion
This study provides a comprehensive summary of the current applications of BCI technology in AD cognitive rehabilitation, emphasizing its significant potential and value in enhancing patients' cognitive function and overall quality of life. BCI technology offers a novel approach to promoting neuroplasticity and improving cognitive function. Future studies should concentrate on the optimization and personalized implementation of BCI technology, taking into account ethical and social considerations, with the aim of facilitating the extensive application of BCI technology in AD cognitive rehabilitation. Moreover, interdisciplinary collaboration and international cooperation are essential for advancing the development of BCI technology.
References
[1]. Li X, Feng X, Sun X, Hou N, Han F, Liu Y. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990-2019. Front Aging Neurosci. 2022 Oct 10;14:937486. doi: 10.3389/fnagi.2022.937486. PMID: 36299608; PMCID: PMC9588915.
[2]. Ji Z, Chen Q, Yang J, Hou J, Wu H, Zhang L. Global, regional, and national health inequalities of Alzheimer's disease and Parkinson's disease in 204 countries, 1990-2019. Int J Equity Health. 2024 Jun 19;23(1):125. doi: 10.1186/s12939-024-02212-5. PMID: 38898437; PMCID: PMC11188225.
[3]. Wang YQ, Jia RX, Liang JH, Li J, Qian S, Li JY, Xu Y. Dementia in China (2015-2050) estimated using the 1% population sampling survey in 2015. Geriatr Gerontol Int. 2019 Nov;19(11):1096-1100. doi: 10.1111/ggi.13778. Epub 2019 Sep 18. PMID: 31535462.
[4]. Christensen DD. Changing the course of Alzheimer's disease: anti-amyloid disease-modifying treatments on the horizon. Prim Care Companion J Clin Psychiatry. 2007;9(1):32-41. doi: 10.4088/pcc.v09n0106. PMID: 17599166; PMCID: PMC1894844.
[5]. Conti Filho CE, Loss LB, Marcolongo-Pereira C, Rossoni Junior JV, Barcelos RM, Chiarelli-Neto O, da Silva BS, Passamani Ambrosio R, Castro FCAQ, Teixeira SF, Mezzomo NJ. Advances in Alzheimer's disease's pharmacological treatment. Front Pharmacol. 2023 Jan 26;14:1101452. doi: 10.3389/fphar.2023.1101452. PMID: 36817126; PMCID: PMC9933512.
[6]. Delrieu J, Ousset PJ, Caillaud C, Vellas B. 'Clinical trials in Alzheimer's disease': immunotherapy approaches. J Neurochem. 2012 Jan;120 Suppl 1:186-193. doi: 10.1111/j.1471-4159.2011.07458.x. Epub 2011 Nov 28. Retraction in: J Neurochem. 2021 Aug;158(3):821. doi: 10.1111/jnc.15435. PMID: 21883222.
[7]. Fan L, Mao C, Hu X, Zhang S, Yang Z, Hu Z, Sun H, Fan Y, Dong Y, Yang J, Shi C, Xu Y. New Insights Into the Pathogenesis of Alzheimer's Disease. Front Neurol. 2020 Jan 10;10:1312. doi: 10.3389/fneur.2019.01312. PMID: 31998208; PMCID: PMC6965067.
[8]. Wu X, Metcalfe B, He S, Tan H, Zhang D. A Review of Motor Brain-Computer Interfaces Using Intracranial Electroencephalography Based on Surface Electrodes and Depth Electrodes. IEEE Trans Neural Syst Rehabil Eng. 2024;32:2408-2431. doi: 10.1109/TNSRE.2024.3421551. Epub 2024 Jul 4. PMID: 38949928.
[9]. Perez-Valero E, Lopez-Gordo MA, Morillas C, Pelayo F, Vaquero-Blasco MA. A Review of Automated Techniques for Assisting the Early Detection of Alzheimer's Disease with a Focus on EEG. J Alzheimers Dis. 2021;80(4):1363-1376. doi: 10.3233/JAD-201455. PMID: 33682717.
[10]. Tayebi H, Azadnajafabad S, Maroufi SF, Pour-Rashidi A, Khorasanizadeh M, Faramarzi S, Slavin KV. Applications of brain-computer interfaces in neurodegenerative diseases. Neurosurg Rev. 2023 May 31;46(1):131. doi: 10.1007/s10143-023-02038-9. PMID: 37256332.
[11]. Ríos AS, Oxenford S, Neudorfer C, Butenko K, Li N, Rajamani N, Boutet A, Elias GJB, Germann J, Loh A, Deeb W, Wang F, Setsompop K, Salvato B, Almeida LB, Foote KD, Amaral R, Rosenberg PB, Tang-Wai DF, Wolk DA, Burke AD, Salloway S, Sabbagh MN, Chakravarty MM, Smith GS, Lyketsos CG, Okun MS, Anderson WS, Mari Z, Ponce FA, Lozano AM, Horn A. Optimal deep brain stimulation sites and networks for stimulation of the fornix in Alzheimer's disease. Nat Commun. 2022 Dec 14;13(1):7707. doi: 10.1038/s41467-022-34510-3. PMID: 36517479; PMCID: PMC9751139.
Cite this article
Yang,W. (2024). Brain-computer interfaces in cognitive rehabilitation for Alzheimer's disease. Theoretical and Natural Science,64,163-169.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Li X, Feng X, Sun X, Hou N, Han F, Liu Y. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990-2019. Front Aging Neurosci. 2022 Oct 10;14:937486. doi: 10.3389/fnagi.2022.937486. PMID: 36299608; PMCID: PMC9588915.
[2]. Ji Z, Chen Q, Yang J, Hou J, Wu H, Zhang L. Global, regional, and national health inequalities of Alzheimer's disease and Parkinson's disease in 204 countries, 1990-2019. Int J Equity Health. 2024 Jun 19;23(1):125. doi: 10.1186/s12939-024-02212-5. PMID: 38898437; PMCID: PMC11188225.
[3]. Wang YQ, Jia RX, Liang JH, Li J, Qian S, Li JY, Xu Y. Dementia in China (2015-2050) estimated using the 1% population sampling survey in 2015. Geriatr Gerontol Int. 2019 Nov;19(11):1096-1100. doi: 10.1111/ggi.13778. Epub 2019 Sep 18. PMID: 31535462.
[4]. Christensen DD. Changing the course of Alzheimer's disease: anti-amyloid disease-modifying treatments on the horizon. Prim Care Companion J Clin Psychiatry. 2007;9(1):32-41. doi: 10.4088/pcc.v09n0106. PMID: 17599166; PMCID: PMC1894844.
[5]. Conti Filho CE, Loss LB, Marcolongo-Pereira C, Rossoni Junior JV, Barcelos RM, Chiarelli-Neto O, da Silva BS, Passamani Ambrosio R, Castro FCAQ, Teixeira SF, Mezzomo NJ. Advances in Alzheimer's disease's pharmacological treatment. Front Pharmacol. 2023 Jan 26;14:1101452. doi: 10.3389/fphar.2023.1101452. PMID: 36817126; PMCID: PMC9933512.
[6]. Delrieu J, Ousset PJ, Caillaud C, Vellas B. 'Clinical trials in Alzheimer's disease': immunotherapy approaches. J Neurochem. 2012 Jan;120 Suppl 1:186-193. doi: 10.1111/j.1471-4159.2011.07458.x. Epub 2011 Nov 28. Retraction in: J Neurochem. 2021 Aug;158(3):821. doi: 10.1111/jnc.15435. PMID: 21883222.
[7]. Fan L, Mao C, Hu X, Zhang S, Yang Z, Hu Z, Sun H, Fan Y, Dong Y, Yang J, Shi C, Xu Y. New Insights Into the Pathogenesis of Alzheimer's Disease. Front Neurol. 2020 Jan 10;10:1312. doi: 10.3389/fneur.2019.01312. PMID: 31998208; PMCID: PMC6965067.
[8]. Wu X, Metcalfe B, He S, Tan H, Zhang D. A Review of Motor Brain-Computer Interfaces Using Intracranial Electroencephalography Based on Surface Electrodes and Depth Electrodes. IEEE Trans Neural Syst Rehabil Eng. 2024;32:2408-2431. doi: 10.1109/TNSRE.2024.3421551. Epub 2024 Jul 4. PMID: 38949928.
[9]. Perez-Valero E, Lopez-Gordo MA, Morillas C, Pelayo F, Vaquero-Blasco MA. A Review of Automated Techniques for Assisting the Early Detection of Alzheimer's Disease with a Focus on EEG. J Alzheimers Dis. 2021;80(4):1363-1376. doi: 10.3233/JAD-201455. PMID: 33682717.
[10]. Tayebi H, Azadnajafabad S, Maroufi SF, Pour-Rashidi A, Khorasanizadeh M, Faramarzi S, Slavin KV. Applications of brain-computer interfaces in neurodegenerative diseases. Neurosurg Rev. 2023 May 31;46(1):131. doi: 10.1007/s10143-023-02038-9. PMID: 37256332.
[11]. Ríos AS, Oxenford S, Neudorfer C, Butenko K, Li N, Rajamani N, Boutet A, Elias GJB, Germann J, Loh A, Deeb W, Wang F, Setsompop K, Salvato B, Almeida LB, Foote KD, Amaral R, Rosenberg PB, Tang-Wai DF, Wolk DA, Burke AD, Salloway S, Sabbagh MN, Chakravarty MM, Smith GS, Lyketsos CG, Okun MS, Anderson WS, Mari Z, Ponce FA, Lozano AM, Horn A. Optimal deep brain stimulation sites and networks for stimulation of the fornix in Alzheimer's disease. Nat Commun. 2022 Dec 14;13(1):7707. doi: 10.1038/s41467-022-34510-3. PMID: 36517479; PMCID: PMC9751139.









