1. Introduction
The issue of Gastrointestinal Diseases has received considerable critical attention. In 1990, there were an estimated 3.32 million cases, which rose to 4.90 million by 2019. Detailed analysis reveals that China and the USA lead with 911,405 and 762,890 cases, corresponding to rates of 66.9 and 245.3 cases per 100,000 individuals, respectively. Despite this increase, further evaluation indicates that age-standardized prevalence rates, mortality, and Disability-Adjusted Life Years (DALYs) for Inflammatory Bowel Disease (IBD) have escalated in 13 out of 21 regions, according to the Global Burden of Disease database. Consequently, IBD is poised to become a significant public health challenge due to the increasing prevalence, mortality, and DALY figures[1]. Enter Probiotics, live microorganisms, have emerged as potential therapeutic agents for various gastrointestinal disorders. The delicate balance of the gut microbiota is crucial for optimal digestive health, and disruptions to this ecosystem are implicated in conditions like Inflammatory Bowel Disease (IBD). Probiotics have long since been utilised and can be found on fermented foods such as cheese or milk. It is not until 1907 that Russian Zoologist Élie Metchnikoff discovered the bacteria’s therapeutic effects which are present in a few genuses of bacteria.
Several probiotic genera have demonstrated efficacy in modulating IBD pathogenesis. Lactobacillus species, renowned for their lactic acid production, have shown promise in regulating gut microbiota composition and regulating inflammation. Additionally, Saccharomyces, particularly Saccharomyces boulardii, has emerged as a therapeutic option due to its anti-inflammatory properties and ability to strengthen the intestinal barrier. [2]
IBD patients develop microbial dysbiosis with a decrease in short chained fatty acids(Il-6,8,10 etc.) and an increase in Proteobacteria(Prepares the gut for colonisation). Normally, SCFAs are a group of acids which are used to As a result, the down regulation of Muc2, RELMB proteins and E-cadherin lead to a thin mucosal layer as loose cells with openings or gaps present on the epithelial lining. Furthermore, The immune systems’ CD-14(receptors which are found to detect and respond to bacterial infections) expressing macrophages is decreased in the area. In addition, dendritic cells present a defective form of CX3CR1, leading to macrophages unable to produce IL-10 and restimulate regulatory T-cells [3-6,9] impairing Autophagy. This in all creates an imbalance between effector and regulatory T-cells in the IBD mucosa, leading to a chain reaction and uncontrolled activation of different T-cells lineages that migrate to the inflamed intestine(as shown in figure 1).
IBD can be caused by many different factors, from the interaction of cytokines or immune factors. Previous research has hinted a new cause of environmental factors, habits such as smoking, unhealthy diet or alcohol has led to the increase of omega 6 fatty acids, long chain fatty acids, proteins and digestible carbohydrates, leading to an imbalance of the microbiota and promoting inflammation, eventually leading to the diagnosis of IBD [7]. According to [8], Irritable Bowel Syndrome (IBS) and Inflammatory Bowel Disease (IBD) present comparable symptoms. These symptoms include immune activation, heightened intestinal permeability, increased mucosal serotonin levels, changes in enteric nerve structure and function, and dysbiosis in the gut microbiota observed in IBS.
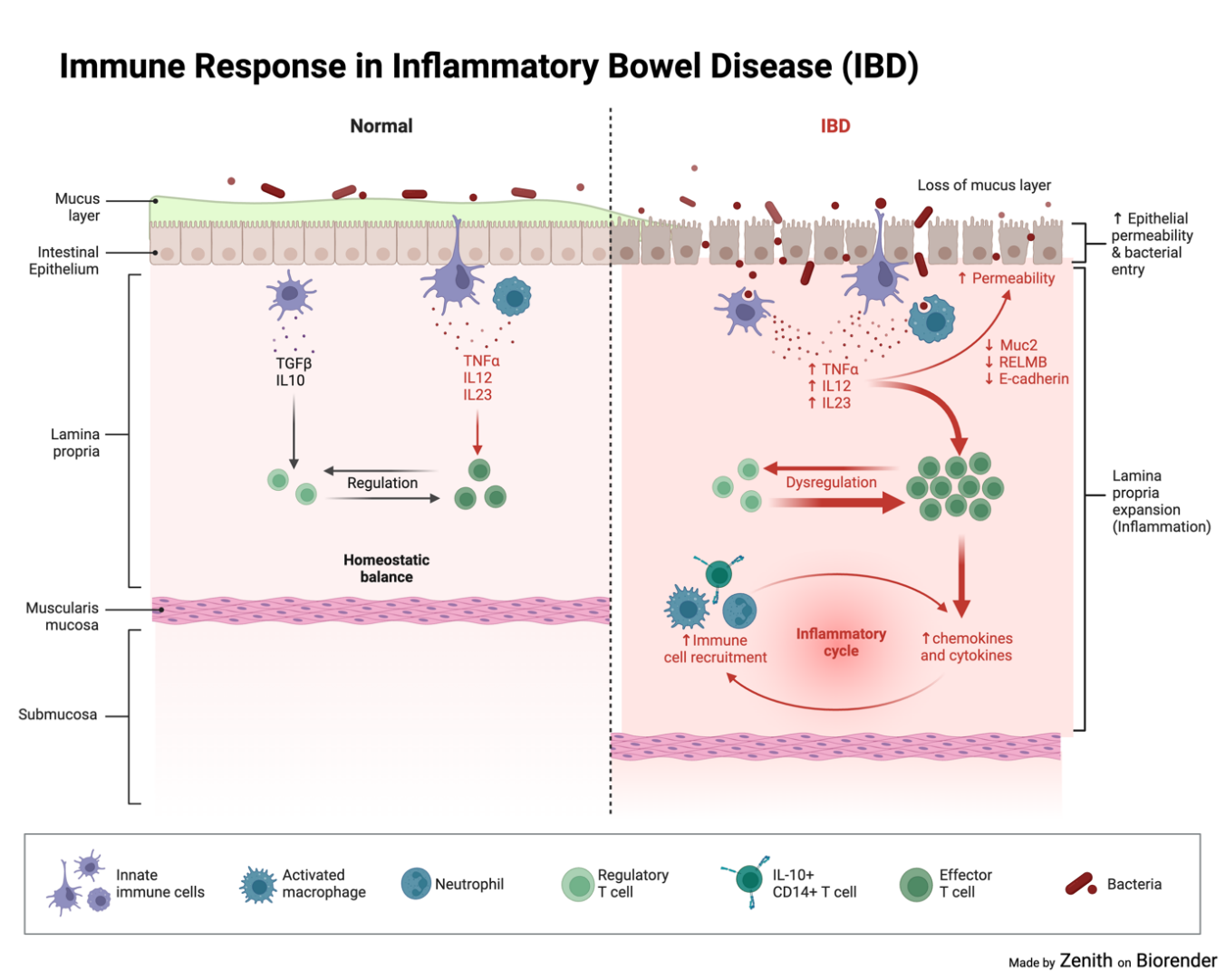
Figure 1. Immune response in inflammatory bowel disease (IBD). Bacterial invasion as a result of lacking proteins for cell maintenance and regulation of the mucosal layer.
This section has reviewed the basic information of some probiotics and some main genuses used for the research for treatment of gastrointestinal disorders. The next part will be about it’s mechanism and several probiotics used to treat the disease.
2. Discovering the Probiotics against IBD
For this study, 3 genuses of bacteria followed by their species(some may have it’s variant) using the relevance of searches we found on ProBioQuest and PubMed. After that, using literature data, we compared it’s efficacy and safety. We then will explain our conclusions using any trends or patterns we see.
2.1. Mechanisms of Action
After searching for suitable probiotics, we settled on 3 probiotics which may produce the best results. The following probiotics are: 1. Lactobacillus Rhamnosus 2.Lactobacillus Plantarum 3.E.coli Nissle 1917. 1 and 2 belongs to the Lactobacillus genus but under different species(Rhamnosus and Plantarum) while 3 is a variation of E.coli genetically modified to treat the human intestinal tract
2.1.1. Lactobacillus Rhamosus. Lactobacillus rhamnosus is a specie of bacteria from the Lactobacillus genus. When faced with an inflammatory disease, Lactobacillus Rhamnosus has been shown to regulate and reduce inflammation in epithelial cells due to it inhibiting the activation of the NF-kB signalling pathway (as shown in Figure 2), a pathway used to activate an inflammatory response through the degradation of proteins and subunits to access the nucleus and perform gene transcription, influencing various cellular functions [10, 11]. Lactobacillus rhamnosus has also been seen regulating key pro-inflammatory, anti inflammatory and immune regulation cytokines such as TNF-A, IL-6,IL-10 and IL-1B [12,13]
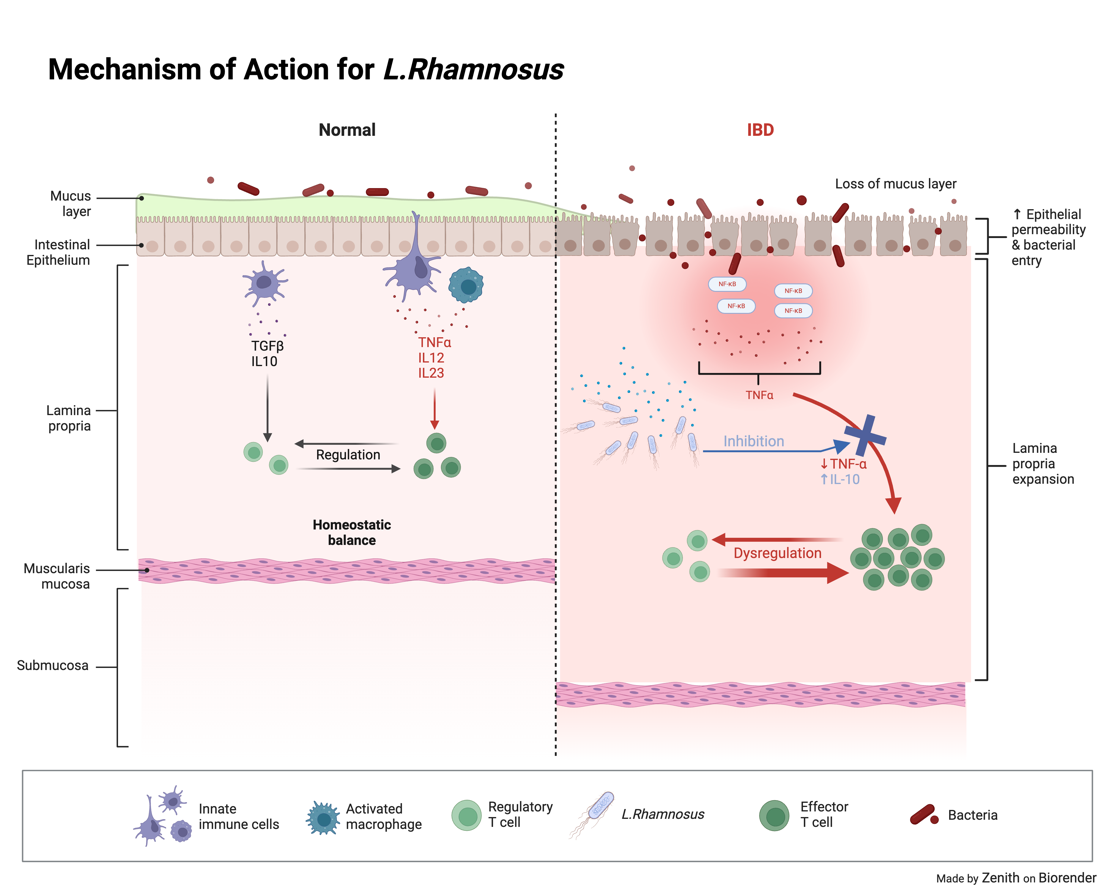
Figure 2. Mechanism of action for L.Rhamnosus. L.Rhamnosus inhibits the NF-kB pathway as well as preventing Dysregulation.
2.1.2. Lactobacillus Plantarum. Lactobacillus plantarum is another specie of bacteria from the Lactobacillus family. It is 0.5-0.8 micrometres in diameter and 8 micrometres in length. Similar to Lactobacillus rhamosus, Lactobacillus planatrum has been seen to induce an endotoxin tolerance phenotype in intestinal cells, leading to the increase in endotoxin resistance as well as a decrease in pro-inflammatory cytokines [14] due to the propionic acid production of the species contributing of the maintenance of gut homeostasis[15]. It can also increase Il-10 synthesis as well as secretion of t-cells from the inflamed areas, this provides ameliorate inappropriate inflammation[16] all the while increasing the tight-junction proteins (Zonula occludens-1 and occludin) to strengthen the intestinal barrier and closes the gap between the epithelial lining(as shown in Figure 3).
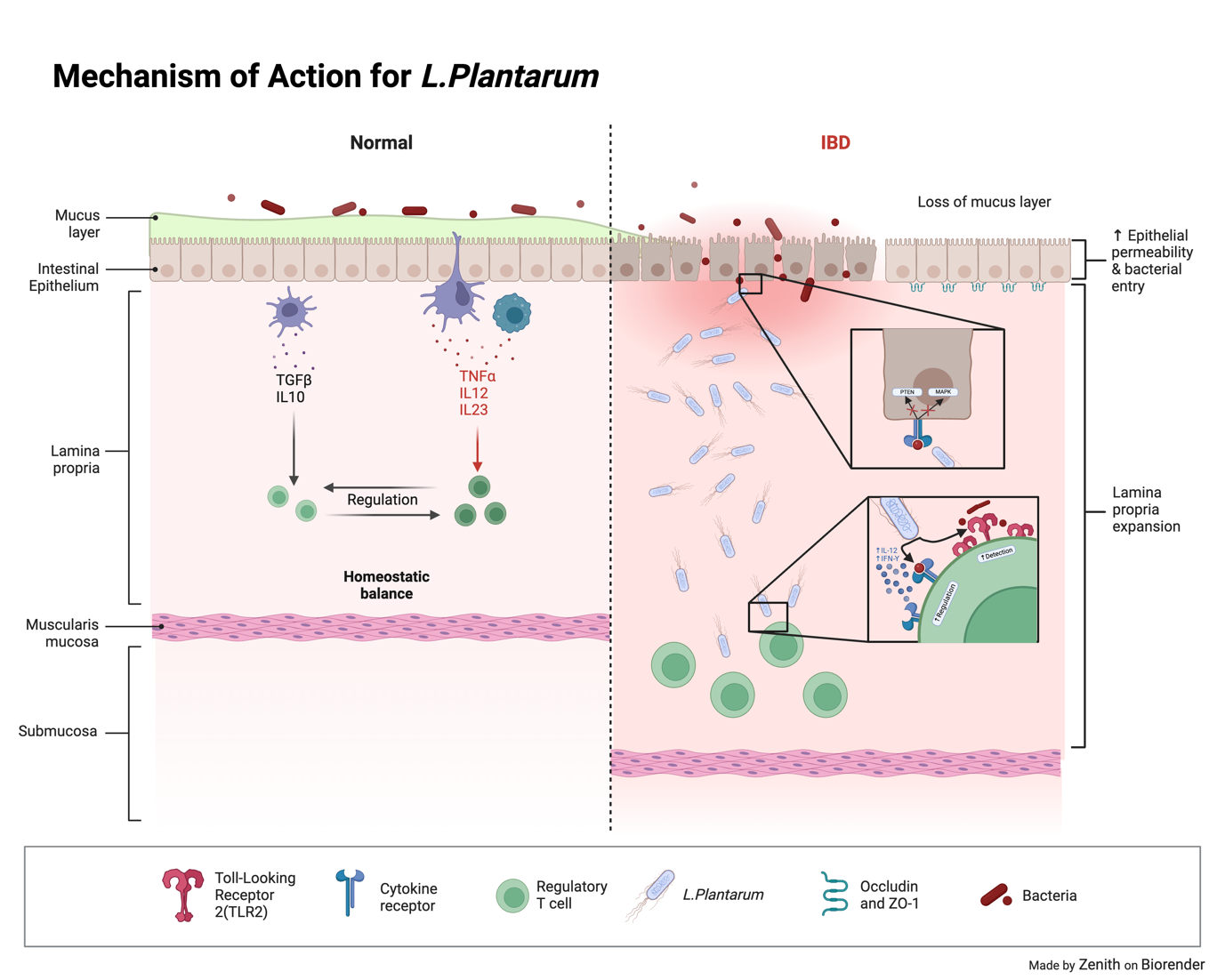
Figure 3. Mechanism of action for L.Plantarum. L.Plantarum inhibits the MAPK, PTEN and increases regulation and detection of foreign bacteria
2.1.3. E.coli Nissle 1917. E.coli Nissle is a genetically modified strain of p1robiotic bacteria. It is known as the serotype O6:K5:H1. E.coli Nissle works by secreting EGF into the surrounding area through the Hly-A secretion system. The Hly-A secretion system is a simple system which consists of 3 parts. HlyB(Hemolysin B) is a protein used to receive energy for the system because it contains a ATP-binding cassette. HlyD, a protein acts as a linker between the inner and outer membranes of Gram-negative bacteria, providing a passageway for EGF to be secreted freely into the environments [17,18] ToIC is a porin in the outer membrane of Gram negative bacteria. They form channels, in the form of beta barrels, to allow the diffusion of ions and molecules in and out of the membrane [19]. This system allows for the efficient diffusion of EGF molecules inside the gut. EGF can then stimulate and promote ERK1/2 and MAPK pathways, pathways which promote cell migration, proliferation and wound healing, ensuring that the damaged areas can repair and regenerate [20]. Moreover, EGF can inhibit NF-kB pathways as well as NO2 production through PI3K signalling and prevent further inflammation [21]
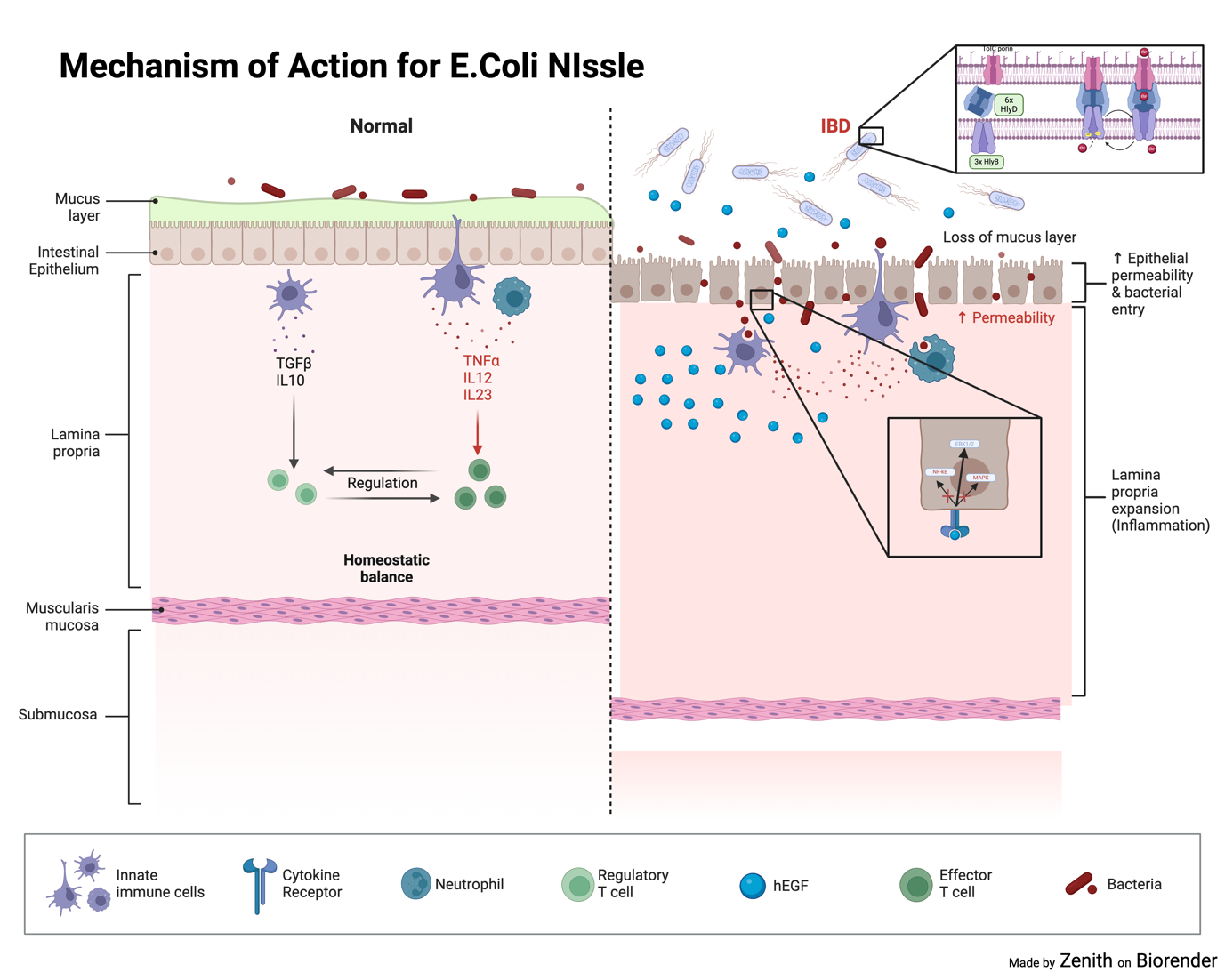
Figure 4. Mechanism of action for E.coli Nissle. E.coli Nissle excretes EGF through the Secretion System, EGF then inhibits MAPK and NF-kB pathways but increases ERK1/2 to speed up repair of epithelial cells
2.2. Safety and Efficacy
Regarding the safety and efficacy of these probiotics, We utilised literature data found from Research articles and Original Articles to determine the safety and efficacy of probiotics. The 4 probiotics selected are Lactobacillus rhamnosus (L.Rhamnosus), Lactobacillus plantarum L-137, E.coli Nissle (Mutaflor)
2.2.1. L.Rhamnosus. In this study conducted by [22], They evaluated the safety and efficacy of Lactobacillus rhamnosus against a placebo in infants aged 4-48 months with Atopic Dermatitis. They measured the overall efficacy and safety of the drug using mainly the Hanifin and Rajka criteria, deciding a score based on the behavioural and symptoms visible on the patient as well as the patient’s history of atopy, otherwise known as Score of Atopic Dermatitis(SCORAD) [23]. These patients were randomised into a group receiving 1 capsule of placebo(n=32) and another receiving 1 capsule of L.Rhamnosus(n=30) per day with a complete follow-up at week 2,4,6. Patients were also categorised into an Intent-to-Treat(ITT) as well as a Per-Protocol(PP), patients with a 80% remission rate are categorised into this group.
After 8 weeks, The average change in SCORAD from baseline at week 8 was −21.69 ± 16.56 for the L.Rhamnosus group and −12.35 ± 12.82 for the placebo group within the ITT population (p = 0.005, Mann-Whitney test). In the PP population, the mean change in SCORAD from baseline was −23.20 ± 15.24 for the L.Rhamnosus group and −12.35 ± 12.82 for the placebo group (p = 0.002, Mann-Whitney test).
Table 1. Mean Change of SCORAD in ITT and PP populations tested with L.Rhamnosus and Placebo [22].
ITT population | PP population | |||
LR | Placebo | LR | LR | |
Mean±SD | -21.69 ± 16.56 | −12.35 ± 12.82 | -23.20 ± 15.24 | -12.69 ± 12.82 |
Median | −18.19 | −10.97 | −18.86 | −10.97 |
Mann-Whitney Value | 0.005 | 0.002 | ||
A comparative analysis of mean changes in intensity between the intention-to-treat (ITT) and per-protocol (PP) groups revealed significant differences. Specifically, scores for the low-dose (LR) group were substantially lower than those for the placebo group, indicating a more pronounced reduction in intensity among patients receiving the LR treatment.
2.2.2. L.Plantarum L-137. In order to test for efficacy of L.Plantarum L-137, A study conducted by [24] used heat killed L.Plantarum L-137 ( L-137) to conduct a single arm, non-standardized, open label study in healthy participants. In order to conduct this, the patients were split into two groups: long-term and high-dose. The scientists then measured the patients’ Anthropometric, hematological, biochemical, and urinary measures taken at 3, 6 and 12 months for both groups. A total of 44 patients were selected to participate (29 for long-term and 15 for high-dose) and the data collected was compared with a baseline(measured with a paired t test).
2.2.2.1. Long term efficacy. After 12 months, the only significant measure found was the measurements of fecal microbiota; the results are as follows:
Table 2. L-137 effects on the composition of fecal microbiome [24].
Clostridium sub-cluster XIVa | Control Group | 3 months | 6 months | 12 months |
18.50 ± 7.40 | 16.70 ± 6.90 | 16.30 ± 5.70 | *15.70 ± 5.00 | |
Phylum Firmicutes | 36.20 ± 9.10 | 33.70 ± 8.40 | 33.80 ± 10.30 | *32.20 ± 8.10 |
Phylum Bacteroidetes | 43.80 ± 12.60 | 46.40 ± 15.20 | *47.90 ± 15.80 | *49.60 ± 15.00 |
F/B ratio | 0.76 ± 0.29 | 0.66 ± 0.27 | 0.71 ± 0.49 | *0.60 ± 0.25 |
Compared to control group, Clostridium sub-cluster XIV levels were significantly lower at 12 months. Meanwhile, Bacteroides levels rose substantially, leading to a greater Bacteroidetes-to-Firmicutes ratio. Accordingly, the F-to-B ratio decreased at 12 months compared to control group. Notably, the level of short chain fatty acids increased and is significantly more higher compared to control group. The results are as follows:
Table 3. L-137 effects on the consumption on SCFAs [24].
|
These results show that all four short-chain fatty acids (n-butyric, iso-butyric, n-valeric, and iso-valeric) showed elevated levels at both 6-month and 12-month compared to control group. This results in an improved gut health and gut microbiota possibly due to its stimulatory effect. However, the development of such processes was no longer significant due to the period of time they developed.
2.2.3. E.coli Nissle. To evaluate the effectiveness of E. coli Nissle as an adjunct therapy, a study referenced as [25] employed commercial E. coli (Mutaflor) alongside 5-aminosalicylic acid treatment in patients with Ulcerative Colitis (UC). Researchers performed a multicenter, double-blind, randomized, placebo-controlled trial. They divided 133 patients into two groups, administering EcN and a placebo, and assessed outcomes using the Inflammatory Bowel Disease Questionnaire (IBDQ). The average scores for each group were calculated. The study reported the primary endpoint of IBD scores and secondary endpoint of clinical remission rates. Over the 8-week period, 15 participants withdrew, resulting in a final cohort of 118 patients. The findings are summarized as follows:
2.2.3.1. Primary Endpoint IBDQ Scores: The primary endpoint of the study, measuring the improvement in IBDQ scores among patients with mild-to-moderate ulcerative colitis UC (30 [51.7%] vs. 31 [51.7%]; PP, p = 1.0; ITT, p = 0.86), did not reveal a significant difference between the ECN and placebo groups. However, both groups exhibited notable improvements in their IBDQ scores compared to baseline levels at the 8-week mark. This suggests that factors beyond the direct effects of ECN, such as elevated EGF levels in the local environment, may have contributed to the observed improvements.
When analyzing the magnitude of declines in IBDQ scores, a significantly higher proportion of patients in the placebo group experienced substantial reductions (≥16 points) compared to the ECN group. This finding may indicate a potential advantage of ECN in mitigating disease progression.
Table 4. Primary Endpoints (Measuring the IBDQ score of patients) [25].
Variable | Per Protocol | Intention to treat | ||||
E.coli Nissle (n=58) | Placebo (n=60) | P value | E.coli Nissle (n=67) | Placebo (n=66) | P value | |
Increase | 30(51.7) | 31(51.7) | 1.00 | 30(44.8) | 31(47) | 0.86 |
Decrease | 1 | 8 | 0.03 | 1 | 8 | 0.02 |
IBDQ Score | ||||||
At Study initation | 159.7±30.2 | 158.7±31.2 | 0.85 | 159.1±30.7 | 158.9±30.3 | 0.93 |
At 8 weeks | 181.3±29.3 | 177.7±28.9 | 0.50 | |||
2.2.3.2. Secondary Endpoint: Partial Mayo Score A secondary analysis focused on the partial Mayo score revealed a significantly higher percentage of patients in the ECN group experiencing substantial improvements at the 4-week mark compared to the placebo group (39.7% vs. 21.7%. p=0.04). This suggests that ECN may have a more pronounced effect on specific aspects of disease activity, as measured by the partial Mayo score.
3. Discussion
Despite there being three probiotics being tested, and many of them showing similarities, each one of them can be used for a different purpose or method of consumption. For example, Due to the absence of materials, I think L.Rhamnosus would be best used for infants aged 4 months to 3 years. However, The treatment of Atopic Dermatitis with L.Rhamnosus remains controversial. There is evidence that shows SCORAD decreasing after consuming probiotics.[26,27]. At the same time, there is also evidence that shows the increase of SCORAD [28]. Besides this, the data showed a overall decrease in mean SCORAD and reached a level of significance lower than 0.005.
L. plantarum L-137 exhibited beneficial effects without adverse reactions. Over a 12-month period, ex vivo proliferation by T-cells indicated a slight drop in terms of rates but remained well above baseline levels. Moreover, following the consumption of L-137, researchers observed an increase in Bacteroides and a decrease in the Firmicutes phylum. This alteration correlated with a transient increase in short-chain fatty acids within fecal samples, notably affecting the Firmicutes to Bacteroidetes (F/B) ratio. These modifications contributed to alleviating obesity and related metabolic disorders by enhancing microbiota equilibrium. This could be due to a significant implication of elevated SCFA levels is their association with metabolic health, which have been linked with reduced inflammation from their stimulatory effect[29,30]. Additionally, produced by intestinal microbes during fermentation are vital for regulating various processes. These encompass the maintenance of tissue-specific maintenance, gastrointestinal motility, gut barrier strength, management of colitis, and effects on energy and lipid metabolism, tumorigenesis, as well as immune responses.
Finally, I believe that E.coli Nissle will be the best probiotic that yields the best results when added as a booster. For example, In the span of 8 weeks, The mean score of the IBDQ showed no variation after 8 weeks. However, the percentage of patients with reduced IBDQ scores fell from 1 [1.7%] compared to 8 [13.3%]; (analysed from PP, p = 0.03; analysed from ITT, p = 0.02). Furthermore, a significantly greater number of patients in the EcN group exhibited clinical improvement at 4 weeks (23 [39.7%] versus 13 [21.7%], p = 0.04) and achieved endoscopic subsidence at 8 weeks (26 [46.4%] versus 16 [27.1%], p = 0.03). This could be due to the fact that
Although the research on these probiotics has yielded positive outcomes, it's challenging to compare these studies due to variations in design, goals, and methods. To gain a clearer understanding, future studies should follow consistent guidelines and measurements. This will help researchers better assess the effectiveness of probiotics in different situations.
References
[1]. Wang, R. et al. (2023) Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the global burden of disease study 2019, BMJ Open. Available at: https://bmjopen.bmj.com/content/13/3/e065186 .
[2]. Pothoulakis, C. (2009). Review article: anti‐inflammatory mechanisms of action of saccharomyces boulardii. Alimentary Pharmacology &Amp; Therapeutics, 30(8), 826-833. https://doi.org/10.1111/j.1365-2036.2009.04102.x
[3]. Mazzini, E., Massimiliano, L., Penna, G., & Rescigno, M. (2014). Oral tolerance can be established via gap junction transfer of fed antigens from cx3cr1+ macrophages to cd103+ dendritic cells. Immunity, 40(2), 248-261. https://doi.org/10.1016/j.immuni.2013.12.012
[4]. Aleksandrova, K., Romero-Mosquera, B., & Hernández, V. (2017). Diet, gut microbiome and epigenetics: emerging links with inflammatory bowel diseases and prospects for management and prevention. Nutrients, 9(9), 962. https://doi.org/10.3390/nu9090962
[5]. Ramos, G. P. and Papadakis, K. A. (2019). Mechanisms of disease: inflammatory bowel diseases. Mayo Clinic Proceedings, 94(1), 155-165. https://doi.org/10.1016/j.mayocp.2018.09.013
[6]. Mudter, J. and Neurath, M. F. (2007). Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflammatory Bowel Diseases, 13(8), 1016-1023. https://doi.org/10.1002/ibd.20148
[7]. Dixon, L., Kabi, A., Nickerson, K. P., & McDonald, C. (2015). Combinatorial effects of diet and genetics on inflammatory bowel disease pathogenesis. Inflammatory Bowel Diseases, 21(4), 912-922. https://doi.org/10.1097/mib.0000000000000289
[8]. Spiller, R. C. and Major, G. (2016). Ibs and ibd — separate entities or on a spectrum?. Nature Reviews Gastroenterology &Amp; Hepatology, 13(10), 613-621. https://doi.org/10.1038/nrgastro.2016.141
[9]. Djavaheri-Mergny, M. and Codogno, P. (2007). Autophagy joins the game to regulate nf-κb signaling pathways. Cell Research, 17(7), 576-577. https://doi.org/10.1038/cr.2007.58
[10]. Lin, L., Wu, C., & Hu, K. (2012). Tissue plasminogen activator activates nf-κb through a pathway involving annexin a2/cd11b and integrin-linked kinase. Journal of the American Society of Nephrology, 23(8), 1329-1338. https://doi.org/10.1681/asn.2011111123
[11]. Oeckinghaus, A. and Ghosh, S. (2009). The nf- b family of transcription factors and its regulation. Cold Spring Harbor Perspectives in Biology, 1(4), a000034-a000034. https://doi.org/10.1101/cshperspect.a000034
[12]. Zhao, L., Zhang, D., Liu, Y., Zhang, Y., Meng, D., Xu, Q., … & Wang, S. (2022). Quantitative pcr assays for the strain-specific identification and enumeration of probiotic strain lacticaseibacillus rhamnosus x253. Foods, 11(15), 2282. https://doi.org/10.3390/foods11152282
[13]. Peña, J. and Versalovic, J. (2003). Lactobacillus rhamnosus gg decreases tnf-alpha production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cellular Microbiology, 5(4), 277-285. https://doi.org/10.1046/j.1462-5822.2003.t01-1-00275.x
[14]. Karczewski, J. M., Troost, F. J., Konings, I., Dekker, J., Kleerebezem, M., Brummer, R. J., … & Wells, J. M. (2010). Regulation of human epithelial tight junction proteins by lactobacillus plantarum in vivo and protective effects on the epithelial barrier. American Journal of Physiology-Gastrointestinal and Liver Physiology, 298(6), G851-G859. https://doi.org/10.1152/ajpgi.00327.2009
[15]. Asoudeh-Fard, A., Barzegari, A., Dehnad, A., Bastani, S., Golchin, A., & Omidi, Y. (2017). Lactobacillus plantarum induces apoptosis in oral cancer kb cells through upregulation of pten and downregulation of mapk signalling pathways. BioImpacts, 7(3), 193-198. https://doi.org/10.15171/bi.2017.22
[16]. Pathmakanthan, S., Li, C. K., Cowie, J., & Hawkey, C. J. (2004). lactobacillus plantarum 299: beneficial in vitro immunomodulation in cells extracted from inflamed human colon. Journal of Gastroenterology and Hepatology, 19(2), 166-173. https://doi.org/10.1111/j.1440-1746.2004.03181.x
[17]. Baek, Y., Lee, J., Lee, D., Xu, Y., & Ha, N. (2023). Modeling study of the hlybd-tolc hemolysin secretion system using the ai-based structural prediction and molecular dynamics simulation. Korean Society for Structural Biology, 11(2), 21-27. https://doi.org/10.34184/kssb.2023.11.2.21
[18]. Kim, J., Song, S., Lee, S., Lee, K., & Ha, N. (2016). Crystal structure of a soluble fragment of the membrane fusion protein hlyd in a type i secretion system of gram-negative bacteria. Structure, 24(3), 477-485. https://doi.org/10.1016/j.str.2015.12.012
[19]. Sansom, M. S. and Kerr, I. D. (1995). Transbilayer pores formed by beta-barrels: molecular modeling of pore structures and properties. Biophysical Journal, 69(4), 1334-1343. https://doi.org/10.1016/s0006-3495(95)80000-7
[20]. Reuter, K. C., Loitsch, S., Dignaß, A., Steinhilber, D., & Stein, J. M. (2012). Selective non-steroidal glucocorticoid receptor agonists attenuate inflammation but do not impair intestinal epithelial cell restitution in vitro. PLoS ONE, 7(1), e29756. https://doi.org/10.1371/journal.pone.0029756
[21]. Mehta, V. B. and Besner, G. E. (2005). Heparin-binding epidermal growth factor-like growth factor inhibits cytokine-induced nf-κb activation and nitric oxide production via activation of the phosphatidylinositol 3-kinase pathway. The Journal of Immunology, 175(3), 1911-1918. https://doi.org/10.4049/jimmunol.175.3.1911
[22]. Wu, Y., Wu, W., Hung, C., Ku, M., Liao, P., Sun, H., … & Ko, J. (2017). Evaluation of efficacy and safety of lactobacillus rhamnosus in children aged 4–48 months with atopic dermatitis: an 8-week, double-blind, randomized, placebo-controlled study. Journal of Microbiology, Immunology and Infection, 50(5), 684-692. https://doi.org/10.1016/j.jmii.2015.10.003
[23]. Umborowati, M. A., Jastika, F. R., Hendaria, M. P., Anggraeni, S., Damayanti, Sari, M., … & Prakoeswa, C. R. S. (2024). Description of hanifin-rajka criteria and skin hydration in adult patients with mild-moderate atopic dermatitis at tertiary hospital. Berkala Ilmu Kesehatan Kulit Dan Kelamin, 36(1), 20-25. https://doi.org/10.20473/bikk.v36.1.2024.20-25
[24]. Nakai, H., Murosaki, S., Yamamoto, Y., Furutani, M., Matsuoka, R., & Hirose, Y. (2021). Safety and efficacy of using heat-killed lactobacillus plantarum l-137: high-dose and long-term use effects on immune-related safety and intestinal bacterial flora. Journal of Immunotoxicology, 18(1), 127-135. https://doi.org/10.1080/1547691x.2021.1979698
[25]. Park, S., Kang, S. B., Kim, S. S., Kim, T. O., Myung, J., Im, J. P., … & Park, D. I. (2022). Additive effect of probiotics (mutaflor) on 5-aminosalicylic acid therapy in patients with ulcerative colitis. The Korean Journal of Internal Medicine, 37(5), 949-957. https://doi.org/10.3904/kjim.2021.458
[26]. Gerasimov, S. V., Vasjuta, V. V., Myhovych, O. O., & Bondarchuk, L. I. (2010). Probiotic supplement reduces atopic dermatitis in preschool children. American Journal of Clinical Dermatology, 11(5), 351-361. https://doi.org/10.2165/11531420-000000000-00000
[27]. Weston, S., Halbert, A., Richmond, P., & Prescott, S. L. (2005). Effects of probiotics on atopic dermatitis: a randomised controlled trial. Archives of Disease in Childhood, 90(9), 892-897. https://doi.org/10.1136/adc.2004.060673
[28]. Rosenfeldt, V., Benfeldt, E., Nielsen, S. D., Michaelsen, K. F., Jeppesen, D. L., Valerius, N. H., … & Pærregaard, A. (2003). Effect of probiotic lactobacillus strains in children with atopic dermatitis. Journal of Allergy and Clinical Immunology, 111(2), 389-395. https://doi.org/10.1067/mai.2003.389
[29]. Kong, D., Schipper, L., & Dijk, G. v. (2021). Distinct effects of short chain fatty acids on host energy balance and fuel homeostasis with focus on route of administration and host species. Frontiers in Neuroscience, 15. https://doi.org/10.3389/fnins.2021.755845
[30]. Baothman, O. A., Zamzami, M. A., Taher, I., Abubaker, J., & Abu‐Farha, M. (2016). The role of gut microbiota in the development of obesity and diabetes. Lipids in Health and Disease, 15(1). https://doi.org/10.1186/s12944-016-0278-4
Cite this article
Kwong,Z. (2024). The Application of Probiotics in Gastrointestinal Diseases. Theoretical and Natural Science,74,25-34.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2024 Workshop: Computational Proteomics in Drug Discovery and Development from Medicinal Plants
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Wang, R. et al. (2023) Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the global burden of disease study 2019, BMJ Open. Available at: https://bmjopen.bmj.com/content/13/3/e065186 .
[2]. Pothoulakis, C. (2009). Review article: anti‐inflammatory mechanisms of action of saccharomyces boulardii. Alimentary Pharmacology &Amp; Therapeutics, 30(8), 826-833. https://doi.org/10.1111/j.1365-2036.2009.04102.x
[3]. Mazzini, E., Massimiliano, L., Penna, G., & Rescigno, M. (2014). Oral tolerance can be established via gap junction transfer of fed antigens from cx3cr1+ macrophages to cd103+ dendritic cells. Immunity, 40(2), 248-261. https://doi.org/10.1016/j.immuni.2013.12.012
[4]. Aleksandrova, K., Romero-Mosquera, B., & Hernández, V. (2017). Diet, gut microbiome and epigenetics: emerging links with inflammatory bowel diseases and prospects for management and prevention. Nutrients, 9(9), 962. https://doi.org/10.3390/nu9090962
[5]. Ramos, G. P. and Papadakis, K. A. (2019). Mechanisms of disease: inflammatory bowel diseases. Mayo Clinic Proceedings, 94(1), 155-165. https://doi.org/10.1016/j.mayocp.2018.09.013
[6]. Mudter, J. and Neurath, M. F. (2007). Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflammatory Bowel Diseases, 13(8), 1016-1023. https://doi.org/10.1002/ibd.20148
[7]. Dixon, L., Kabi, A., Nickerson, K. P., & McDonald, C. (2015). Combinatorial effects of diet and genetics on inflammatory bowel disease pathogenesis. Inflammatory Bowel Diseases, 21(4), 912-922. https://doi.org/10.1097/mib.0000000000000289
[8]. Spiller, R. C. and Major, G. (2016). Ibs and ibd — separate entities or on a spectrum?. Nature Reviews Gastroenterology &Amp; Hepatology, 13(10), 613-621. https://doi.org/10.1038/nrgastro.2016.141
[9]. Djavaheri-Mergny, M. and Codogno, P. (2007). Autophagy joins the game to regulate nf-κb signaling pathways. Cell Research, 17(7), 576-577. https://doi.org/10.1038/cr.2007.58
[10]. Lin, L., Wu, C., & Hu, K. (2012). Tissue plasminogen activator activates nf-κb through a pathway involving annexin a2/cd11b and integrin-linked kinase. Journal of the American Society of Nephrology, 23(8), 1329-1338. https://doi.org/10.1681/asn.2011111123
[11]. Oeckinghaus, A. and Ghosh, S. (2009). The nf- b family of transcription factors and its regulation. Cold Spring Harbor Perspectives in Biology, 1(4), a000034-a000034. https://doi.org/10.1101/cshperspect.a000034
[12]. Zhao, L., Zhang, D., Liu, Y., Zhang, Y., Meng, D., Xu, Q., … & Wang, S. (2022). Quantitative pcr assays for the strain-specific identification and enumeration of probiotic strain lacticaseibacillus rhamnosus x253. Foods, 11(15), 2282. https://doi.org/10.3390/foods11152282
[13]. Peña, J. and Versalovic, J. (2003). Lactobacillus rhamnosus gg decreases tnf-alpha production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cellular Microbiology, 5(4), 277-285. https://doi.org/10.1046/j.1462-5822.2003.t01-1-00275.x
[14]. Karczewski, J. M., Troost, F. J., Konings, I., Dekker, J., Kleerebezem, M., Brummer, R. J., … & Wells, J. M. (2010). Regulation of human epithelial tight junction proteins by lactobacillus plantarum in vivo and protective effects on the epithelial barrier. American Journal of Physiology-Gastrointestinal and Liver Physiology, 298(6), G851-G859. https://doi.org/10.1152/ajpgi.00327.2009
[15]. Asoudeh-Fard, A., Barzegari, A., Dehnad, A., Bastani, S., Golchin, A., & Omidi, Y. (2017). Lactobacillus plantarum induces apoptosis in oral cancer kb cells through upregulation of pten and downregulation of mapk signalling pathways. BioImpacts, 7(3), 193-198. https://doi.org/10.15171/bi.2017.22
[16]. Pathmakanthan, S., Li, C. K., Cowie, J., & Hawkey, C. J. (2004). lactobacillus plantarum 299: beneficial in vitro immunomodulation in cells extracted from inflamed human colon. Journal of Gastroenterology and Hepatology, 19(2), 166-173. https://doi.org/10.1111/j.1440-1746.2004.03181.x
[17]. Baek, Y., Lee, J., Lee, D., Xu, Y., & Ha, N. (2023). Modeling study of the hlybd-tolc hemolysin secretion system using the ai-based structural prediction and molecular dynamics simulation. Korean Society for Structural Biology, 11(2), 21-27. https://doi.org/10.34184/kssb.2023.11.2.21
[18]. Kim, J., Song, S., Lee, S., Lee, K., & Ha, N. (2016). Crystal structure of a soluble fragment of the membrane fusion protein hlyd in a type i secretion system of gram-negative bacteria. Structure, 24(3), 477-485. https://doi.org/10.1016/j.str.2015.12.012
[19]. Sansom, M. S. and Kerr, I. D. (1995). Transbilayer pores formed by beta-barrels: molecular modeling of pore structures and properties. Biophysical Journal, 69(4), 1334-1343. https://doi.org/10.1016/s0006-3495(95)80000-7
[20]. Reuter, K. C., Loitsch, S., Dignaß, A., Steinhilber, D., & Stein, J. M. (2012). Selective non-steroidal glucocorticoid receptor agonists attenuate inflammation but do not impair intestinal epithelial cell restitution in vitro. PLoS ONE, 7(1), e29756. https://doi.org/10.1371/journal.pone.0029756
[21]. Mehta, V. B. and Besner, G. E. (2005). Heparin-binding epidermal growth factor-like growth factor inhibits cytokine-induced nf-κb activation and nitric oxide production via activation of the phosphatidylinositol 3-kinase pathway. The Journal of Immunology, 175(3), 1911-1918. https://doi.org/10.4049/jimmunol.175.3.1911
[22]. Wu, Y., Wu, W., Hung, C., Ku, M., Liao, P., Sun, H., … & Ko, J. (2017). Evaluation of efficacy and safety of lactobacillus rhamnosus in children aged 4–48 months with atopic dermatitis: an 8-week, double-blind, randomized, placebo-controlled study. Journal of Microbiology, Immunology and Infection, 50(5), 684-692. https://doi.org/10.1016/j.jmii.2015.10.003
[23]. Umborowati, M. A., Jastika, F. R., Hendaria, M. P., Anggraeni, S., Damayanti, Sari, M., … & Prakoeswa, C. R. S. (2024). Description of hanifin-rajka criteria and skin hydration in adult patients with mild-moderate atopic dermatitis at tertiary hospital. Berkala Ilmu Kesehatan Kulit Dan Kelamin, 36(1), 20-25. https://doi.org/10.20473/bikk.v36.1.2024.20-25
[24]. Nakai, H., Murosaki, S., Yamamoto, Y., Furutani, M., Matsuoka, R., & Hirose, Y. (2021). Safety and efficacy of using heat-killed lactobacillus plantarum l-137: high-dose and long-term use effects on immune-related safety and intestinal bacterial flora. Journal of Immunotoxicology, 18(1), 127-135. https://doi.org/10.1080/1547691x.2021.1979698
[25]. Park, S., Kang, S. B., Kim, S. S., Kim, T. O., Myung, J., Im, J. P., … & Park, D. I. (2022). Additive effect of probiotics (mutaflor) on 5-aminosalicylic acid therapy in patients with ulcerative colitis. The Korean Journal of Internal Medicine, 37(5), 949-957. https://doi.org/10.3904/kjim.2021.458
[26]. Gerasimov, S. V., Vasjuta, V. V., Myhovych, O. O., & Bondarchuk, L. I. (2010). Probiotic supplement reduces atopic dermatitis in preschool children. American Journal of Clinical Dermatology, 11(5), 351-361. https://doi.org/10.2165/11531420-000000000-00000
[27]. Weston, S., Halbert, A., Richmond, P., & Prescott, S. L. (2005). Effects of probiotics on atopic dermatitis: a randomised controlled trial. Archives of Disease in Childhood, 90(9), 892-897. https://doi.org/10.1136/adc.2004.060673
[28]. Rosenfeldt, V., Benfeldt, E., Nielsen, S. D., Michaelsen, K. F., Jeppesen, D. L., Valerius, N. H., … & Pærregaard, A. (2003). Effect of probiotic lactobacillus strains in children with atopic dermatitis. Journal of Allergy and Clinical Immunology, 111(2), 389-395. https://doi.org/10.1067/mai.2003.389
[29]. Kong, D., Schipper, L., & Dijk, G. v. (2021). Distinct effects of short chain fatty acids on host energy balance and fuel homeostasis with focus on route of administration and host species. Frontiers in Neuroscience, 15. https://doi.org/10.3389/fnins.2021.755845
[30]. Baothman, O. A., Zamzami, M. A., Taher, I., Abubaker, J., & Abu‐Farha, M. (2016). The role of gut microbiota in the development of obesity and diabetes. Lipids in Health and Disease, 15(1). https://doi.org/10.1186/s12944-016-0278-4









