1. Introduction
Cardiovascular diseases (CVDs), especially arteriosclerotic cardiovascular disease (ASCVD) have the highest incidence rate and highest risk of the whole systemic syndrome. According to the latest statistics, over 3.3 hundred million people have suffered from acute and non-contagious diseases. CVD is a huge conceptual disease or a general designation, which includes cardiomyopathic structure disorder, cardio electrophysiological disorder often called arrhythmia, and angiointerventional disease usually correlated with the anomaly of the coronary artery. Above all arrhythmia is the most dangerous kind of CVD. Therefore how to solve or remit arrhythmia is the focus of health problems, and scientists always put effort into overcoming the disease which has never been thought an insolvable problem.
Arrhythmia can be categorized by these types: atrial arrhythmia, ventricular arrhythmia, atrioventricular junction arrhythmia, left and right bundle branches arrhythmia, and atrial-ventricular blocking. There atrial-ventricular blocking is the most dangerous kind of arrhythmia. To solve these problems, Some anti-arrhythmic drugs would be developed. A kind of severe blocking of the Kalium ion channel called IA-type anti-arrhythmic drugs, such as conquine and procainamide. A kind of mild blocking of the calcium ion channel called IB-type anti-arrhythmic drugs, such as lidocaine, phenytoin sodium, and mexiletine. A kind of moderate blocking of the calcium ion channel called IC-type anti-arrhythmic drugs, such as propafenone. A kind of β receptor blocking called II-type anti-arrhythmic drugs, such as propranolol, and metoprolol. A kind of distracting the active potential duration (APD) drugs, such as amiodarone. A kind of calcium ion channel blocking called IV-type anti-arrhythmic drugs, such as iproveratril. These drugs have many side effects especially over-distract the APD and over-blocking or deficient-blocking could cause severe atrial-ventricular blocking, even ventricular tachycardia or ventricular fibrillation which is an extremely critical complication of cardiovascular disorder, or cause systematic disorder such as corneal chromatophore precipitation, thyroid hypofunction, and thymohyperplasia. It can cause more pain for the patients. So the end-stage arrhythmic patient could only use the single or double-chamber iron-pacemaker or pacemaker to distract the quality of cardiovascular mechanism or sustain the correlative homeostasis cardiovascular ability as much as possible. So the researchers put effort into looking for new kind of ways that could perhaps exhaustively solve the problems.
Recently researchers have found that the CRISPR-Cas9 technique can orientable knockout or screen the signal molecule Toll-like receptor 4 (TLR4) from the upstream of the NF-κB signal pathway, The TLR4 may interfere with the crosstalk of the multi-correspond signal pathways and reduce the various signal pathways’ functions, besides it will break down the functional proteins and other immune provocated factors which should be taken part in the exocytosis process, on the other hand, TLR4 enhances these particle compositions releasing, which can improve the human-Mensenchymal Stem Cell (hMSC) contact suppression and reduce the cell interaction even cancel the hMSC division or growth. With that, the immature hMSC will interfere with the normal Cardiovascular Myocytes (CM) providing the outside stable electric signals, which may cause the very severe various cardiac ion-channels damage episode and finally promote the immediate arrhythmia. Through the referent kinds of ribonucleases, for example, high specificity enzyme Cas9 can accurately target the single-guide RNA (sgRNA) which can guide the Cas9 to make the incisions of the coding DNA corresponding to the TLR4 receptor and form the anti-homologous sequences between the DNA incisions after Cas9 resects the reflective DNA sequences, which can be used for the targets of screening. Researchers use the induced-pluripotent stem cells (iPSC) as the receptor of the post-shearing DNA sequences in vitro, take the artificial undifferentiated iPSC back into the cardiovascular tissues to differentiate the oriented-function cells-hMSC in vivo, and drive these cells to hit against the disturbance or even abnormal crosstalk of the intercellular various signals transductions, especially in the NF-κB signal pathway, which also can repairing the damaged and overexpressed CM as well as emitting the normal electrophilic signals, and correct the ion channels disorder as well as cardiac arrhythmia.
The CRISPR Cas9 technique is a microbiologic technique, which can throughout the cytobiologic crosstalks and correlative reactions to solve the pathophysiologic phenotypic CVDs and hypofunctions. Which also can be found to know the process of occurrence and mechanism of the cardiovascular ion channel disorder and arrhythmia.
By finding this method, researchers can take reliable therapy on the arrhythmia, even cure the arrhythmia to a certain extent. It will give the answer to treating the arrhythmia as perfectly as possible.
2. Overview of CRISPR-Cas9 technology
2.1. Mechanism of CRISPR-Cas9 system
CRISPR, meaning clusters of regularly spaced short palindromic repeats, is an acquired immune mechanism widely present in bacteria and archaea to resist foreign pathogens [1,2]. After the first invasion of the pathogen is identified, some of its genetic fragments are integrated into the CRISPR space. The integration positions are arranged from the 3 'to 5' end and separated by repetitive sequences according to the time of the pathogen's first invasion. When the pathogen invades again, the CRISPR gene begins to transcribe to form precursor crRNA (Pre crRNA), and the Cas gene adjacent to CRISPR begins to express to form Cas protein. At the same time, the tracrRNA is also transcribed. Under the joint action of tracrRNA, Cas protein, and RNA endonuclease, the Pre crRNA is cleaved, leaving only the corresponding crRNA of the immune target (Figure 1). Its conjugate binds to the pathogen through the crRNA that is specifically complementary to the invading pathogen. Then, the Cas protein cleaves the target sequence, causing it to be cleaved. Sequence breakage leads to loss of biological activity [3].
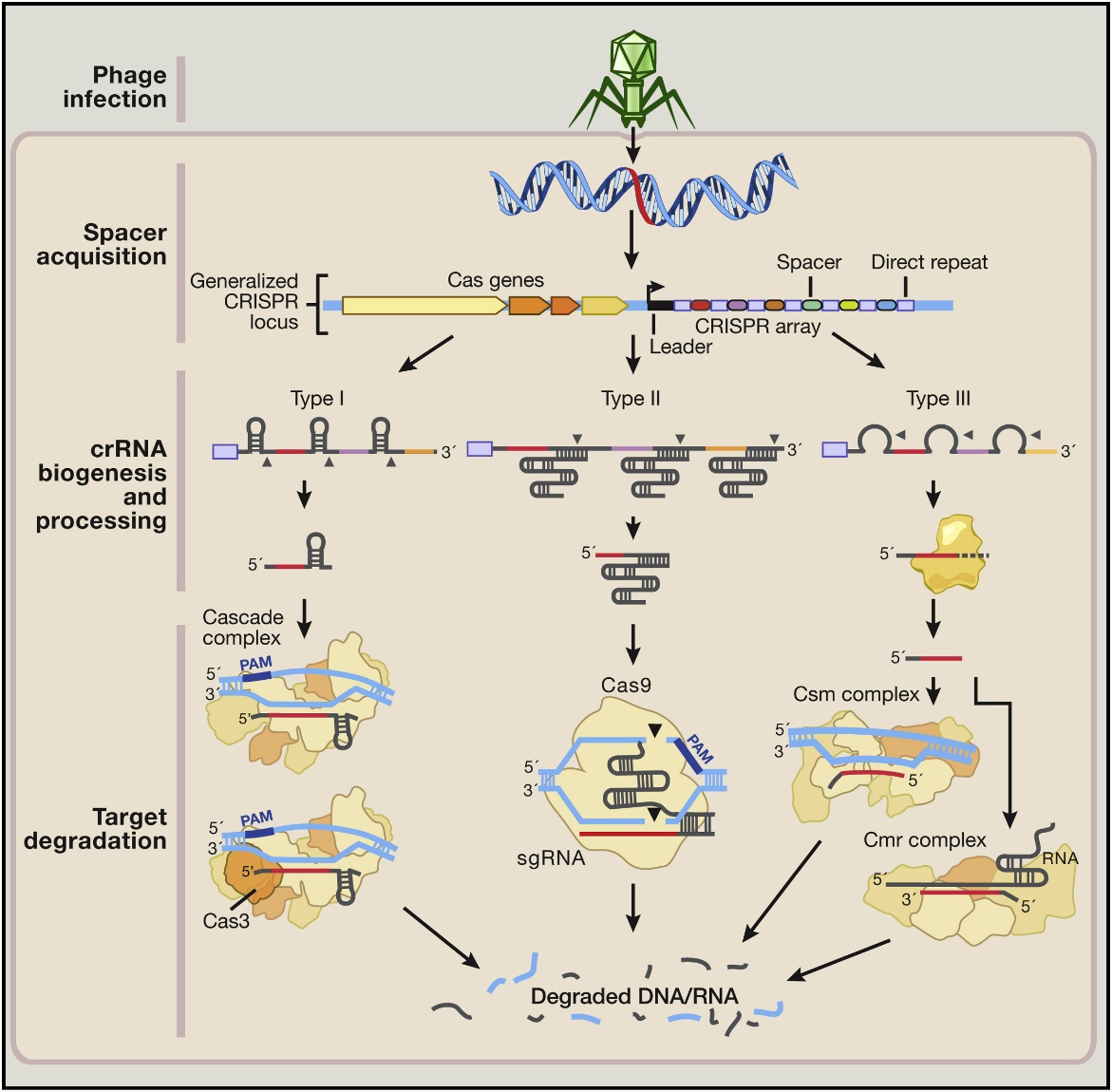
Figure 1: CRISPR-Cas9 mechanism [4]
2.2. Gene editing technology and its application based on the principle of CRISPR-CAS9 system
Based on the CRISPR-Cas9 mechanism, the Jennifer Dou DNA team demonstrated in 2012 that the CRISPR-Cas9 system can cleave DNA in vitro, providing the possibility for subsequent gene editing applications [3]. In 2013, the Cho SW team published a study on targeted genome engineering in human cells using Cas9 RNA guided endonucleases [5]. Subsequently, the number of SCI papers on gene editing rapidly increased around 2016, and derivative technologies related to the CRISPR-Cas9 system gradually emerged [6]. At present, the feasibility of using CRISPR-Cas9 technology for gene editing to treat mature individual diseases has been confirmed in the field of biomedicine [7], and there have been examples of using CRISPR-Cas9 to treat β - thalassemia, clear hepatitis B virus (HBV), etc. [8,9]. Provided new ideas and solutions for treating certain difficult and complicated diseases.
3. Introduction to myocardial cell electrophysiological diseases
3.1. Mechanism of arrhythmia occurrence
Arrhythmia is a process in which the sinoatrial node located at the superior vena cava controls the epicardium and distributes electrical signals to the endocardium through the myocardial cell layer. The production or distribution of cellular electrical signals mediated by the cardiac conduction system is disrupted, leading to myocardial electrophysiological disorders. It can be roughly divided into various disease manifestations such as sinus arrhythmia, atrial arrhythmia, atrioventricular junction arrhythmia, ventricular arrhythmia, and heart conduction block. It is controlled by pathological processes such as myocardial cell fibrosis, formation of myocardial scar tissue, myocardial hypertrophy or ventricular enlargement.
3.2. Defects of traditional treatment for arrhythmia
Arrhythmia drugs need to be used according to different conditions, and it is difficult to sustainably solve certain types of arrhythmia problems. Therefore, patients with arrhythmia, especially those with high atrioventricular block, often need to install pacemakers. The metallic properties of pacemakers are difficult to wear, have a lifespan, affect surrounding blood vessels, and immune rejection issues, which pose certain difficulties in clinical application. Therefore, there is a need for a sustained alternative treatment to avoid or reduce the occurrence of these problems.
4. Application of CRISPR technology in myocardial cell electrophysiology
Using CRISPR technology to knock down or screen the negative regulatory receptor (Toll like receptor family) gene TLR4 in hMSCs, followed by the negative regulation of TLR4 secretion group (this part can be verified using proteomic analysis techniques), to further negatively regulate the release of inflammatory factors, proteases, etc., in order to improve the survival rate of hMSCs, reduce the interference of hMSCs on the electrical signals emitted by mature stem cells in the myocardial layer, and reduce the probability of patients developing various types of cardiac arrhythmias (as part of electrophysiology).
4.1. CRISPR knockout and screening of TLR4 gene
Using CRISPR technology, design ribonucleoprotein Cas9 complementary to the TLR4 target sequence, and design two types of sgRNA. The first type of sgRNA binds to Cas9 protein to accurately locate the position of TLR4 target gene exon 3, methylate it, and then cleave it; Another type of sgRNA that binds to Cas9 protein does not guide Cas9 protein cleavage [10], but instead prevents gene alternative splicing. The two types of sgRNA interact with Cas9 protein to completely cleave TLR4 gene located at specific gene loci with the highest success rate.
In addition, CRISPR can also be used to screen between hMSC cell lines that have been knocked out of the TLR4 gene [11]. A sequence difference control experiment can be conducted between the sequencing experimental group that has been knocked out of the TLR4 gene using CRISPR technology and the control group that has not been knocked out of the TLR4 gene using CRISPR technology. Proteomic techniques can be used to detect the membrane protein receptor TLR4 expressed in both groups, and the experimental group sequence that has been successfully knocked out of the TLR4 gene by CRISPR gene knockout technology can be screened.
4.2. Negative regulatory part of TLR4 secretion group
Due to the crucial role of the cell surface TLR4 receptor encoded by the TLR4 gene in initiating the NF - κ B signaling pathway, excessive activation of the NF - κ B signaling pathway may lead to the initiation of various secretory physiological processes [12], such as stimulating cytokines (e.g. IL-1 α, IL-1 β, IFN - γ) The release of various extracellular proteins and their related downstream inflammatory factors, the release of proteases (such as metalloproteinases MMP, envelope proteins EV, etc.), the release of vascular endothelial growth factor inhibitors (VEGF-I), and the interference between signaling pathways, combined with receptors such as STT3A, can cross regulate the secretion of various extracellular proteins between different signaling pathways (such as Wnt, etc.), and may also participate in the regulation of reactive oxygen species. Therefore, knocking out TLR4 cell surface receptors may disrupt TLR4 mediated extracellular protein secretion [13] and minimize the cross regulation of secretion of various extracellular proteins. This can also be achieved by using proteins. Rigorous monitoring of omics technology.
4.3. Key conditions for sustained survival of myocardial MSCs
The immune response between the transplant vector and hMSC; Mediation of inflammatory factors; The release of proteases and low cell density are several key conditions that affect the sustained survival of myocardial MSCs. Knocking out the TLR4 gene can negatively regulate the secretion of extracellular proteins mediated by TLR4 and the positive and cross expression interference of cell signal transduction, thereby reducing the inflammatory response mediated by cytokines, the release of proteases, the immune response mediated by the Wnt signaling pathway and increasing cell proliferation mediated by VEGF. The sustained survival of myocardial MSCs is of great value, and whether myocardial MSCs are normal is the key factor that directly affects whether subsequent myocardial cell electrophysiological disorders can be effectively corrected.
4.4. Correction of cardiac electrophysiological disorders
The accumulation of reactive oxygen species (ROS) leads to a decrease in angiogenesis, while the upregulation of ECM and MMP matrix metalloproteinases expression leads to fibrosis and remodeling of myocardial cells, which are two important mechanisms causing electrical signal disruption in phenotype mature myocardial cells (CM) [14]. The secretion of extracellular proteins and positive regulation of related cellular signaling pathways mediated by TLR4 receptors can be obtained. Knocking out TLR4 receptors through CRISPR gene editing technology can reduce the release of VEGF-I, increase VEGF release, and reduce the expression of reactive oxygen species, which can increase angiogenesis. Moreover, the knockout of TLR4 receptors mentioned above leads to a reduction in the positive effects of extracellular proteins and related cell signaling transduction, which can maximize the possibility of MSC sustained survival. This plays an important role in the reprogramming of fibrotic scar tissue [15]. Therefore, it can reduce the interference of immature MSCs on mature CM layer electrical signals and lower the incidence of various cardiac arrhythmias in patients. However, knocking out the TLR4 gene may lead to delayed cellular responses to body reactions and inflammatory responses, increasing the risk of infection. As mentioned earlier, if the TLR4 gene on the cell surface is continuously stimulated, it will lead to excessive activation of the NF - κ B signaling pathway, causing many disorders of secretory physiological activities. So, the next experiment will explore how to reduce this negative reaction.
5. Conclusion
The myocardium is a mature cell in the quiescent phase, which was previously believed to be a nonrenewable terminal differentiated cell. However, with the development of technology, myocardial MSCs can be constructed and screened for anti-implantation in the body through CRISPR gene knockout and other methods in vitro, which may play an important role in myocardial cell electrophysiological disorders and arrhythmia. This article analyzes the application of CRISPR in myocardial cell electrophysiology from the perspectives of molecular, cellular, histopathological, and pathophysiological aspects. However, there is currently a lack of specific research methods to further validate the corresponding viewpoints. Future research can focus on using more advanced and safer treatment methods in clinical medicine to help more patients who may have myocardial electrophysiological problems have a better quality of life.
Authors Contribution
All the authors contributed equally and their names were listed in alphabetical order.
References
[1]. Makarova, K. S., Wolf, Y. I., Alkhnbashi, O. S., et al. (2015). An updated evolutionary classification of CRISPR-Cas systems. Nature Reviews Microbiology, 13(11), 722-736.
[2]. Lin, S. R., Yang, H. C., Kuo, Y. T., et al. (2014). The CRISPR/Cas9 system facilitates clearance of the intrahepatic HBV templates in vivo. Molecular Therapy Nucleic Acids, 3(8), e186.
[3]. Jinek, M., Chylinski, K., Fonfara, I., et al. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science, 337(6096), 816-821.
[4]. Hsu, P. D., Lander, E. S., & Zhang, F. (2014). Development and applications of CRISPR-Cas9 for genome engineering. Cell, 157(6), 1262-1278.
[5]. Cho, S. W., Kim, S., Kim, J. M., & Kim, J. S. (2013). Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nature Biotechnology, 31(3), 230-232.
[6]. Chen, Y., et al. (2021). Research progress and challenges of gene editing technology. World Science and Technology Research and Development, 43(1), 8-23.
[7]. Yin, H., Xue, W., & Chen, S. D., et al. (2014). Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nature Biotechnology, 32(6), 551-553.
[8]. Xie, F., Ye, L., & Chang, J. C., et al. (2014). Seamless gene correction of β-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and PiggyBac. Genome Research, 24(9), 1526-1533.
[9]. Lin, S. R., Yang, H. C., Kuo, Y. T., et al. (2014). The CRISPR/Cas9 system facilitates clearance of the intrahepatic HBV templates in vivo. Molecular Therapy Nucleic Acids, 3(8), e186.
[10]. Hou, X., Fan, W., Zeng, J., Gao, Z., Wan, J., & Liao, B. (2024). Generation of an ISL1 homozygous knockout stem cell line (WAe009-A-1G) by episomal vector-based CRISPR/Cas9 system. Stem Cell Research, 76, 103376.
[11]. Han, J. L., Heinson, Y. W., Pozo, M. R., Li, W., & Entcheva, E. (2024). Paradoxical effects of ZIM3, a CRISPRi effector, on human induced pluripotent stem-cell-derived cardiomyocyte electrophysiology. PNAS Nexus, 3(3), e027.
[12]. Freitas, G. P., Lopes, H. B., Souza, A. T. P., Gomes, M. P. O., Quiles, G. K., Gordon, J., Tye, C., Stein, J. L., Stein, G. S., Lian, J. B., Beloti, M. M., & Rosa, A. L. (2021). Mesenchymal stem cells overexpressing BMP-9 by CRISPR-Cas9 present high in vitro osteogenic potential and enhance in vivo bone formation. Gene Therapy, 28(12), 748-759.
[13]. Schary, Y., Rotem, I., Caller, T., et al. (2023). CRISPR-Cas9 editing of TLR4 to improve the outcome of cardiac cell therapy. Scientific Reports, 13, 4481.
[14]. Roland, T. J., & Song, K. (2024). Advances in the generation of constructed cardiac tissue derived from induced pluripotent stem cells for disease modeling and therapeutic discovery. Cells, 13(3), 250.
[15]. Hartmann, N., Knierim, M., Maurer, W., Dybkova, N., Zeman, F., Hasenfuß, G., Sossalla, S., & Streckfuss-Bömeke, K. (2024). NaV1.8 as proarrhythmic target in a ventricular cardiac stem cell model. International Journal of Molecular Sciences, 25(11), 6144.
Cite this article
Han,Y.;Huang,Z.;Zhang,B. (2025). Application of CRISPR Technology in Cardiomyocyte Electrophysiology. Theoretical and Natural Science,82,12-18.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Makarova, K. S., Wolf, Y. I., Alkhnbashi, O. S., et al. (2015). An updated evolutionary classification of CRISPR-Cas systems. Nature Reviews Microbiology, 13(11), 722-736.
[2]. Lin, S. R., Yang, H. C., Kuo, Y. T., et al. (2014). The CRISPR/Cas9 system facilitates clearance of the intrahepatic HBV templates in vivo. Molecular Therapy Nucleic Acids, 3(8), e186.
[3]. Jinek, M., Chylinski, K., Fonfara, I., et al. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science, 337(6096), 816-821.
[4]. Hsu, P. D., Lander, E. S., & Zhang, F. (2014). Development and applications of CRISPR-Cas9 for genome engineering. Cell, 157(6), 1262-1278.
[5]. Cho, S. W., Kim, S., Kim, J. M., & Kim, J. S. (2013). Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nature Biotechnology, 31(3), 230-232.
[6]. Chen, Y., et al. (2021). Research progress and challenges of gene editing technology. World Science and Technology Research and Development, 43(1), 8-23.
[7]. Yin, H., Xue, W., & Chen, S. D., et al. (2014). Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nature Biotechnology, 32(6), 551-553.
[8]. Xie, F., Ye, L., & Chang, J. C., et al. (2014). Seamless gene correction of β-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and PiggyBac. Genome Research, 24(9), 1526-1533.
[9]. Lin, S. R., Yang, H. C., Kuo, Y. T., et al. (2014). The CRISPR/Cas9 system facilitates clearance of the intrahepatic HBV templates in vivo. Molecular Therapy Nucleic Acids, 3(8), e186.
[10]. Hou, X., Fan, W., Zeng, J., Gao, Z., Wan, J., & Liao, B. (2024). Generation of an ISL1 homozygous knockout stem cell line (WAe009-A-1G) by episomal vector-based CRISPR/Cas9 system. Stem Cell Research, 76, 103376.
[11]. Han, J. L., Heinson, Y. W., Pozo, M. R., Li, W., & Entcheva, E. (2024). Paradoxical effects of ZIM3, a CRISPRi effector, on human induced pluripotent stem-cell-derived cardiomyocyte electrophysiology. PNAS Nexus, 3(3), e027.
[12]. Freitas, G. P., Lopes, H. B., Souza, A. T. P., Gomes, M. P. O., Quiles, G. K., Gordon, J., Tye, C., Stein, J. L., Stein, G. S., Lian, J. B., Beloti, M. M., & Rosa, A. L. (2021). Mesenchymal stem cells overexpressing BMP-9 by CRISPR-Cas9 present high in vitro osteogenic potential and enhance in vivo bone formation. Gene Therapy, 28(12), 748-759.
[13]. Schary, Y., Rotem, I., Caller, T., et al. (2023). CRISPR-Cas9 editing of TLR4 to improve the outcome of cardiac cell therapy. Scientific Reports, 13, 4481.
[14]. Roland, T. J., & Song, K. (2024). Advances in the generation of constructed cardiac tissue derived from induced pluripotent stem cells for disease modeling and therapeutic discovery. Cells, 13(3), 250.
[15]. Hartmann, N., Knierim, M., Maurer, W., Dybkova, N., Zeman, F., Hasenfuß, G., Sossalla, S., & Streckfuss-Bömeke, K. (2024). NaV1.8 as proarrhythmic target in a ventricular cardiac stem cell model. International Journal of Molecular Sciences, 25(11), 6144.









