1. Introduction
Carcinogenic factors, including alcohol, tobacco, and various chemical carcinogens, can affect local tissue cells, causing changes in the genes of these cells.This subsequently affects the normal physiological functions of the cells, resulting in the development of cancer. Subsequently, it can also have an impact on the metastasis of cancer. There are four subtypes of fibroblast growth factor receptors, which are associated with multiple signaling pathways. Therefore, they play an important role in the normal life activities of cells [1]. FGFR inhibitors can inhibit the FGFR-related cell pathways by binding to fibroblast growth factor receptors, thereby suppressing the physiological activities of cells. Currently, FGFR inhibitors can be classified into selective FGFR receptor inhibitors and non-selective FGFR inhibitors. Currently, FGFR inhibitors exhibit therapeutic efficacy against several malignancies. FGFR1 amplification is present in certain cases of non-small cell lung cancer (NSCLC), and since several FGFR inhibitors possess angiogenesis-inhibiting properties, several FGFR inhibitors, including nintedanib, anlotinib, and erdafitinib, have been utilized in the treatment of NSCLC. Currently, experiments have been conducted to study the therapeutic effects and safety of these three drugs, and some of them have been officially put into use [2-4]. Based on the existing literature and data, this paper analyzes FGFR inhibitors and their applications in NSCLC. Lung cancer is an extremely common tumor disease in contemporary society, boasting a remarkably high mortality rate. It is believed that with the continuous development and research of drugs, in the future, people will acquire a more in-depth understanding of NSCLC, and NSCLC will be treated more effectively [5].
2. FGFR and FGF
FGFR is a receptor possessing a tyrosine kinase domain. Upon FGFR binding to its specific ligand, FGF, FGFR undergoes dimerization and autophosphorylation, subsequently activating relevant intracellular signaling pathways, thereby contributing significantly to essential physiological processes in the human body, including cell growth, cell migration, and angiogenesis[1]. The primary intracellular signaling pathways activated following FGFR stimulation include the mitogen-activated protein kinase (MAPK) pathway, the phosphatidylinositol-3-kinase/serine/threonine kinase (PI3K/AKT) pathway, the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway, and the signal transducer and activator of transcription (STAT) pathway (illustrated in Figure 1) [6].
There are mainly four types of fibroblast growth factor receptors, which are FGFR1, FGFR2, FGFR3, and FGFR4 respectively. They have three structures, namely the extracellular domain, the single-channel transmembrane domain, and the intracellular domain [6]. The extracellular domain is composed of three immunoglobulin-like domains, namely D1, D2, and D3(as shown in Figure 1). Between D1 and D2, there is an acid box (AB), which is an amino acid sequence rich in glutamic acid, aspartic acid, and serine. D2 and D3 play important roles in ligand binding and its specificity. D2 has an HS-binding site, and the bases in the D2 domain assist in the binding of FGF to FGFR. In addition, in FGFR1-3, the b and c subtypes generated after the selective splicing of the D3 domain are also the reasons for the generation of FGF-binding specificity [1,6]
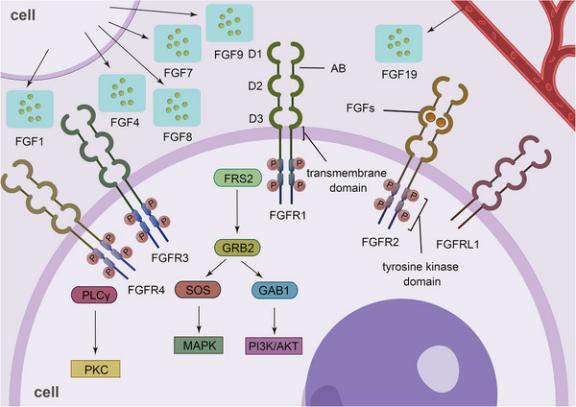
Figure 1: The structure of FGFR and its related signaling pathways [1]
There are four types of FGFR receptors in all, namely FGFR1, FGFR2, FGFR3 and FGFR4. The FGFR is composed of three immunoglobulin-like domains and an intracellular tyrosine kinase domain. Upon binding of FGFR to FGF, the tyrosine kinase domain experiences autophosphorylation and dimerization processes. Subsequently, this activates the activation of the intracellular signaling pathway, consequently exerting an influence on the physiological functions and activities of the cells [1].
FGFR inhibitors can be classified into selective FGFR inhibitors and non-selective inhibitors according to their action targets and objectives. Meanwhile, they can also be divided into reversible inhibitors and irreversible inhibitors based on their functions [1,7]. Among them, non-selective FGFR inhibitors include ponatinib, lenvatinib, nintedanib, olverembatinib, dovitinib, lucitanib, and derazantinib [1]. Ponatinib is primarily utilized for treating Philadelphia chromosome-positive refractory chronic myeloid leukemia, demonstrating significant therapeutic efficacy. Ponatinib is mostly metabolized in the liver via the CYP 3A4 pathway. Its metabolic intermediates may cause liver damage, and there may be drug interactions when it is used in combination with CYP 3A4-inducing drugs or CYP 3A4-inhibiting drugs [8]. Lenvatinib is a multi-target inhibitor. It mainly inhibits angiogenesis by suppressing the intracellular signaling pathways of VEGF and FGF. The dual inhibition of FGFR and VEGFR by lenvatinib has enhanced its antitumor activity against HCC to a certain extent [9]. Nintedanib has anti-fibrotic properties. Since FGF mediates the pro-fibrotic process and is related to the pathogenesis of idiopathic pulmonary fibrosis (IPF), Nintedanib can be used to treat IPF [10]. Osimertinib, a third-generation tyrosine kinase inhibitor (TKI), has demonstrated significant clinical success in treating chronic myeloid leukemia (CML) patients with the T315I mutation. It can bind with high affinity to both the BCR-ABL1 and the adenosine triphosphate (ATP) binding sites of BCR-ABL1 [11]. Dovitinib, a small-molecule tyrosine kinase inhibitor, can target FGFR. It has been demonstrated to possess potent tumor inhibitory effects in preclinical animal models and clinical trials. Dovitinib can exert its tumor suppressive effects by activating Src homology region 2 domain-containing phosphatase-1 (SHP-1) and blocking the induction of phosphorylated signal transducer and activator of transcription 3 (P-STAT3). Moreover, it can exert anti-tumor effects by activating programmed cell death [12]. Lucitanib functions as a multi-target inhibitor, and its targeted receptors include fibroblast growth factor receptors 1, 2, and 3 (FGFR1, FGFR2, and FGFR 3). In the treatment of advanced cancers, the amplification status of FGFR1 and FGF is often used as a biomarker. Experimental findings have shown that cancer cell lines harboring mutations or undergoing amplifications in fibroblast growth FGFR1 and FGFR 2 exhibit a higher degree of sensitivity to lucitanib [13]. Derazantinib, also referred to as ARQ087, is an inhibitor of FGFR. It functions as an ATP-competitive inhibitor, primarily targeting fibroblast growth factor receptors FGFR1 through FGFR3. Derazantinib can influence several FGFR-dependent human cancer cell lines and xenograft tumor models in both in vitro and in vivo settings [14]. Moreover, selective FGFR inhibitors including futibatinib, erdafitinib, LY2874455, pemigatinib, AZD4547, rogaratinib, E7090, and debio 1347 [1]. Futibatinib is an irreversible FGFR inhibitor that can act on FGFR1-4. It is a highly specific inhibitor targeting FGFR, which acts through a covalent binding mechanism. This mechanism is predominantly demonstrated by the formation of covalent bonds with the conserved cysteine residues present within the kinase domain of FGFR, and this binding is irreversible. Futibatinib not only exhibits great activity against the acquired resistance of the FGFR kinase domain but also shows activity against various cancers with FGFR mutations. When using futibatinib, adverse reactions such as hyperphosphatemia, diarrhea, constipation, fatigue, dry mouth, and alopecia may occur [15]. Erdafitinib is an oral FGFR inhibitor with the primary targets being FGFR1-4. It can be used for the treatment of advanced, unresectable, and metastatic urothelial carcinoma. Currently, the efficacy of erdafitinib in treating other cancers with FGFR mutations is being evaluated. When used, it is prone to cause side effects, including a variety of symptoms such as hyperphosphatemia, stomatitis, diarrhea, dry mouth, decreased appetite, and dysgeusia [16] LY2874455 is a pan-FGFR inhibitor. It can bind to the adenosine triphosphate (ATP) binding pocket and overcome the resistance conferred by the FGFR4 V550L mutation [17]. In addition, LY2874455 also has the effect of radiosensitization. However, whether it can be combined with radiotherapy still requires further clinical verification [18]. Pemigatinib is indicated for the treatment of myeloid or lymphoid cancers exhibiting FGFR rearrangement. The side effects linked to pemigatinib administration encompass hyperphosphatemia, weariness, nausea, dizziness, baldness, and more symptoms. Additional severe adverse effects including eye toxicity [19].
3. FGFR inhibitors in NSCLC
Lung cancer is a prevalent neoplastic illness. In 2008, it was the most prevalent cancer among males worldwide and the fourth most prevalent cancer among women. Lung cancer is not just linked to smoking; it may also be associated with occupational exposure to toxins, such as asbestos and others [20].
Lung cancer include small cell lung cancer and non-small cell lung cancer. Non-small cell lung cancer is the predominant kind of lung cancer. Additionally, non-small cell lung cancer (NSCLC) can be subdivided into squamous NSCLC and non-squamous carcinoma [21].
Amplification of FGFR1 is commonly seen in squamous cell carcinoma, and its detection rate inNSCLC is 6% [22]. The amplification of FGFR1 activates many pathways, hence facilitating the onset and progression of cancer. Consequently, the development of medicines that target FGFR may represent a significant strategy for the treatment of NSCLC [23]. Angiogenesis is crucial for cancer growth and maintenance, and nintedanib inhibits angiogenesis by binding to the ATP-binding site of the kinase domain of pro-angiogenic receptors, hence demonstrating therapeutic efficacy in NSCLC [2].
In the safety assessment of an open-label phase I accelerated dose-escalation trial, the adverse events exhibited during the use of nintedanib were mild or moderate, mainly including nausea, vomiting, diarrhea, fatigue and dizziness [24]. Nintedanib may also be administered in conjunction with cytotoxic chemotherapy, specifically with docetaxel or pemetrexed. In the phase 3 LUME-Lung1 research, following the commencement of first-line treatment, the median overall survival for 199 patients in the docetaxel+nintedanib cohort was 10.9 months, surpassing that of the placebo group. Therefore, the combined use of docetaxel and nintedanib can prolong the overall survival of patients [25]. Anlotinib is an oral tyrosine - kinase inhibitor that targets FGFR1-4 and has been approved for the third - line or higher - line treatment of advanced NSCLC [3]. In the phase III randomized clinical trial of ALTER 0303, both the overall survival and progression-free survival of anlotinib were higher than those of the placebo group. Furthermore, the objective response rate (9.2%) and disease control rate (81%) of anlotinib surpassed those of the placebo group [26]. A randomized, double-blind, multicenter, placebo-controlled phase II trial evaluated the safety and efficacy of anlotinib as a third-line or higher treatment for patients with advanced NSCLC.
The trial results demonstrated an advantage in progression-free survival (PFS). The PFS of anlotinib in this trial was 4.8 months (95% confidence interval, 3.5-6.0), surpassing that of the placebo group (1.2 months, 95% confidence interval, 0.7-1.6). In this experiment, 55 of the 60 patients in the anlotinib group experienced grade 1 to 4 side events, which were more prevalent than in the placebo group. This trial demonstrated that anlotinib exhibits favorable tolerability in both third-line and first-line treatments [3]. A single-arm, open-label, phase IIa trial designed to assess the clinical effectiveness, safety, and pharmacokinetics of erdafitinib in Asian patients with advanced NSCLC. In this trial, the disease control rate for NSCLC patients was 25%, and the safety profile of erdafitinib was deemed acceptable, demonstrating good tolerance among patients with advanced solid tumors [27]. PD173074 exhibits strong complementarity to the tyrosine kinase domain of FGFR1 and can impede angiogenesis in preclinical murine studies. Moreover, in individuals with NSCLC, the expression of multidrug resistance-associated protein 7(MRP7) is elevated. This protein is part of the ATP-binding cassette (ABC) transporter family and imparts resistance to certain anti-tumor agents. PD173074 enhances the intracellular retention of anti-tumor agents by inhibiting MRP7 and diminishing their efflux [4].
4. Discussion
When using FGFR inhibitors, multiple precautions should be noted. One of them is their target - related toxicity. Hyperphosphatemia, and toxicities related to nails, eyes, skin, and the gastrointestinal tract are the most common target-related toxicities of FGFR inhibitors. When using erdafitinib to treat NSCLC patients, patients may develop hyperphosphatemia and central serous retinopathy. In the most severe cases, the serum phosphate of patients with hyperphosphatemia may exceed 9.0 mg/dl or there may be a significant change in baseline renal function, and hypercalcemia may also occur. For patients with central serous retinopathy, the visual acuity of the affected eye is 20/200 or worse[27]. At present, anlotinib has obtained approval in China for the management of advanced NSCLC cases in which the disease has advanced following the administration of at least two different lines of prior treatment. However, regarding the use of anlotinib, further research is needed on predictive biomarkers of anlotinib to identify the patients who are most suitable for anlotinib treatment[20]. When using FGFR inhibitors to treat patients, drug resistance may occur. The gatekeeper mutation can cause spatial inhibition of the ATP pocket, which prevents TKI drugs such as FGFR inhibitors from entering, thus leading to drug resistance[1]. Alongside the development of drug resistance via particular mutations like the gatekeeper mutation, the activation of alternative signaling pathways may also obstruct the FGFR signaling pathway.
For example, when the MET signaling pathway is up-regulated, ERK/MAPK may be reactivated [28]. Therefore, in future research, how to reduce the impact of drug resistance on experiments may also be a very important direction.
Immunotherapy is also gradually being incorporated into the treatment of NSCLC. Related research to be conducted in the future may prove that the combined use of immune checkpoint inhibitors and FGFR inhibitors has a good therapeutic effect on NSCLC [2].
5. Conclusion
FGFR is a tyrosine kinase receptor intricately linked to cellular proliferation, migration, and angiogenesis. Its aberrant activation is associated with the onset and progression of several malignancies. FGFR inhibitors are categorized into selective and non-selective types, and they have anti-tumor actions by obstructing the FGFR signaling pathway. FGFR1 amplification is relatively prevalent in NSCLC. Certain FGFR inhibitors, including nintedanib, anlotinib, and erdafitinib, have exhibited efficacy and safety in clinical trials. However, the use of FGFR inhibitors also faces issues such as target-related toxicity and drug resistance. Future research needs to further explore the mechanisms of drug resistance and optimize treatment strategies, such as combining immunotherapy, to enhance the application value of FGFR inhibitors in the treatment of NSCLC.
References
[1]. Liu Q, Huang J, Yan W, Liu Z, Liu S, Fang W. (2020). FGFR families: biological functions and therapeutic interventions in tumors. MedComm. Sep 23;4(5):e367.
[2]. Bronte, G., Passiglia, F., Galvano, A., Barraco, N., Listì, A., Castiglia, M., ... & Russo, A. (2016). Nintedanib in NSCLC: evidence to date and place in therapy. Therapeutic Advances in Medical Oncology, 8(3), 188-197.
[3]. Han, B., Li, K., Zhao, Y., Li, B., Cheng, Y., Zhou, J., ... & Wu, G. (2018). Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). British journal of cancer, 118(5), 654-661.
[4]. Anreddy, N., Patel, A., et al. (2014). PD173074, a selective FGFR inhibitor, reverses MRP7 (ABCC10)-mediated MDR. Acta Pharmaceutica Sinica B, 4(3), 202-207.
[5]. Yu, G., Shen, Y., Xu, X., & Zhong, F. (2020). Anlotinib for refractory advanced non-small-cell lung cancer: A systematic review and meta-analysis. PloS one, 15(11), e0242982.
[6]. Edirisinghe, O., Ternier, G., Alraawi, Z., & Suresh Kumar, T. K. (2024). Decoding FGF/FGFR Signaling: Insights into Biological Functions and Disease Relevance. Biomolecules, 14(12), 1622.
[7]. Katoh, M. (2016). FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis. International journal of molecular medicine, 38(1), 3-15.
[8]. De la Garza-Salazar F, Colunga-Pedraza PR, Gómez-Almaguer D. 2023. Cytochrome P450 inhibition to decrease dosage and costs of venetoclax and ibrutinib: A proof-of-concept case study. Br J Clin Pharmacol. 89(2):898-902. doi: 10.1111/bcp.15590. Epub 2022 Nov 22. PMID: 36354135.
[9]. Zhao, Y., Zhang, Y. N., Wang, K. T., & Chen, L. (2020). Lenvatinib for hepatocellular carcinoma: From preclinical mechanisms to anti-cancer therapy. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 1874(1), 188391.
[10]. Lamb, Y. N. (2021). Nintedanib: a review in fibrotic interstitial lung diseases. Drugs, 81, 575-586.
[11]. Qian, H., Gang, D., He, X., & Jiang, S. (2022). A review of the therapeutic role of the new third-generation TKI olverembatinib in chronic myeloid leukemia. Frontiers in Oncology, 12, 1036437.
[12]. Chiu, Y. H., Lee, Y. Y., Huang, K. C., Liu, C. C., & Lin, C. S. (2019). Dovitinib triggers apoptosis and autophagic cell death by targeting SHP‐1/p‐STAT3 signaling in human breast cancers. Journal of Oncology, 2019(1), 2024648.
[13]. Zhang, Y., Luo, F., Ma, Y. X., Liu, Q. W., Yang, Y. P., Fang, W. F., ... & Zhang, L. (2022). A phase Ib study of lucitanib (AL3810) in a cohort of patients with recurrent and metastatic nasopharyngeal carcinoma. The Oncologist, 27(6), e453-e462.
[14]. Raggi, C., Fiaccadori, K., Pastore, M., Correnti, M., Piombanti, B., Forti, E., ... & Invernizzi, P. (2019). Antitumor activity of a novel fibroblast growth factor receptor inhibitor for intrahepatic cholangiocarcinoma. The American Journal of Pathology, 189(10), 2090-2101.
[15]. Javle, M., King, G., Spencer, K., & Borad, M. J. (2023). Futibatinib, an irreversible FGFR1-4 inhibitor for the treatment of FGFR-aberrant tumors. The oncologist, 28(11), 928-943.
[16]. Bethesda, M. D. (2009). National Institute of Diabetes and Digestive and Kidney Diseases 2009. US Renal Data System: USRDS 2009 Annual Data Report.
[17]. Dehghanian, F., & Alavi, S. (2021). Molecular mechanisms of the anti-cancer drug, LY2874455, in overcoming the FGFR4 mutation-based resistance. Scientific Reports, 11(1), 16593.
[18]. Darwis NDM, Horigome E, Li S, Adachi A, Oike T, Shibata A, Hirota Y, Ohno T. 2022. Radiosensitization by the Selective Pan-FGFR Inhibitor LY2874455. Cells. 11(11):1727. doi: 10.3390/cells11111727. PMID: 35681425; PMCID: PMC9179643.
[19]. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. (2012). Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases.
[20]. Jemal, A., Bray, F., Center, M. M., Ferlay, J., Ward, E., & Forman, D. (2011). Global cancer statistics. CA: a cancer journal for clinicians, 61(2), 69-90.
[21]. Piperdi, B., Merla, A., & Perez-Soler, R. (2014). Targeting angiogenesis in squamous non-small cell lung cancer. Drugs, 74, 403-413.
[22]. Ferguson, H. R., Smith, M. P., & Francavilla, C. (2021). Fibroblast growth factor receptors (FGFRs) and noncanonical partners in cancer signaling. Cells, 10(5), 1201.
[23]. Cihoric, N., Savic, S., Schneider, S., Ackermann, I., Bichsel-Naef, M., Schmid, R. A., ... & Tapia, C. (2014). Prognostic role of FGFR1 amplification in early-stage non-small cell lung cancer. British journal of cancer, 110(12), 2914-2922.
[24]. Mross, K., Stefanic, M., Gmehling, D., Frost, A., Baas, F., Unger, C., ... & Kaiser, R. (2010). Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clinical Cancer Research, 16(1), 311-319.
[25]. Reck, M., Kaiser, R., Mellemgaard, A., Douillard, J. Y., Orlov, S., Krzakowski, M., ... & Novello, S. (2014). Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. The lancet oncology, 15(2), 143-155.
[26]. Han, B., Li, K., Wang, Q., Zhang, L., Shi, J., Wang, Z., ... & Sun, Y. (2018). Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non–small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA oncology, 4(11), 1569-1575.
[27]. Park JO, Feng YH, Su WC, Oh DY, Keam B, Shen L, Kim SW, Liu X, Liao H, Qing M, Zhang C, Qian J, Tang X, Li P, Triantos S, Sweiti H. 2024. Erdafitinib in Asian patients with advanced solid tumors: an open-label, single-arm, phase IIa trial. BMC Cancer. 24(1):1006. doi: 10.1186/s12885-024-12584-0. PMID: 39138436; PMCID: PMC11323360
[28]. Krook MA, Reeser JW, Ernst G, Barker H, Wilberding M, Li G, Chen HZ, Roychowdhury S. 2021. Fibroblast growth factor receptors in cancer: genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br J Cancer. 124(5):880-892. doi: 10.1038/s41416-020-01157-0. Epub 2020 Dec 3. PMID: 33268819; PMCID: PMC7921129.
Cite this article
Shi,Z. (2025). Fibroblast Growth Factor Receptor Inhibitors and Their Application in Non-Small Cell Lung Cancer. Theoretical and Natural Science,99,109-114.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Liu Q, Huang J, Yan W, Liu Z, Liu S, Fang W. (2020). FGFR families: biological functions and therapeutic interventions in tumors. MedComm. Sep 23;4(5):e367.
[2]. Bronte, G., Passiglia, F., Galvano, A., Barraco, N., Listì, A., Castiglia, M., ... & Russo, A. (2016). Nintedanib in NSCLC: evidence to date and place in therapy. Therapeutic Advances in Medical Oncology, 8(3), 188-197.
[3]. Han, B., Li, K., Zhao, Y., Li, B., Cheng, Y., Zhou, J., ... & Wu, G. (2018). Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). British journal of cancer, 118(5), 654-661.
[4]. Anreddy, N., Patel, A., et al. (2014). PD173074, a selective FGFR inhibitor, reverses MRP7 (ABCC10)-mediated MDR. Acta Pharmaceutica Sinica B, 4(3), 202-207.
[5]. Yu, G., Shen, Y., Xu, X., & Zhong, F. (2020). Anlotinib for refractory advanced non-small-cell lung cancer: A systematic review and meta-analysis. PloS one, 15(11), e0242982.
[6]. Edirisinghe, O., Ternier, G., Alraawi, Z., & Suresh Kumar, T. K. (2024). Decoding FGF/FGFR Signaling: Insights into Biological Functions and Disease Relevance. Biomolecules, 14(12), 1622.
[7]. Katoh, M. (2016). FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis. International journal of molecular medicine, 38(1), 3-15.
[8]. De la Garza-Salazar F, Colunga-Pedraza PR, Gómez-Almaguer D. 2023. Cytochrome P450 inhibition to decrease dosage and costs of venetoclax and ibrutinib: A proof-of-concept case study. Br J Clin Pharmacol. 89(2):898-902. doi: 10.1111/bcp.15590. Epub 2022 Nov 22. PMID: 36354135.
[9]. Zhao, Y., Zhang, Y. N., Wang, K. T., & Chen, L. (2020). Lenvatinib for hepatocellular carcinoma: From preclinical mechanisms to anti-cancer therapy. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 1874(1), 188391.
[10]. Lamb, Y. N. (2021). Nintedanib: a review in fibrotic interstitial lung diseases. Drugs, 81, 575-586.
[11]. Qian, H., Gang, D., He, X., & Jiang, S. (2022). A review of the therapeutic role of the new third-generation TKI olverembatinib in chronic myeloid leukemia. Frontiers in Oncology, 12, 1036437.
[12]. Chiu, Y. H., Lee, Y. Y., Huang, K. C., Liu, C. C., & Lin, C. S. (2019). Dovitinib triggers apoptosis and autophagic cell death by targeting SHP‐1/p‐STAT3 signaling in human breast cancers. Journal of Oncology, 2019(1), 2024648.
[13]. Zhang, Y., Luo, F., Ma, Y. X., Liu, Q. W., Yang, Y. P., Fang, W. F., ... & Zhang, L. (2022). A phase Ib study of lucitanib (AL3810) in a cohort of patients with recurrent and metastatic nasopharyngeal carcinoma. The Oncologist, 27(6), e453-e462.
[14]. Raggi, C., Fiaccadori, K., Pastore, M., Correnti, M., Piombanti, B., Forti, E., ... & Invernizzi, P. (2019). Antitumor activity of a novel fibroblast growth factor receptor inhibitor for intrahepatic cholangiocarcinoma. The American Journal of Pathology, 189(10), 2090-2101.
[15]. Javle, M., King, G., Spencer, K., & Borad, M. J. (2023). Futibatinib, an irreversible FGFR1-4 inhibitor for the treatment of FGFR-aberrant tumors. The oncologist, 28(11), 928-943.
[16]. Bethesda, M. D. (2009). National Institute of Diabetes and Digestive and Kidney Diseases 2009. US Renal Data System: USRDS 2009 Annual Data Report.
[17]. Dehghanian, F., & Alavi, S. (2021). Molecular mechanisms of the anti-cancer drug, LY2874455, in overcoming the FGFR4 mutation-based resistance. Scientific Reports, 11(1), 16593.
[18]. Darwis NDM, Horigome E, Li S, Adachi A, Oike T, Shibata A, Hirota Y, Ohno T. 2022. Radiosensitization by the Selective Pan-FGFR Inhibitor LY2874455. Cells. 11(11):1727. doi: 10.3390/cells11111727. PMID: 35681425; PMCID: PMC9179643.
[19]. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. (2012). Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases.
[20]. Jemal, A., Bray, F., Center, M. M., Ferlay, J., Ward, E., & Forman, D. (2011). Global cancer statistics. CA: a cancer journal for clinicians, 61(2), 69-90.
[21]. Piperdi, B., Merla, A., & Perez-Soler, R. (2014). Targeting angiogenesis in squamous non-small cell lung cancer. Drugs, 74, 403-413.
[22]. Ferguson, H. R., Smith, M. P., & Francavilla, C. (2021). Fibroblast growth factor receptors (FGFRs) and noncanonical partners in cancer signaling. Cells, 10(5), 1201.
[23]. Cihoric, N., Savic, S., Schneider, S., Ackermann, I., Bichsel-Naef, M., Schmid, R. A., ... & Tapia, C. (2014). Prognostic role of FGFR1 amplification in early-stage non-small cell lung cancer. British journal of cancer, 110(12), 2914-2922.
[24]. Mross, K., Stefanic, M., Gmehling, D., Frost, A., Baas, F., Unger, C., ... & Kaiser, R. (2010). Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clinical Cancer Research, 16(1), 311-319.
[25]. Reck, M., Kaiser, R., Mellemgaard, A., Douillard, J. Y., Orlov, S., Krzakowski, M., ... & Novello, S. (2014). Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. The lancet oncology, 15(2), 143-155.
[26]. Han, B., Li, K., Wang, Q., Zhang, L., Shi, J., Wang, Z., ... & Sun, Y. (2018). Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non–small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA oncology, 4(11), 1569-1575.
[27]. Park JO, Feng YH, Su WC, Oh DY, Keam B, Shen L, Kim SW, Liu X, Liao H, Qing M, Zhang C, Qian J, Tang X, Li P, Triantos S, Sweiti H. 2024. Erdafitinib in Asian patients with advanced solid tumors: an open-label, single-arm, phase IIa trial. BMC Cancer. 24(1):1006. doi: 10.1186/s12885-024-12584-0. PMID: 39138436; PMCID: PMC11323360
[28]. Krook MA, Reeser JW, Ernst G, Barker H, Wilberding M, Li G, Chen HZ, Roychowdhury S. 2021. Fibroblast growth factor receptors in cancer: genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br J Cancer. 124(5):880-892. doi: 10.1038/s41416-020-01157-0. Epub 2020 Dec 3. PMID: 33268819; PMCID: PMC7921129.









