1. Introduction
Circadian rhythms are a biological rhythm that repeats about every 24 hours. It regulates important physiological behaviors such as sleep, eating, hormone production and cognitive activity. This rhythm is mainly dominated by the suprachiasmatic nucleus (SCN) located in the hypothalamus, which regulates the biological clock according to external light signals and thus maintains the stability of the circadian rhythm [1]. Although a great many studies have demonstrated that sleep plays a crucial role in memory consolidation [2], an increasing amount of evidence indicates that circadian rhythms per se play an important part in learning and memory, even without any alteration in the length of sleep. As an important structure in the brain that is responsible for spatial and declarative memory, there exists a functional connection between the hippocampus and the SCN at the levels of nerve and hormone. Recent experiments have shown that disruption of SCN rhythms can impair hippocampal-related learning and lead to a decline in long-term enhancement (LTP), a physiological marker of neuroplasticity and memory coding [3,4]. The emergence of these memory impairments is not contingent upon sleep deprivation; rather, it is directly associated with the disruption of rhythmic central function. A profound understanding of the mechanism by which circadian rhythm influences learning is not only conducive to comprehending the mechanism of memory formation from the perspective of basic neuroscience, but also of great significance for practical applications. For example, night shift workers, students with irregular schedules, and older adults with neurodegenerative diseases often experience varying degrees of cognitive impairment due to circadian rhythm disturbances. This paper will explore the potential mechanisms by which circadian rhythms regulate memory formation, synthesize animal and human research evidence, and discuss current and possible future interventions to mitigate memory deficits caused by rhythm disturbances.
2. Biological mechanisms of circadian influence on memory
Circadian rhythms modulate memory through circuit-level and molecular mechanisms. At the heart of this regulation is the SCN which acts as the brain’s master clock. While the SCN does not participate directly in memory formation, it communicates to cognitive circuitry, especially to the hippocampus through hormonal pathways, modulation via neurotransmitters, as well as through intermediate hypothalamic structures where the SCN sends projections.
One suggested route is that the SCN may indirectly modulate hippocampal function via hypothalamic relays, e.g., subparaventricular zone (sPVz) and dorsomedial hypothalamus (DMH). These regions mediate arousal and autonomic rhythms and may serve as intermediaries through which the SCN (suprachiasmatic nucleus) exerts modulation on cortical and limbic activity. In addition, orexin-expressing neurons in the lateral hypothalamus could connect circadian and cognitive processes, especially those related to wakefulness and attention. Melatonin secretion has been also attributed to regulating hippocampal neuroplasticity by influencing sleep timing in rodents[5], although this process may not be present in all rodent strains, such as C57BL/6J.
As shown in Figure 1, the SCN receives photic information from the retina through the retinohypothalamic tract (RHT) and projects through a multi-synaptic pathway to hypothalamic nuclei involved in sleep-wake regulation, including the VLPO, TMN, and PVN. These nuclei might then modulate the hippocampus via diffuse modulatory systems or circadian-timed hormone secretion. Although the anatomical connectome between SCN and hippocampus is not well characterized, strong evidence supports functional regulation by rhythmic changes in hippocampal LTP and clock gene transcription[6,7].
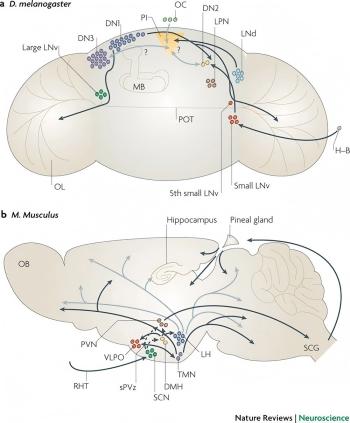
At the molecular level, the major components of core clock genes, such as BMAL1, CLOCK, PER1/2, and CRY1/2, are rhythmically expressed in the hippocampus and have an influence on synaptic plasticity. For example, deletion of BMAL1 was shown to disrupt LTP and compromise memory consolidation [8].These genes govern the generation of transcriptional loops that impact neurotransmission, such as GABAergic and glutamatergic signaling, which are crucial for learning.
In Drosophila, an invertebrate, the connections between rhythm neurons and memory centers, such as the mushroom body, are clearer. As shown in Figure 1a, these rhythm neurons receive light input and send time signals to downstream neural circuits. In contrast, Figure 1b shows that the connections between SCN and memory-related structures in mammals are more indirect, and the specific mechanisms are still being further explored [5].
3. Experimental evidence
3.1. Animal studies
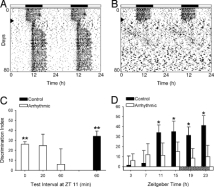
In rodents, experimental studies have shown that a functional circadian system is required for hippocampal-dependent memory formation, regardless of sleep [3], as well and the metabolism of certain nutrients [4]. Ruby et al. [3] employed a dark disruptive phase shift (DPS) protocol in Siberian hampsters (Phodopus sungorus), resulting in a complete annulment of circadian rhythmicity with no loss of sleep quantity. The protocol involved a light pulse during the night (ZT12-X) followed by a 3-hr delay in the light–dark (LD) cycle. Control hamsters exhibited strong circadian activity patterns, whereas DPS-treated animals were arrhythmic after a few days (Figure 2A–B).
Neurocognitive test using the Novel Object Recognition (NOR) task. Performance, defined as the discrimination index (DI), reflecting a preference for the novel over the familiar object (ssc.tamc.edu.tw), was used to determine memory. As shown in Figure 2C, control animals demonstrated robust recognition memory at a 60 min delay (P < 0.001), whereas arrhythmic animals did not discriminate the novel object at 20 or 60 min (P > 0.05), but performed normally at 0-minute delay. The data indicates that short-term memory preservation is intact but memory consolidation and retention are disrupted in the absence of functional SCN.
The performance also differed throughout the circadian cycle. Control animals tested at a range of Zeitgeber Times (ZT; Fig. 2D) showed a time-of-day dependent change in DI, while arrhythmic animals showed no significant performance at any time point. This data highlights that circadian phase, not simply sleep status, modulates memory function.
More work by Snider et al. supports these behavioral results at the neural level. Using electrophysiological recordings, they found that LTP, a cellular correlate of memory, was significantly reduced in arrhythmic animals, but LTP was restored following SCN ablation[6]. This shows that the SCN itself, when dysfunctional, actively disrupts hippocampal plasticity and that its removal can relieve this inhibition.
Pharmacological manipulation also provides insight into SCN-cognition interaction. Colas et al. [4] showed that pentylenetetrazole (PTZ)-induced GABA_A receptor blockade restores the memory of a Down syndrome mouse model. Similarly, Ruby et al. [3] reported that PTZ administration significantly promoted NOR performance in arrhythmic hamsters across a broad background of ongoing circadian disruption. This indicates that excessive inhibitory output from the SCN may provide an inhibitory effect on cognitive circuits, and it is worth investigating this pathway for therapeutic purposes.
3.2. Human studies
The research also supports the significant role of circadian rhythm regulation in human cognitive function. Valdez et al. found that attention, working memory, and executive function have obvious time - related effects, usually peaking in the morning and evening [9]. These patterns are in line with the circadian rhythm in the body, not just with the waking hours.
Cognitive impairments are well documented in populations experiencing chronic circadian disruption, including shift workers. Wright et al. [10] showed that although total sleep time is comparable, shift workers have deficits in short-term memory, attention, and information processing speed. These impairments are associated with circadian misalignment, rather than sleep deprivation per se [11].
Another relevant context in which circadian dysfunction affects cognition is aging. Mander et al. [12] found that aged individuals with impaired SCN output exhibited simultaneous deficits in sleep quality and hippocampal function. These findings were further expanded by Hood and Amir [13], who provided compelling evidence suggesting that SCN degeneration during aging is partly responsible for defects in circadian stability and memory, exacerbating neurodegenerative processes.
4. Consequences and interventions
Timed circadian disruption is an increasingly recognized contributing risk factor to cognitive decline across populations, including shift workers, adolescents sleeping out of rhythm, and elders.Such outcomes may be attributed not only to an inadequate amount of sleep time but also to the desynchrony between internal circadian timing and external demands.
Effects of circadian disruption on cognition are most prominently seen in the context of shift work. Workers on the night or rotating shifts usually show a desynchrony between their endogenous circadian pacemaker and the imposed work–sleep schedule. Even when total sleep time is controlled for, this results in cognitive deficits in attention, working memory, and decision-making speed[14]. These results indicate that circadian misalignment, but not sleep deprivation per se, is a significant contributor to impaired cognitive functioning in shift-working populations.
Circadian disruption is also a strong contributor to cognitive decline during aging. In older individuals chronobiological deficits include attenuated SCN output, diminished amplitude rhythms, phase delays of core body temperature and melatonin secretion[13]. These physiological changes correlate with impaired memory, especially on hippocampal dependent tasks. In fact, longitudinal studies examining older healthy individuals have reported associations between long-term circadian disruption and higher β-amyloid deposition and accelerated memory impairments[15]. These findings underscore the relevance of maintaining circadian integrity to cognitive health across the lifespan.
Considering the negative implications of circadian disruption, several intervention strategies have been devised to reduce its effect on cognition. The most powerful of these is light therapy, especially the use of short-wavelength (blue) light. Sleepless: The scope of blue light evidence is limited to insomniacs, and waking it during the ultra morning or early day can improve both alertness and cognitive ability, even in blinds who cannot consciously see[16] These effects are mediated by melanopsin-containing retinal ganglion cells that project directly to the SCN, reinforcing an increasing recognition of the role of circadian mechanisms in the control of cognition.
A second strategy is the use of chronobiotic agents, including melatonin and its agonists, that may manipulate the timing of circadian rhythms. Melatonin, taken when used properly and at the right time, has had some success in treating jet lag, shift work disorder, and delayed sleep phase syndrome, with indirect benefits for mood and cognitive clarity[17]. Yet in older adults, reduced endogenous production and end organ desensitization may limit the efficacy of melatonin.
In addition to pharmacological and photic manipulations, behavioral strategies are also key elements in circadian alignment.Regular sleep - wake schedules, physical activity during the daytime, and exposure to natural light will enhance circadian entrainment and help strengthen cognitive resilience. These methods are of great significance for older adults, as the restructuring of lifestyle could counteract the degradation of endogenous rhythms [18].
In conclusion, circadian disruption represents an apparent danger to learning and memory; however, multiple types of non-invasive and low-risk interventions, especially those that involve light exposure or behaviour timing, can remedy rhythm-cognition misalignment. As contemporary lifestyles increasingly disrupt biological timing systems, these findings hint at a means of safeguarding and enhancing cognitive performance across all ages.
5. Conclusion
Circadian rhythms, maintained by the suprachiasmatic nucleus (SCN), are critical for sleep, hormone release, and cognition. This review elucidated the evidences from both animal and human studies that indicate that circadian rhythmicity, independent of sleep duration, is essential for learning and memory. SCN dysfunction leads to impairments in hippocampal-dependent memory performance and synaptic plasticity and restoration of circadian integrity improves cognitive outcomes. These findings emphasize the brain's reliance on internal timing strategies to support memory consolidation and attentional control.
However, this review must not be without limitations. While this work strongly implicates circadian rhythms in memory formation, the actual number of studies addressing the causal role of circadian rhythms in memory formation is still relatively small. Many of these findings rely on specific animal models like Siberian hamsters or genetically engineered mice, which might not translate perfectly to us. Furthermore, differences in experimental protocols between studies complicate the investigation of subjective circadian effects unconformity with alternate factors, including stress or sleep disruption.
Future research needs to utilize heterogeneous populations and standardized methods to expand the evidence. Understanding how circadian oscillators interact with cognitive circuits at the molecular level is able to reveal new therapeutic targets. Ultimately, a better understanding of how these circadian rhythms regulate memory will be crucial for formulating strategies to delay cognitive decline across all ages.
References
[1]. Fernandez F, Lu D, Ha P, Costacurta P, Chavez R, Heller HC, Ruby NF. Dysrhythmia in the suprachiasmatic nucleus inhibits memory processing. Science. 2014;346(6211): 854–857.
[2]. Born J, Wilhelm I. System consolidation of memory during sleep. Psychol Res. 2012;76(2): 192–203.
[3]. Ruby NF, Hwang CE, Wessells C, Fernandez F, Zhang P, Sapolsky R, Heller HC. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci U S A. 2008;105(40): 15593–15598.
[4]. Colas D, Chuluun B, Warrier D, Blank M, Wetmore DZ, Buckmaster P, et al. Short-term treatment with the GABA_A antagonist pentylenetetrazole produces a sustained procognitive benefit in a mouse model of Down’s syndrome. Br J Pharmacol. 2013;169(5): 763–773.
[5]. Gerstner JR, Yin JC. Circadian rhythms and memory formation. Nat Rev Neurosci. 2010;11(8): 577–588.
[6]. Snider KH, Sullivan KA, Obrietan K. Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms. 2016;31(2): 107–117.
[7]. Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS. Circadian modulation of hippocampal long-term potentiation. J Biol Rhythms. 2005;20(3): 225–236.
[8]. Gerstner JR, Perron IJ, Pack AI, et al. BMAL1 regulates long-term synaptic plasticity and circadian modulation of hippocampal oscillations. Nat Neurosci. 2014;17(3): 356–364.
[9]. Valdez P, Ramírez C, García A. Circadian rhythms in cognitive performance: implications for neuropsychological assessment. ChronoPhysiology and Therapy. 2012;2: 81–92.
[10]. Wright KP, Lowry CA, LeBourgeois MK. Circadian and wakefulness-sleep modulation of cognition in humans. Sleep Med Clin. 2012;7(4): 469–480.
[11]. Chellappa SL, Morris CJ, Scheer FAJL. Daily circadian misalignment impairs human cognitive performance in the real world. Neurobiol Learn Mem. 2019;160: 114–120.
[12]. Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci. 2016;39(8): 552–566.
[13]. Hood S, Amir S. The aging clock: Circadian rhythms and later life. J Clin Invest. 2017;127(2): 437–446.
[14]. Boivin DB, Boudreau P. Impacts of shift work on sleep and circadian rhythms. Pathol Biol (Paris). 2014;62(5): 292–301.
[15]. Winer JR, Mander BA, Helfrich RF, et al. Sleep disturbance forecasts β-amyloid accumulation and memory decline in older adults. Neuron. 2019;103(2): 320–331. e5.
[16]. Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13(10): 429–438.
[17]. Arendt J. Managing jet lag: Some of the problems and possible new solutions. Sleep Med Rev. 2009;13(4): 249–256.
[18]. Hood S, Amir S. Biological clocks and the aging brain. Handb Clin Neurol. 2019;167: 209–225.
Cite this article
Su,X. (2025). Influence of Circadian Rhythm on Learning and Memory. Theoretical and Natural Science,113,163-168.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Fernandez F, Lu D, Ha P, Costacurta P, Chavez R, Heller HC, Ruby NF. Dysrhythmia in the suprachiasmatic nucleus inhibits memory processing. Science. 2014;346(6211): 854–857.
[2]. Born J, Wilhelm I. System consolidation of memory during sleep. Psychol Res. 2012;76(2): 192–203.
[3]. Ruby NF, Hwang CE, Wessells C, Fernandez F, Zhang P, Sapolsky R, Heller HC. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci U S A. 2008;105(40): 15593–15598.
[4]. Colas D, Chuluun B, Warrier D, Blank M, Wetmore DZ, Buckmaster P, et al. Short-term treatment with the GABA_A antagonist pentylenetetrazole produces a sustained procognitive benefit in a mouse model of Down’s syndrome. Br J Pharmacol. 2013;169(5): 763–773.
[5]. Gerstner JR, Yin JC. Circadian rhythms and memory formation. Nat Rev Neurosci. 2010;11(8): 577–588.
[6]. Snider KH, Sullivan KA, Obrietan K. Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms. 2016;31(2): 107–117.
[7]. Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS. Circadian modulation of hippocampal long-term potentiation. J Biol Rhythms. 2005;20(3): 225–236.
[8]. Gerstner JR, Perron IJ, Pack AI, et al. BMAL1 regulates long-term synaptic plasticity and circadian modulation of hippocampal oscillations. Nat Neurosci. 2014;17(3): 356–364.
[9]. Valdez P, Ramírez C, García A. Circadian rhythms in cognitive performance: implications for neuropsychological assessment. ChronoPhysiology and Therapy. 2012;2: 81–92.
[10]. Wright KP, Lowry CA, LeBourgeois MK. Circadian and wakefulness-sleep modulation of cognition in humans. Sleep Med Clin. 2012;7(4): 469–480.
[11]. Chellappa SL, Morris CJ, Scheer FAJL. Daily circadian misalignment impairs human cognitive performance in the real world. Neurobiol Learn Mem. 2019;160: 114–120.
[12]. Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci. 2016;39(8): 552–566.
[13]. Hood S, Amir S. The aging clock: Circadian rhythms and later life. J Clin Invest. 2017;127(2): 437–446.
[14]. Boivin DB, Boudreau P. Impacts of shift work on sleep and circadian rhythms. Pathol Biol (Paris). 2014;62(5): 292–301.
[15]. Winer JR, Mander BA, Helfrich RF, et al. Sleep disturbance forecasts β-amyloid accumulation and memory decline in older adults. Neuron. 2019;103(2): 320–331. e5.
[16]. Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13(10): 429–438.
[17]. Arendt J. Managing jet lag: Some of the problems and possible new solutions. Sleep Med Rev. 2009;13(4): 249–256.
[18]. Hood S, Amir S. Biological clocks and the aging brain. Handb Clin Neurol. 2019;167: 209–225.









