1. Introduction
The development of science and technology and the progress of human society bring about a series of problems. The improvement of people's living standard is accompanied by diseases caused by obesity, such as diabetes and so on. In the study and study of diabetes, the author noted the concomitant diseases of diabetes, the most common of which is HF. The incidence of HF is strongly associated with T2DM. The article specifically studied the relationship between the development of heart failure and T2DM through literature analysis. Also, the essay utilized survey method to see if the treatment of T2DM can alleviate heart failure since T2DM is one of the causes of heart failure, and the two are closely linked. The author wants to explore a new approach to treating HF,through the treatment of T2DM to breakthrough the treatment of HF. With this new method, there are chances to reduce the risk of HF caused by T2DM.
2. Introduction to heart failure (HF) related to T2DM
Almost 870,000 individuals are struck down with cardiovascular disease yearly, and nearly six million Americans currently have the condition. Congestive heart failure is the most common reason for hospitalization in adults over 65[1]. Heart failure (HF), often referred to as congestive coronary artery disease (CCAD) and (congestive) heart arrhythmia (CHA), is a collection of symptoms brought on by the heart's inability to perform its role as a pump to facilitate the circulatory system's function. Its symptoms and manifestations are caused by architectural and/or operational cardiac abnormalities that preventing the heart from bleeding or expelling blood normally with single heartbeat [2]. Heart failure is a symptom brought on by other diseases, not a disease in and of itself.
Ventricular remodeling is the main pathophysiological change of chronic HF, and its formation process is complex. Various mechanisms such as hyperglycemia, oxidative stress, inflammation, apoptosis or fibrosis can lead to ventricular remodeling [3-5]. These pathological changes closely related to T2DM may lead to the occurrence of T2DM-related HF. According to studies, left ventricular hypertrophy, inflammation, increased synthesis of extracellular matrix, poor cardiac metabolism, and myocardial cell death are all signs of ventricular remodeling brought on by T2DM [5].
Oxidative stress and hyperglycemia are traits of T2DM. The imbalance of elevated reactive oxygen species production and/or decreased cellular antioxidant ability, both of which can result in T2DM cardiomyopathy, is the main source of oxidative stress. At the same time, heart damage-related myocardial inflammation can be brought on by oxidative stress [3]. Due to hyperglycemia and oxidative stress, advanced glycation end-products (AGEs) aggregate much more in Type 2 diabetes mellitus. AGEs can alter vascular wall homeostasis through a variety of mechanisms, leading to chronic vascular inflammation that leads to rapidly progressive T2DM-related atherosclerosis, resulting in impaired myocardial blood supply. The increase of AGEs may also change structural proteins, lead to the cross-linking of collagen molecules and weaken the degradation ability of collagen, resulting in increased fibrosis, increased myocardial hardness and decreased cardiac relaxation [4], which increases the risk of heart failure.
The myocardial energy metabolism of T2DM patients is abnormal. Cardiac energy metabolic remodeling is considered to be one of the important mechanisms leading to HF, including changes in cardiac substrate utilization, mitochondrial dysfunction, reduction of high-energy phosphate and blocked energy transport, etc. [6]. On the other hand, T2DM is related to myocardial lipid accumulation. Changes in substrate utilization, such as increased free fatty acids, can lead to lipid accumulation and lipid toxic damage in cardiomyocytes [4].
3. The high risk of developing heart failure for people with type 2 diabetes
In fact, about 33 percent of patients hospitalized for heart failure in the United States also have diabetes [7]. Heart failure may be brought on by complications such as high blood pressure or coronary heart disease, but there are exceptions [7]. According to the research of Mayo Clinic Proceedings, when age, high blood pressure, sex, coronary artery disease and diastolic dysfunction were similar, those who had diabetes experienced heart failure at a rate of 21% compared to those who did not have diabetes at a rate of 11%[8]. Persons with diabetes have a substantially higher risk of developing heart failure than people without diabetes, even when their ejection fraction is normal and there are no known structural abnormalities of the heart [8]. Heart failure can develop for various reasons, including diabetes [8]. People with type 2 diabetes (T2D) frequently experience HF as their first cardiovascular (CV) event [9]. Even those who meet American Diabetes Association (ADA) and the World Health Organization (WHO) standards for pre-diabetes increases the risk of HF by 9 to 58% [10].
The frequency of microalbuminuria, a sign of vascular disorder, is significantly higher in HF patients diabetes without [11] or having it than in the general population, where it ranges from 10% to 20% in those with T2D[12–13]. Microalbuminuria is a well-known, potent, and a distinct indicator of elevated CV risk and overall mortality since it doubles the chance for a CV event in persons with T2D.
The estimated glomerular filtration rate serves as a benchmark for evaluating renal function (eGFR). The progression of HF in patients with both types of diabetes has been shown to be highly frequent and longitudinally predicted by eGFR reduction, and eGFR declines are a more significant indicator of worse outcomes in HF [14].
In various CV outcome trials, patients suffering Dyslipidemia and a low eGFR experienced an approximately 2-fold rise in the HHF rate[15]. Higher eGFR was associated with lower survival rates, according to an observational study[16] looking at persons with T2D and newly developed HF. In T2D participants, 2.2 is frequently estimated as the incidence rate for Vascular complications for every split in the basal eGFR [17].
4. Introduction to type 2 diabetes and treatment of therapy related to HF
By 2035, the number of persons with type 2 diabetes mellitus (T2DM), a worldwide epidemic that is currently predicted to afflict 382 million people [18], will have increased dramatically to over 592 million [19]. An estimated 30.2 million adults, or 12.2%, in the United States alone were projected to have diabetes mellitus in 2015; however, 7.2 million (23.3%) of those individuals were either unaware of their condition or did not disclose it [20]. T1DM and T2DM are both heterogeneous disorders with a wide range of clinical presentations and disease progressions. 90% to 95% of all instances of diabetes mellitus are T2DM. Hyperglycemia, insulin resistance, and a relative impairment in insulin production are the hallmarks of type 2 diabetes mellitus. Patients with type 2 diabetes may experience variable degrees of impaired insulin production and insulin action (insulin resistance) [21]. With rising levels of obesity and age, type 2 diabetes is a frequent illness whose incidence rises significantly [22]. Overweightness affects most T2DM patients in the US (>80%) [23]. Day-long increases in the plasma free fatty acid content in T2DM patients, whether they are lean or very obese, are a common finding [24]. These elevations do not generally subside after a mixed meal or oral glucose load. Type 2 diabetes mellitus (T2DM), which accounts for more than 90% of cases, is significantly more common than type 1 diabetes mellitus (T1DM) or gestational diabetes. Our knowledge of how T2DM develops and progresses has significantly advanced over the last few decades. Its major cause is a slow decline in the ability of pancreatic cells to secrete insulin, which is frequently accompanied with pre-existing insulin sensitivity in motor neurons, the hepatitis, and peripheral tissues [25]. Insulin is a strong lipolysis inhibitor that also prevents the hormone sensitive lipase enzyme from extracting FFA from the adipose tissues. The capacity of insulin to inhibit lipolysis and lower plasma FFA concentrations is significantly diminished in T2DM patients, as evidenced by poor suppression of radioactive palmitate turnover [26].
It is possible that factors other than glycemia may contribute to the increased risk of heart failure in people with diabetes mellitus, and there may be independent mechanisms linking antihyperglycemic medications and left ventricle (LV) remodeling, as evidenced by the findings indicate blood glucose reduction may not be sufficient to stop an increase in HF-related hospitalization and mortality. A complicated underlying and related pathophysiology likely to happen and might even promote towards HF in the setting of diabetic mellitus [27], the changes in anatomical structure and function alone that diabetic cardiomyopathy causes. Some of these pathophysiological factors may be susceptible to pharmaceutical therapy. The image below depicts these paths, which will be discussed later. (Figure 1).
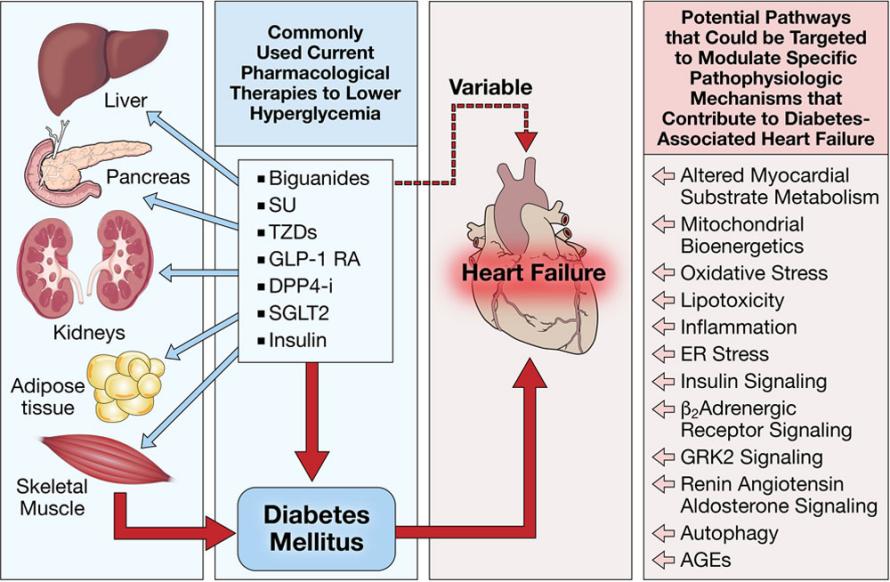
Figure 1. Pathways of complex underlying and interrelated pathophysiology contributing to HF while referring to type 2 diabetes.
Possible therapeutic targets and current antihyperglycemic drugs that potentially manage heart failure brought on by diabetes mellitus. The multi-organ disease state of diabetes mellitus is marked by hyperglycemia and dyslipidemia. Normoglycemia may be achieved with current treatments, although the risks and results of heart failure can vary. Alternative targets that may be treatable with drugs and that raise heart failure risk in individuals with diabetic mellitus are listed [28].
SGLT2 inhibitor is a treatment drug for type 2 diabetes. International clinical study results show that Empagliflozin is also effective in the management of heart failure, so in addition to the indication for diabetes, it also has the indication for heart failure. Reduced the risk of hospitalization for heart failure or cardiovascular death by 25% and postponed the rate of reduction in filamentous filtration (eGFR) by a factor of four, according to the multinational clinical trial published in 2021 [29].
As blood sugar lowering agents, SGLT2 inhibitors work by inhibiting SGLT2 transport into egg white located in proximal renal tubule. Reduce renal tubular reabsorption of filtered glucose and sodium, and then excrete glucose through urine, resulting in natriuresis (sodium treatment). At the same time, afferent arteriole constriction was promoted, and sodium and chloride ions were increased to induce dense spot constriction, and the feedback mechanism of renal tubules was restored and regulated, thus achieving the effect of hypoglycemia. Due to their simultaneous reduction of glomerular blood pressure, proteinuria and renal hyperfiltration rate [30], they may have a role in reducing or improving renal disease in diabetes [31].
Sglt-2 synchronously transport sodium and glucose in the proximal renal tubules, so sGLT-2 inhibitors reduce sodium reabsorption in addition to glucose reabsorption. However, since distal renal tubules can still reabsorb sodium, there is no significant hyponatremia, although urinary sodium excretion is slightly increased. However, after long-term use of SGLT-2 inhibitors, mild weight loss was observed, along with reductions in plasma volume and total sodium levels. Therefore, SGLT-2 inhibitors may improve HF through sodium excretion [32]. Additionally, SGLT-2 inhibitors can lower insulin levels, which increases ketone body production. Ketone bodies are involved in the energy metabolism of the myocardial, and a little increase in ketone bodies helps the heart's energy metabolism function more efficiently [33]. In addition, the hematocrit can rise because of the osmotic diuretic impact of SGLT-2 inhibitors, which boosts the heart's supply of oxygen [34]. Both of these effects help the heart's energy metabolism.
5. Conclusion
In the analysis of clinical data, this paper found that type 2 diabetics are at an extremely serious danger of getting heart failure, and the prevention, control and treatment of diabetes-induced heart failure are also highly valued in clinic. As a concomitant disease highly associated with type 2 diabetes, the treatment of heart failure has attracted more and more attention in the treatment of type 2 diabetes. The possibility of utilizing type 2 diabetes therapy to alleviate heart failure had been verified effective. Drugs used to treat type 2 diabetes are already being used to treat heart failure and have shown remarkable results. Treating diabetes with heart failure-related therapies can also partially curb the likelihood of heart failure risk in diabetics.
Due to the complex pathogenesis of type 2 diabetes, the process that triggers cardiac failure is likewise quite complicated, which increases the difficulty and complexity of the research and treatment of heart failure with the treatment of type 2 diabetes. Type 2 diabetes requires specialized clinical care, which makes treating heart failure associated with it more challenging. Tough requirements for human, material, and financial resources are also present. Because of this, there are still issues to be solved and space for advancement in the therapy of heart failure using the management of type 2 diabetes. This is a crucial component of ongoing study and offers one potential direction. It is essential to manage renal failure with type 2 diabetes, and its success also offers suggestions and workable examples for the management of other diseases.
Acknowledgement
Thanks to the guidance of Dr Andrew J Murray. Without the guidance and support of Dr Andrew J Murray, this paper could not have been completed so smoothly.
References
[1]. Heart Failure(Congestive Heart Failure), 100 Years of Cleveland Clinic, 800.223.2273
[2]. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. (2016). "2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC". Eur Heart J. 37 (27): 2129–2200. doi:10.1093/eurheartj/ehw128. PMID 27206819
[3]. MarwickTH, RitchieR, ShawJE, et al. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy [J]. J Am Coll Cardiol, 2018, 71 (3): 339-351. DOI:10.1016/j.jacc.2017.11.0019
[4]. BuggerH, AbelED. Molecular mechanisms of diabetic cardiomyopathy [J]. Diabetologia, 2014, 57 (4): 660-671. DOI: 10.1007/s00125-014-3171-6.
[5]. BurchfieldJS, XieM, HillJA. Pathological ventricular remodeling: mechanisms: part 1 of 2 [J]. Circulation, 2013, 128 (4): 388-400. DOI: 10.1161/CIRCULATIONAHA.113.001878.
[6]. BerteroE, MaackC. Metabolic remodelling in heart failure [J]. Nat Rev Cardiol, 2018, 15 (8): 457-470. DOI: 10.1038/s41569-018-0044-6.
[7]. MAYO CLINIC.2020.1. https://mandarin.mayoclinic.org/press-room/diabetes-independently-heart-failure-2020-01-08/
[8]. Mayo Clinic Proceedings,Population study: Diabetes is an independent predictor of heart failure development
[9]. Birkeland KI, Bodegard J, Eriksson JW, Norhammar A, Haller H, Linssen GCM, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020;22(9):1607–18.
[10]. Cai X, Liu X, Sun L, He Y, Zheng S, Zhang Y, et al. Prediabetes and the risk of heart failure: A meta-analysis. Diabetes Obes Metab. 2021;23(8):1746–53.
[11]. Damman K, Hillege HL, van Veldhuisen DJ. Albuminuria in heart failure: a CHARMing new risk factor? Lancet. 2009;374(9689):506–8.
[12]. van de Wal RM, Asselbergs FW, Plokker HW, Smilde TD, Lok D, van Veldhuisen DJ, et al. High prevalence of microalbuminuria in chronic heart failure patients. J Card Fail. 2005;11(8):602–6.
[13]. Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol. 2006;17(8):2106–11.
[14]. Mullens W, Damman K, Testani JM, Martens P, Mueller C, Lassus J, et al. Evaluation of kidney function throughout the heart failure trajectory – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22(4):584–603.
[15]. Sacre JW, Magliano DJ, Shaw JE. Heart failure hospitalisation relative to major atherosclerotic events in type 2 diabetes with versus without chronic kidney disease: a meta-analysis of cardiovascular outcomes trials. Diabetes Metab. 2021;47(5):101249.
[16]. Lawson CA, Seidu S, Zaccardi F, McCann G, Kadam UT, Davies MJ, et al. Outcome trends in people with heart failure, type 2 diabetes mellitus and chronic kidney disease in the UK over twenty years. EClin Med. 2021;32:9.
[17]. Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol JASN. 2009;20(8):1813–21.
[18]. Guariguata, L, Whiting, DR, Hambleton, I, Beagley, J, Linnenkamp, U, Shaw, JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002 Crossref. PubMed.
[19]. Zimmet, P, Alberti, KG, Magliano, DJ, Bennett, PH. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol. 2016;12:616–622. doi: 10.1038/nrendo.2016.105 Crossref. PubMed.
[20]. Diabetes - WHO | World Health Organization
[21]. R Paul Robertson, MDMiriam S Udler, MD, PhD. Pathogenesis of type 2 diabetes mellitus. Dec 14, 2021. https://www.uptodate.com/contents/pathogenesis-of-type-2-diabetes-mellitus#H40
[22]. Harris MI. Impaired glucose tolerance in the U.S. population. Diabetes Care 1989; 12:464.
[23]. Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, Marks JS. Diabetes trends in the United States, 1990-1998. Diabetes Care. 2000;23:1278–1283. [PubMed]
[24]. Reaven GM, Hollenbeck C, Jeng C-Y, Wu MS, Chen Y-DI. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 hours in patients with NIDDM. Diabetes. 1988;37:1020–1024. [PubMed]
[25]. DeFronzo, R. A. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58, 773–795 (2009). A classic review of the aetiology of T2DM, with a therapeutic approach based on its pathophysiology.
[26]. Jansson P-A, Larsson A, Smith U, Lonroth P. Lactate release from the subcutaneous tissue in lean and obese men. J Clin Invest. 1994;93:240–246. [PMC free article] [PubMed]
[27]. Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–671. Crossref. PubMed.
[28]. Helena C. Kenny and E. Dale Abel. Heart Failure in Type 2 DiabetesMellitus. Impact of Glucose-Lowering Agents, Heart Failure Therapies, and Novel Therapeutic Strategies. 2019;124:121–141
[29]. Correction to: Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation. 2021-01-26, 143 (4). ISSN 0009-7322. doi:10.1161/cir.0000000000000954.
[30]. DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 2017; 13: 11-26.
[31]. Hao Lizhi, Zhang Yayi, Yang Chunyi et al. American Diabetes Association 2018 Standard Management Recommendations for diabetic nephropathy. Chinese Journal of Internal Medicine 2018; 29:250-61.
[32]. WilcoxCS. Antihypertensive and renal mechanisms of SGLT2 (sodium-glucose linked transporter 2) inhibitors [J]. Hypertension, 2020, 75 (4): 894-901. DOI: 10.1161/HYPERTENSIONAHA.119.11684.
[33]. NewmanJC, VerdinE. β-hydroxybutyrate: much more than a metabolite [J]. Diabetes Res Clin Pract, 2014, 106 (2): 173-181. DOI: 10.1016/j.diabres.2014.08.009.
[34]. MartensP, MathieuC, VerbruggeFH. Promise of SGLT2 inhibitors in heart failure: diabetes and beyond [J]. Curr Treat Options Cardiovasc Med, 2017, 19 (3): 23. DOI: 10.1007/s11936-017-0522-x.
Cite this article
Huang,Q. (2023). Analysis of the possibility of effectiveness that therapeutic techniques for diabetes in the treatment of heart failure. Theoretical and Natural Science,6,185-190.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the International Conference on Modern Medicine and Global Health (ICMMGH 2023)
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Heart Failure(Congestive Heart Failure), 100 Years of Cleveland Clinic, 800.223.2273
[2]. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. (2016). "2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC". Eur Heart J. 37 (27): 2129–2200. doi:10.1093/eurheartj/ehw128. PMID 27206819
[3]. MarwickTH, RitchieR, ShawJE, et al. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy [J]. J Am Coll Cardiol, 2018, 71 (3): 339-351. DOI:10.1016/j.jacc.2017.11.0019
[4]. BuggerH, AbelED. Molecular mechanisms of diabetic cardiomyopathy [J]. Diabetologia, 2014, 57 (4): 660-671. DOI: 10.1007/s00125-014-3171-6.
[5]. BurchfieldJS, XieM, HillJA. Pathological ventricular remodeling: mechanisms: part 1 of 2 [J]. Circulation, 2013, 128 (4): 388-400. DOI: 10.1161/CIRCULATIONAHA.113.001878.
[6]. BerteroE, MaackC. Metabolic remodelling in heart failure [J]. Nat Rev Cardiol, 2018, 15 (8): 457-470. DOI: 10.1038/s41569-018-0044-6.
[7]. MAYO CLINIC.2020.1. https://mandarin.mayoclinic.org/press-room/diabetes-independently-heart-failure-2020-01-08/
[8]. Mayo Clinic Proceedings,Population study: Diabetes is an independent predictor of heart failure development
[9]. Birkeland KI, Bodegard J, Eriksson JW, Norhammar A, Haller H, Linssen GCM, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020;22(9):1607–18.
[10]. Cai X, Liu X, Sun L, He Y, Zheng S, Zhang Y, et al. Prediabetes and the risk of heart failure: A meta-analysis. Diabetes Obes Metab. 2021;23(8):1746–53.
[11]. Damman K, Hillege HL, van Veldhuisen DJ. Albuminuria in heart failure: a CHARMing new risk factor? Lancet. 2009;374(9689):506–8.
[12]. van de Wal RM, Asselbergs FW, Plokker HW, Smilde TD, Lok D, van Veldhuisen DJ, et al. High prevalence of microalbuminuria in chronic heart failure patients. J Card Fail. 2005;11(8):602–6.
[13]. Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol. 2006;17(8):2106–11.
[14]. Mullens W, Damman K, Testani JM, Martens P, Mueller C, Lassus J, et al. Evaluation of kidney function throughout the heart failure trajectory – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22(4):584–603.
[15]. Sacre JW, Magliano DJ, Shaw JE. Heart failure hospitalisation relative to major atherosclerotic events in type 2 diabetes with versus without chronic kidney disease: a meta-analysis of cardiovascular outcomes trials. Diabetes Metab. 2021;47(5):101249.
[16]. Lawson CA, Seidu S, Zaccardi F, McCann G, Kadam UT, Davies MJ, et al. Outcome trends in people with heart failure, type 2 diabetes mellitus and chronic kidney disease in the UK over twenty years. EClin Med. 2021;32:9.
[17]. Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol JASN. 2009;20(8):1813–21.
[18]. Guariguata, L, Whiting, DR, Hambleton, I, Beagley, J, Linnenkamp, U, Shaw, JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002 Crossref. PubMed.
[19]. Zimmet, P, Alberti, KG, Magliano, DJ, Bennett, PH. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol. 2016;12:616–622. doi: 10.1038/nrendo.2016.105 Crossref. PubMed.
[20]. Diabetes - WHO | World Health Organization
[21]. R Paul Robertson, MDMiriam S Udler, MD, PhD. Pathogenesis of type 2 diabetes mellitus. Dec 14, 2021. https://www.uptodate.com/contents/pathogenesis-of-type-2-diabetes-mellitus#H40
[22]. Harris MI. Impaired glucose tolerance in the U.S. population. Diabetes Care 1989; 12:464.
[23]. Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, Marks JS. Diabetes trends in the United States, 1990-1998. Diabetes Care. 2000;23:1278–1283. [PubMed]
[24]. Reaven GM, Hollenbeck C, Jeng C-Y, Wu MS, Chen Y-DI. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 hours in patients with NIDDM. Diabetes. 1988;37:1020–1024. [PubMed]
[25]. DeFronzo, R. A. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58, 773–795 (2009). A classic review of the aetiology of T2DM, with a therapeutic approach based on its pathophysiology.
[26]. Jansson P-A, Larsson A, Smith U, Lonroth P. Lactate release from the subcutaneous tissue in lean and obese men. J Clin Invest. 1994;93:240–246. [PMC free article] [PubMed]
[27]. Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–671. Crossref. PubMed.
[28]. Helena C. Kenny and E. Dale Abel. Heart Failure in Type 2 DiabetesMellitus. Impact of Glucose-Lowering Agents, Heart Failure Therapies, and Novel Therapeutic Strategies. 2019;124:121–141
[29]. Correction to: Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation. 2021-01-26, 143 (4). ISSN 0009-7322. doi:10.1161/cir.0000000000000954.
[30]. DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 2017; 13: 11-26.
[31]. Hao Lizhi, Zhang Yayi, Yang Chunyi et al. American Diabetes Association 2018 Standard Management Recommendations for diabetic nephropathy. Chinese Journal of Internal Medicine 2018; 29:250-61.
[32]. WilcoxCS. Antihypertensive and renal mechanisms of SGLT2 (sodium-glucose linked transporter 2) inhibitors [J]. Hypertension, 2020, 75 (4): 894-901. DOI: 10.1161/HYPERTENSIONAHA.119.11684.
[33]. NewmanJC, VerdinE. β-hydroxybutyrate: much more than a metabolite [J]. Diabetes Res Clin Pract, 2014, 106 (2): 173-181. DOI: 10.1016/j.diabres.2014.08.009.
[34]. MartensP, MathieuC, VerbruggeFH. Promise of SGLT2 inhibitors in heart failure: diabetes and beyond [J]. Curr Treat Options Cardiovasc Med, 2017, 19 (3): 23. DOI: 10.1007/s11936-017-0522-x.









