1. Introduction
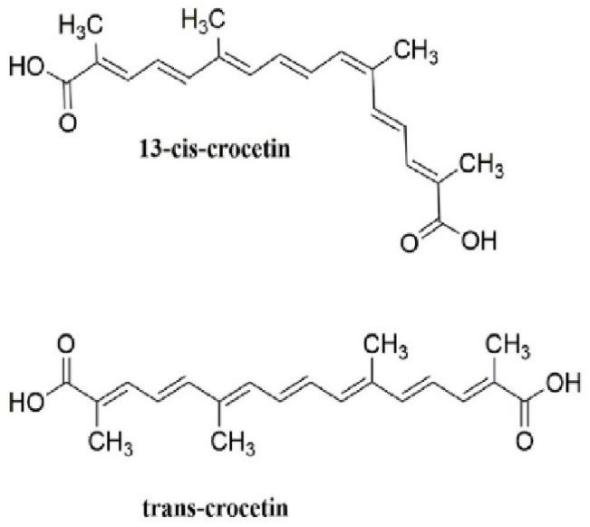
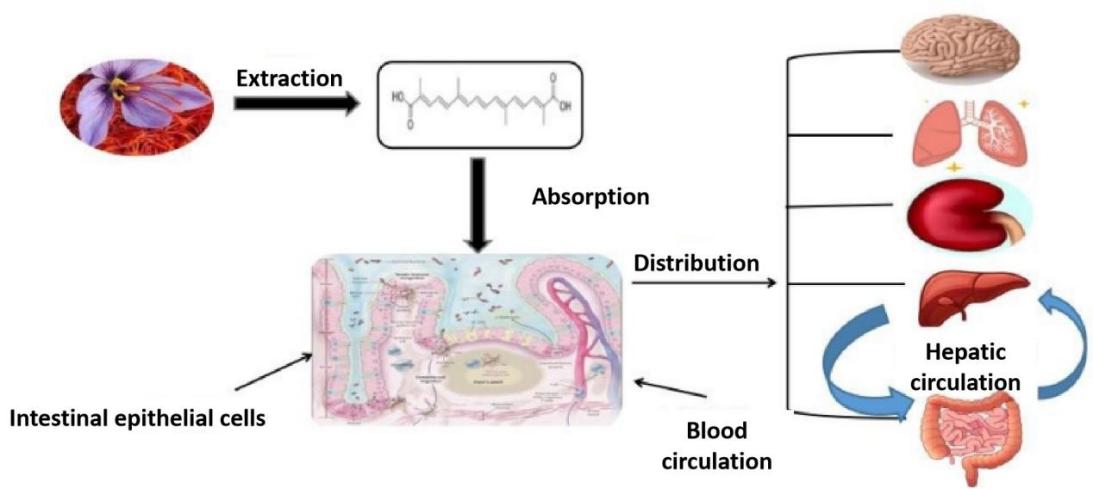
Crocus Sativus L. is a bulbous herb of the genus Crocus in the Iridaceae family. It is native to Mediterranean countries such as Iran, Greece, Spain, Turkey, and is now grown in China, Japan, India, and other countries [1]. Crocetin is one of the main components of the precious medicinal herb saffron and is a low molecular-weight carotenoid compound. It is produced in biological systems through the hydrolysis of crocetin glycoside as a bioactive metabolite [2]. Crocetin has a polyunsaturated conjugated acid structure, four side-chain methyl groups and seven conjugated double bonds, so it has both cis- and trans- structures as shown in Fig.1 [3]. Due to the presence of a long chain of conjugated carbon-carbon double bonds, it is unstable to heat, light and pH [4]. Crocetin is soluble only in pyridine and dimethyl sulfoxide, and has poor solubility in water and most organic solvents [5]. It is a non-toxic, non-mutagenic natural substance that has been widely used as a food additive since ancient times. Crocetin has been reported to have a wide range of pharmacological effects. It has protective effects on the heart, liver and nerves, and also has antioxidant, antidepressant, antiviral, anti-cancer, atherosclerotic, anti-diabetic and memory-enhancing properties. Its pharmacokinetics is shown in Fig. 2. In addition, many animal model studies have confirmed that crocetin, due to its potential antioxidant activity [6], can inhibit the production of ROS and inflammatory cascade reactions to slow down heart damage caused by bleeding or resus citation [7].
In recent years, a large number of scholars have studied the use of new technologies to incorporate crocetin and its derivatives into liposomes, microcapsules, Multiple emulsion, nanoparticles, etc, to improve the solubility, stability, bioavailability and activity of crocetin.
2. Microcapsule
Microcapsule is a method of enhancing drug stability by encapsulating a drug using a polymer material consisting of a core and inert polymers. Due to the long conjugated structure of crocetin, the unsaturated molecular structure can adversely affect stability during handling and storage. Zhou et al. [8] prepared crocetin microcapsules by spray drying using cyclodextrin, gum arabic and maltodextrin as wall materials. The results showed that the half-life of the microcapsules was significantly prolonged and the stability was significantly improved at 35℃ and low oxygen conditions.
Nanosponges are fine reticular structures, and due to their porous structure and small size, hydrophilic and lipophilic drugs can be easily loaded into nanosponges, thereby enhancing bioavailability and solubility. A large number of researchers have prepared nanosponges from cyclodextrins, which show hydrophobic cavities and hydrophilic surfaces in the structure and have strong complexing ability, thereby greatly improving solubility, stability and bioavailability. Cyclodextrins can also maintain the stability of emulsions and increase the foaming property of solutions. In addition, it protects certain food components that are sensitive to oxygen, heat, and light degradation. Ka et al. [9] studied a water-soluble inclusion complex composed of crocetin and γ-cyclodextrin. The results showed that the inclusion rate of the crocetin-γ-cyclodextrin inclusion complex was over 95%, the bioavailability of the inclusion complex was stronger, and it could promote the crossing of crocetin through the blood-brain barrier into the brain, providing some information for drug research in the treatment of Alzheimer's disease. Trotta et al. [10] prepared nanosponges using carbamate crosslinkers and cyclodextrin as the raw material. The study showed a significant improvement in the stability of the nanosponges. In addition, nanosponges can also be used to remove contaminants, odors, and compounds that produce unpleasant smells.
3. Multiple emulsion
Multiple emulsion is complex emulsion systems formed by further emulsification of common emulsions, which can be classified into water-in-oil systems and oil-in-water systems based on the properties of the continuous phase and the dispersed phase. Structurally, it presents a unique structure of "two films and three phases" with multiple compartments, so it can dissolve drugs of different properties separately in different phases, and it can also play roles such as isolation protection and controlled release. Ahmad et al. [11] prepared the Tibetan red adhesive by titration with a nanoemulsion particle size of 89.64 nm. Intranasal injection significantly improved neurobehavior, antioxidant activity and histopathology in rats with cerebral ischemia, and could be used as a drug delivery system to treat cerebral ischemia. Esfanjani et al. [12] used spray drying to prepare W/O/W complex emulsions with whey protein concentrate/pectin as stabilizer, with saffron encapsulation rate of 62.55%. Faridi et al. [13] prepared W/O type microemulsions using crocetin extract, sunflower oil and Spain 80 as raw materials, with an encapsulation rate of 96.66%. Mehrnia et al. [14] optimized the traditional multi- emulsion preparation process to produce small particle size crocetin acid side W/O type nanoemulsions using polyglycerol polyricinoleate and nonionic surfactant Pan 80. The same method was then used to prepare W/O nanoemulsions, which were dispersed in three different polymers: Angum gum (AG), WPC, and gum Arabic (GA). In vitro release experiments in the intestinal environment showed that WPC was the fastest and AG was the slowest in terms of crocetin release rate, indicating that AG has a sustained- release effect. In addition, Yang [15] prepared crocetin nanoformulations using the double emulsion evaporation technique and found that it could alleviate diabetic nephropathy through antifibrotic and anti-inflammatory effects.
4. Nanoparticles
By improving pharmacokinetics and addressing the shortcomings of native drugs, nanoparticle-based drug delivery systems offer a promising strategy for enhancing drug delivery efficiency. Nanoparticles are solid colloidal particles with a particle size of 10 to 100 nm, made from natural or synthetic polymer materials.
4.1. Solid lipid nanoparticles
Liposomes are novel formulations that use lipids as carriers to adsorb drugs onto the surface of nanoparticles. The lipids in liposomes have a natural affinity structure, which can bind to some poorly soluble drugs and significantly increase the solubility of poorly soluble drugs. Puglia [16] prepared saffronic acid-solid lipid nanoparticles using the quasi-emulsified solvent diffusion method, and the results showed that the nanodispersant had good homogeneity and stability, with encapsulation rates ranging from 80 to 94%. Mertes [17] invented a novel type of liposome nanoparticles coated with crocetin, which was found to have reoxygenation activity and enhanced oxygenation in vascular tissue, with potential clinical benefits in the treatment of COVID-19 patients. Khameneh [18] used high-pressure homogenization combined with high-shear homogenization and ultrasound to prepare lipid nanoparticles using Tween 80, monostearic acid glyceride and different amounts of crocetin as raw materials, and evaluated their sun protection and moisturizing properties. The findings suggest that the formulation is a promising carrier, which can be applied externally to saffron as a UV blocker. Mousavi et al. [19] used the dehydration rehydration method to encapsulate crocetin in nano-liposomes and prepared three formulations with particle sizes ranging from 150 to 200 nm and encapsulation rates from 4.5 to 7.5%. The results showed that liposomes containing crocetin had better cytotoxicity. Rastgoo et al. [20] prepared four different ratios of nano-liposomes. Then colorimetry was used to determine the IC50 values of saffron glycosides alone and in all four formulations. Based on the results of in vitro experiments, the best prescriptions were screened out for in vivo experiments, and the anti-tumor activity of crocetin encapsulated in nano-liposomes against colon cancer cells was evaluated. The results showed that the IC50 value of crocetin encapsulated in nano-liposomes in colon cancer cells was higher than that of crocetin alone. Li et al. [21] investigated the effects of preparing nano liposomes using three different purities of soy lecithin on food application performance. The results showed that both nano- liposome encapsulation and chitosan coating could enhance the antioxidant and anti- inflammatory properties of crocetin and increase its stability. Compared with high- purity soy lecithin, 70% purity soy lecithin offers better value for money and is more valuable in food applications.
4.2. Lactate-glycolic acid copolymer nanoparticles
Polylactic acid-co-hydroxyacetic acid (PLGA) is a biodegradable and biocompatible polyester approved by the Food and Drug Administration. It is often used as a carrier material for nanospheres in sustained-release drug research. Langroodi et al. [22] prepared crocetin PLGA nanoparticles by the W/O/W reemulsion method. The nanoparticles were spherical and evenly distributed, and the crocetin PLGA nanoparticles had sustained-release effects. crocetin PLGA nanoparticles have a stronger inhibitory effect. Hafezi et al. [23] prepared crocetin nanocapolymers using crocetin and polylactic acyl-glycolic acid polymers, and used single emulsion/solvent evaporation to prepare PLGA nanoparticles with encapsulation efficiency reaching 97%. The results showed that PLGA nanoparticles containing crocetin had higher cytotoxicity inhibition and were more conducive to inhibiting breast cancer cells. Navid et al. [24] prepared nanoparticle PLGA-Crt NPs and showed that crocetin nanoparticles could overcome drug resistance in human ovarian cancer and enhance its anti-cancer effect by blocking MRP2 transporter gene expression and effervescent function.
4.3. Silver nanoparticles
In some cases, the free release of silver ions in silver nanoparticles can induce cell death in mammalian or microbial cells, so silver nanoparticles are used as common antibacterial agents. However, dangerous materials such as hydrazine and polyvinylpyrrolidone are used in the synthesis of AgNPs, and the chemical process can have adverse effects. The plant extract approach, which does not have adverse effects and is more cost-effective, is widely used. Solgi et al. [25] prepared AgNPs using saffron petal extract as a reducing agent and 5 mm AgNO3 as raw material. The study found that silver nanoparticles showed good antibacterial activity against both Gram-positive and Gram-negative bacteria, with higher activity in the genus Gram-positive. Thamer et al. [26] synthesized and optimized silver nanoparticles using the aqueous extract of saffron. In addition, Anahita et al. [27] evaluated the antibacterial activity of saffron extracts and silver nanoparticles. AgNPs, saffron extract and their various minimum bactericidal and inhibitory concentrations against methicillin-resistant Staphylococcus aureus were determined. Taghva and Entezari [28] prepared silver nanoparticles using saffron bulb extracts. The color of the nanoparticles was dark brown, associated with surface plasmon resonance bands. Studies have shown that nanoparticles can kill both Gram- positive and Gram-negative bacteria. Bagherzade [29] et al. prepared AgNPs with significant antibacterial activity against Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacillus subtilis and Shigella pneumoniae using saffron waste liquid and its aqueous extract.
4.4. Nano-gold particles
Gold nanoparticles, with particle sizes ranging from 1 to 100 nm, are easily functionalized, have good biocompatibility, are stable and non-toxic. These properties make AuNPs suitable for drug delivery, biosensing, molecular imaging, etc. Vijayakumar et al. [30] prepared crocetin side gold nanoparticles using tetrachloroauric acid trihydrate and crocetin extract as raw materials. The gold nanoparticles have a stable triangular crystal structure, spherical particles with a particle size of (15±5) nm, in addition, Hoshyar et al. [31] investigated the novel green method of one-step synthesis of gold nanoparticles using crocetin and their anti-cancer activity. The study showed that gold nanoparticles modified with herbal extracts could be used as effective drugs to target tumor cells.
4.5. Magnetic nanoparticles
Magnetic nanoparticles are magnetic nanomaterials with a particle size less than 100 nm. It has a large surface area to volume ratio, can carry a large amount of anti- cancer drugs, and has good biocompatibility, biodegradability, superparamagnetism and other properties, attracting much attention. Studies have found that saffron can inhibit the growth of liver cancer in rats, and these natural polymers can be covered with nanoparticles on the surface to enhance the effect of magnetic nanoparticles, thereby increasing the anti-cancer effect. El-Kharrag et al. [32] constructed a precancerous lesion treatment model using magnetic nanoparticles coated with crocetin, using an improved co-precipitation method, and coated MNPs with glucan and crosslinking agent. The study showed that magnetic nanoparticles coated with crocetin could enhance the anti-cancer effect of crocetin.
4.6. Selenium nanoparticles
Selenium nanoparticles increase enzyme activity and protect DNA from oxidative damage in vitro by scavenging various free radicals. Abdullaev et al. [33] synthesized PEG-SeNPs, and in vitro studies showed that the binding of PEG-SeNPs to crocetin was more significantly enhanced in the acidic conditions of the tumor microenvironment.
4.7. CuO nanoparticles
Stepniak et al. [34] prepared CuO nanoparticles with a particle size of 31 nm. The results showed that the LC50 of CuO NPs in HT22 cells was 25.9 g/ml after 24 hours of treatment, and the effect of CuO NPs on cell death was concentration dependent and time-dependent. In addition, these nanoparticles reduced the activity of some antioxidant or detoxifying enzymes.
5. Solid dispersions
Zhong [35] prepared a solid dispersion of crocetin using a solvent method with crocetin at a dose ratio of 1:4 to PVPK30. Solid dispersion sustained-release tablets of crocetin were prepared with HPMCK4M and HPMC-K15M(6:4) as the skeleton sustained-release materials and MCC as the filler. The pharmacokinetics of crocetin solid dispersion sustained-release tablets in beagle dogs were investigated. The results showed that the drug release rate of the prepared solid dispersions was enhanced, and the drug content of the prepared sustained-release tablets was 98.02%. The average bioavailability of the sustained-release tablets relative to the active pharmaceutical ingredient and the solid dispersion was 255.07 and 217.10 percent, respectively.
6. Crocetin injection
Zhang et al. [36] patented a process for producing crocetin injections. Raw crocetin was dissolved by mixing it with auxiliary materials such as propylene glycol. Subsequently, water for injection and activated carbon were added. A qualified crocetin injection was thus prepared. Following filtration, filling, lamp inspection, and packaging, the final product was obtained. The crocetin injection demonstrated good stability, simple preparation, and low cost. Formulating crocetin as an injection can improve its bioavailability (Zhang et al., 2011). Additionally, a modification involves preparing crocetin salt injections. These injections are obtained by dissolving crocetin salt and sodium chloride in water, which enhances crocetin's bioavailability and therapeutic effects [37].
7. Conclusions and prospect
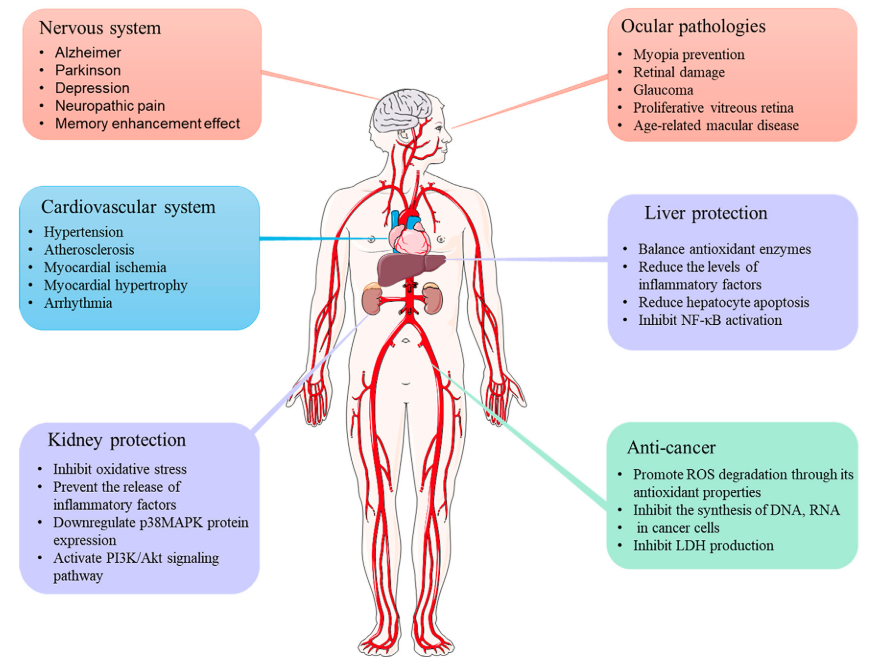
Due to the extensive use of crocetin in recent years as shown in Fig. 3, the medicinal resources of saffron are extremely limited, making it very expensive and earning it the title of "plant gold". Nanomedicines have the characteristics of small particle size, large specific surface area, high surface reactivity, many active centers, and strong adsorption capacity. A large number of studies have shown that incorporating crocetin, crocetin, etc. from saffron into various nanostructured components for application, such as microcapsules, Multiple emulsion, nanoparticles, etc., with the help of nanotechnology strategies, can improve the physicochemical properties of the drugs and significantly increase the solubility and bioavailability of crocetin, as shown in Fig. 2. Among them, silver nanoparticles, gold nanoparticles, selenium nanoparticles, supported nanoparticles, chitosan/sodium alginate nanoparticles, etc., showed significant antibacterial and anti-cancer and anti-tumor effects. Nanotechnology is currently making major breakthroughs and has achieved a series of results. Nanotechnology combined with medicine, because nanomaterials themselves are non-toxic and can be used as drug carriers to improve drug absorption and utilization, achieve efficient targeted drug delivery, extend drug consumption half-life, and reduce harmful side effects on normal tissues, It has promoted the improvement of basic medical technology, the innovation of clinical diagnostic techniques and the improvement of treatment levels. These research results suggest that crocetin has good development and application prospects and is worthy of in-depth study.
References
[1]. Schmidt Mathias BG, Hensel Andreas. Saffron in phytotherapy: pharmacology and clinical uses. Wiener medizinische Wochenschrift. 2007; 27: 5.
[2]. Cnr A, Sbbb C, Ravb C, Ssb A. Chemical analysis of saffron by HPLC based crocetin estimation - ScienceDirect. Journal of Pharmaceutical and Biomedical Analysis.181.
[3]. Peng FC. Protective effects of crocetin on anoxic injury in mice. Chinese Journal of New Drugs. 2007.
[4]. Guo Zi Liang LMX, Li Xiao Lin, . Crocetin: A Systematic Review. Frontiers in Pharmacology. 2022; 14: 13-.
[5]. Eidenberger T. Hydrolysate of crocetin. 2010.
[6]. Tseng TH, Chu CY, Huang JM, Shiow SJ, Wang CJ. Crocetin protects against oxidative damage in rat primary hepatocytes. Cancer Letters. 1995; 97: 61-7.
[7]. Yan J, Qian Z, Sheng L, Zhao B, Yang L, Ji H, et al. Effect of crocetin on blood pressure restoration and synthesis of inflammatory mediators in heart after hemorrhagic shock in anesthetized rats. Shock. 2010; 33: 83-7.
[8]. Zhou H YX, Zhao Q, . Determination of oxygen transmission barrier of microcapsule wall by crocetin deterioration kinetics. Eur Food Res Technol. 2013; 237: 639-46.
[9]. Wong KH, Xie Y, Huang X, Kadota K, Yang Z. Delivering Crocetin across the Blood-Brain Barrier by Using γ-Cyclodextrin to Treat Alzheimer's Disease. Scientific Reports. 2020; 10.
[10]. Trotta F, Cavalli R, Tumiatti W, Zerbinati O, Roggero C, Vallero R. Ultrasound-Assisted Synthesis of Cyclodextrin-Based Nanosponges. 2008.
[11]. Ahmad N, Ahmad R, Naqvi AA, Ashafaq M, Alam A, Ahmad FJ, et al. The effect of safranal loaded mucoadhesive nanoemulsion on oxidative stress markers in cerebral ischemia. Artif Cells Nanomed Biotechnol. 2017.
[12]. Esfanjani AF, Jafari SM, Assadpoor E, Mohammadi A. Nano-encapsulation of saffron extract through double-layered multiple emulsions of pectin and whey protein concentrate. Journal of Food Engineering. 2015; 165: 149-55.
[13]. Esfanjani AF, Jafari SM, Assadpour E. Preparation of a multiple emulsion based on pectin-whey protein complex for encapsulation of saffron extract nanodroplets. Food Chemistry. 2017; 221: 1962-9.
[14]. Mehrnia MA, Jafari SM, Makhmal-Zadeh BS, Maghsoudlou Y. crocetin loaded nano-emulsions: Factors affecting emulsion properties in spontaneous emulsification. International Journal of Biological Macromolecules. 2016; 84: 261-7.
[15]. dong YX. Design and optimization of crocetin loaded PLGA nanoparticles against diabetic nephropathy via suppression of inflammatory biomarkers: a formulation approach to preclinical study. Drug delivery. 2019; 26: 849-59.
[16]. Nanotechnological Approach to Increase the Antioxidant and Cytotoxic Efficacy of crocetin and Crocetin. Planta Medica. 2019; 85: 258-65.
[17]. Mertes PM, Collange O, Coliat P, Banerjee M, Pivot X. Liposomal encapsulation of trans-crocetin enhances oxygenation in patients with COVID-19-related ARDS receiving mechanical ventilation. Journal of Controlled Release. 2021; 336.
[18]. Khameneh B, Halimi V, Jaafari MR, Golmohammadzadeh S. Safranal-loaded solid lipid nanoparticles: evaluation of sunscreen and moisturizing potential for topical applications. Iranian Journal of Basic Medical Sciences. 2015; 18: 58-63.
[19]. Mousavi SH, Moallem SA, Mehri S, Shahsavand S, Nassirli H, Malaekeh-Nikouei B. Improvement of cytotoxic and apoptogenic properties of crocetin in cancer cell lines by its nanoliposomal form. Pharmaceutical Biology. 2011; 49: 1039-45.
[20]. Rastgoo M, Hosseinzadeh H, Alavizadeh H, Abbasi A, Ayati Z, Jaafari M. Antitumor activity of PEGylated nanoliposomes containing crocetin in mice bearing C26 colon carcinoma. Planta Medica. 2013; 79: 447.
[21]. Li Jinglei NJ, Wu Haishan, Park Hyun Jin, Zhao Qingsheng, Yang Liu. Middle purity soy lecithin is appropriate for food grade nanoliposome: Preparation, characterization, antioxidant and anti-inflammatory ability. Food chemistry. 2022; 389.
[22]. Langroodi FA, Ghahestani ZH, Alibolandi M, Ebrahimian M, Hashemi M. Evaluation of the effect of crocetin on antitumor activity of doxorubicin encapsulated in PLGA nanoparticles. Nanomedicine. 2016; 3: 23-34.
[23]. Hafezi Ghahestani Zohreh ALF, Mokhtarzadeh Ahad, Ramezani Mohammad, et al. Evaluation of anti-cancer activity of PLGA nanoparticles containing crocetin Artificial cells, nanomedicine, and biotechnology. 2017; 45: 955-60.
[24]. Navid, Neyshaburinezhad, Fatemeh, Kalalinia, Maryam, Hashemi. Encapsulation of crocetin into poly (lactic-co-glycolic acid) nanoparticles overcomes drug resistance in human ovarian cisplatin-resistant carcinoma cell line (A2780-RCIS). Molecular biology reports.46: 6525-32.
[25]. Solgi, Mousa. Evaluation of plant-mediated Silver nanoparticles synthesis and its application in postharvest Physiology of cut Flowers. Physiology & Molecular Biology of Plants. 2014; 20: 279-85.
[26]. Thamer NA AL. reen synthesis optimization and characterization of silver nanoparticles using aqueous extract of Crocus sativus L. Int J Pharm Bio Sci 2014; 5: 759-70.
[27]. Anahita SZ HRA, Seyyed Mahdi R, . Evaluation of reciprocal pharmaceutical effects and antibacterial activity of silver nanoparticles and metha-nolic extract of Crocus sativus L. Int J Enteric Pathog. 2017; 5: 18-23.
[28]. Arefeh T, Maliheh E. BIOSYNTHESIS AND CHARACTERIZATION OF SILVER NANOPARTICLES USING AQUEOUS EXTRACT OF SAFFRON CORM AND EVALUATION OF THEIR ANTIBACTERIAL AND MUTAGENESIS ACTIVITY. 2017.
[29]. Ghodsieh, Bagherzade, Maryam, Manzari, Tavakoli, Mohmmad, et al. Green synthesis of silver nanoparticles using aqueous extract of saffron(Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asia-pacific Journal of Tropical Biomedical Sciences: English Edition. 2017: 7.
[30]. Vijayakumar R, Devi V, Adavallan K, Saranya D. Green synthesis and characterization of gold nanoparticles using extract of anti-tumor potent Crocus sativus. Physica E Low-dimensional Systems and Nanostructures. 2011; 44.
[31]. Hoshyar R, Khayati GR, Poorgholami M, Kaykhaii M. A novel green one-step synthesis of gold nanoparticles using crocetin and their anti-cancer activities. J Photochem Photobiol B. 2016: 237-42.
[32]. El-Kharrag, Rkia, Amin, Hisaindee, Soleiman, Greish, et al. Development of a therapeutic model of precancerous liver using crocetin-coated magnetite nanoparticles.
[33]. Mary, T., Shanthi, K., Vimala, Soundarapandian. PEG functionalized selenium nanoparticles as a carrier of crocetin to achieve anticancer synergism. RSC ADVANCES. 2016: 22936-49.
[34]. Inkielewicz-Stepniak, Iwona, Radomski, Witold M, Santos-Martinez, Jose M, et al. CuO nanoparticles induce apoptosis by impairing the antioxidant defense and detoxification systems in the mouse hippocampal HT22 cell line: Protective effect of crocetin. Toxicology in vitro: an international journal published in association with BIBRA. 2015.
[35]. Zhong Hui. Research on the Solid Dispersion Sustained-release Tablets of saffron acid. Jiangsu University. 2014.
[36]. Zhang, X. T., Ma, S. W., Wang, L., He, S. J., and Yu, J. Y. 2011. The Preparation Method of Crocetin Injection.
[37]. Wang, H. D., and Li, l. (2015). Crocetin Salt Injection and Preparation Process Thereof.
Cite this article
Li,Y. (2025). Research Progress on Crocetin Solubilization Technology. Theoretical and Natural Science,138,35-42.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: Computational Modelling and Simulation for Biology and Medicine
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Schmidt Mathias BG, Hensel Andreas. Saffron in phytotherapy: pharmacology and clinical uses. Wiener medizinische Wochenschrift. 2007; 27: 5.
[2]. Cnr A, Sbbb C, Ravb C, Ssb A. Chemical analysis of saffron by HPLC based crocetin estimation - ScienceDirect. Journal of Pharmaceutical and Biomedical Analysis.181.
[3]. Peng FC. Protective effects of crocetin on anoxic injury in mice. Chinese Journal of New Drugs. 2007.
[4]. Guo Zi Liang LMX, Li Xiao Lin, . Crocetin: A Systematic Review. Frontiers in Pharmacology. 2022; 14: 13-.
[5]. Eidenberger T. Hydrolysate of crocetin. 2010.
[6]. Tseng TH, Chu CY, Huang JM, Shiow SJ, Wang CJ. Crocetin protects against oxidative damage in rat primary hepatocytes. Cancer Letters. 1995; 97: 61-7.
[7]. Yan J, Qian Z, Sheng L, Zhao B, Yang L, Ji H, et al. Effect of crocetin on blood pressure restoration and synthesis of inflammatory mediators in heart after hemorrhagic shock in anesthetized rats. Shock. 2010; 33: 83-7.
[8]. Zhou H YX, Zhao Q, . Determination of oxygen transmission barrier of microcapsule wall by crocetin deterioration kinetics. Eur Food Res Technol. 2013; 237: 639-46.
[9]. Wong KH, Xie Y, Huang X, Kadota K, Yang Z. Delivering Crocetin across the Blood-Brain Barrier by Using γ-Cyclodextrin to Treat Alzheimer's Disease. Scientific Reports. 2020; 10.
[10]. Trotta F, Cavalli R, Tumiatti W, Zerbinati O, Roggero C, Vallero R. Ultrasound-Assisted Synthesis of Cyclodextrin-Based Nanosponges. 2008.
[11]. Ahmad N, Ahmad R, Naqvi AA, Ashafaq M, Alam A, Ahmad FJ, et al. The effect of safranal loaded mucoadhesive nanoemulsion on oxidative stress markers in cerebral ischemia. Artif Cells Nanomed Biotechnol. 2017.
[12]. Esfanjani AF, Jafari SM, Assadpoor E, Mohammadi A. Nano-encapsulation of saffron extract through double-layered multiple emulsions of pectin and whey protein concentrate. Journal of Food Engineering. 2015; 165: 149-55.
[13]. Esfanjani AF, Jafari SM, Assadpour E. Preparation of a multiple emulsion based on pectin-whey protein complex for encapsulation of saffron extract nanodroplets. Food Chemistry. 2017; 221: 1962-9.
[14]. Mehrnia MA, Jafari SM, Makhmal-Zadeh BS, Maghsoudlou Y. crocetin loaded nano-emulsions: Factors affecting emulsion properties in spontaneous emulsification. International Journal of Biological Macromolecules. 2016; 84: 261-7.
[15]. dong YX. Design and optimization of crocetin loaded PLGA nanoparticles against diabetic nephropathy via suppression of inflammatory biomarkers: a formulation approach to preclinical study. Drug delivery. 2019; 26: 849-59.
[16]. Nanotechnological Approach to Increase the Antioxidant and Cytotoxic Efficacy of crocetin and Crocetin. Planta Medica. 2019; 85: 258-65.
[17]. Mertes PM, Collange O, Coliat P, Banerjee M, Pivot X. Liposomal encapsulation of trans-crocetin enhances oxygenation in patients with COVID-19-related ARDS receiving mechanical ventilation. Journal of Controlled Release. 2021; 336.
[18]. Khameneh B, Halimi V, Jaafari MR, Golmohammadzadeh S. Safranal-loaded solid lipid nanoparticles: evaluation of sunscreen and moisturizing potential for topical applications. Iranian Journal of Basic Medical Sciences. 2015; 18: 58-63.
[19]. Mousavi SH, Moallem SA, Mehri S, Shahsavand S, Nassirli H, Malaekeh-Nikouei B. Improvement of cytotoxic and apoptogenic properties of crocetin in cancer cell lines by its nanoliposomal form. Pharmaceutical Biology. 2011; 49: 1039-45.
[20]. Rastgoo M, Hosseinzadeh H, Alavizadeh H, Abbasi A, Ayati Z, Jaafari M. Antitumor activity of PEGylated nanoliposomes containing crocetin in mice bearing C26 colon carcinoma. Planta Medica. 2013; 79: 447.
[21]. Li Jinglei NJ, Wu Haishan, Park Hyun Jin, Zhao Qingsheng, Yang Liu. Middle purity soy lecithin is appropriate for food grade nanoliposome: Preparation, characterization, antioxidant and anti-inflammatory ability. Food chemistry. 2022; 389.
[22]. Langroodi FA, Ghahestani ZH, Alibolandi M, Ebrahimian M, Hashemi M. Evaluation of the effect of crocetin on antitumor activity of doxorubicin encapsulated in PLGA nanoparticles. Nanomedicine. 2016; 3: 23-34.
[23]. Hafezi Ghahestani Zohreh ALF, Mokhtarzadeh Ahad, Ramezani Mohammad, et al. Evaluation of anti-cancer activity of PLGA nanoparticles containing crocetin Artificial cells, nanomedicine, and biotechnology. 2017; 45: 955-60.
[24]. Navid, Neyshaburinezhad, Fatemeh, Kalalinia, Maryam, Hashemi. Encapsulation of crocetin into poly (lactic-co-glycolic acid) nanoparticles overcomes drug resistance in human ovarian cisplatin-resistant carcinoma cell line (A2780-RCIS). Molecular biology reports.46: 6525-32.
[25]. Solgi, Mousa. Evaluation of plant-mediated Silver nanoparticles synthesis and its application in postharvest Physiology of cut Flowers. Physiology & Molecular Biology of Plants. 2014; 20: 279-85.
[26]. Thamer NA AL. reen synthesis optimization and characterization of silver nanoparticles using aqueous extract of Crocus sativus L. Int J Pharm Bio Sci 2014; 5: 759-70.
[27]. Anahita SZ HRA, Seyyed Mahdi R, . Evaluation of reciprocal pharmaceutical effects and antibacterial activity of silver nanoparticles and metha-nolic extract of Crocus sativus L. Int J Enteric Pathog. 2017; 5: 18-23.
[28]. Arefeh T, Maliheh E. BIOSYNTHESIS AND CHARACTERIZATION OF SILVER NANOPARTICLES USING AQUEOUS EXTRACT OF SAFFRON CORM AND EVALUATION OF THEIR ANTIBACTERIAL AND MUTAGENESIS ACTIVITY. 2017.
[29]. Ghodsieh, Bagherzade, Maryam, Manzari, Tavakoli, Mohmmad, et al. Green synthesis of silver nanoparticles using aqueous extract of saffron(Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asia-pacific Journal of Tropical Biomedical Sciences: English Edition. 2017: 7.
[30]. Vijayakumar R, Devi V, Adavallan K, Saranya D. Green synthesis and characterization of gold nanoparticles using extract of anti-tumor potent Crocus sativus. Physica E Low-dimensional Systems and Nanostructures. 2011; 44.
[31]. Hoshyar R, Khayati GR, Poorgholami M, Kaykhaii M. A novel green one-step synthesis of gold nanoparticles using crocetin and their anti-cancer activities. J Photochem Photobiol B. 2016: 237-42.
[32]. El-Kharrag, Rkia, Amin, Hisaindee, Soleiman, Greish, et al. Development of a therapeutic model of precancerous liver using crocetin-coated magnetite nanoparticles.
[33]. Mary, T., Shanthi, K., Vimala, Soundarapandian. PEG functionalized selenium nanoparticles as a carrier of crocetin to achieve anticancer synergism. RSC ADVANCES. 2016: 22936-49.
[34]. Inkielewicz-Stepniak, Iwona, Radomski, Witold M, Santos-Martinez, Jose M, et al. CuO nanoparticles induce apoptosis by impairing the antioxidant defense and detoxification systems in the mouse hippocampal HT22 cell line: Protective effect of crocetin. Toxicology in vitro: an international journal published in association with BIBRA. 2015.
[35]. Zhong Hui. Research on the Solid Dispersion Sustained-release Tablets of saffron acid. Jiangsu University. 2014.
[36]. Zhang, X. T., Ma, S. W., Wang, L., He, S. J., and Yu, J. Y. 2011. The Preparation Method of Crocetin Injection.
[37]. Wang, H. D., and Li, l. (2015). Crocetin Salt Injection and Preparation Process Thereof.









