1. Introduction
Diabetes mellitus, a chronic metabolic disorder characterized by insufficient insulin production or impaired insulin utilization, is associated with high mortality rates and serious complications, including diabetic foot ulcers (DFUs). Epidemiological data show that approximately 366 million individuals were affected globally in 2013, with diabetes directly causing 1.5 million deaths in 2019 [1]. Among its complications, patients frequently develop chronic wounds such as DFUs and leg ulcers, which exhibit persistently impaired healing. These wounds often lead to hospitalization and are heterogeneous in nature, meaning their treatment and prognosis depend heavily on the use of targeted therapeutic strategies [2]. Current treatment options remain largely inadequate and pose a significant financial burden to patients, with DFUs being the leading cause of lower-limb amputations. Notably, diabetic wounds account for 50–70% of all limb amputations, equating to one diabetes-related leg amputation every 30 seconds globally [3, 4]. As a result, there is an urgent need to develop novel therapeutic approaches for diabetic wound healing.
Recent advancements in nanotechnology have revolutionized the design and development of drug delivery systems for diabetic wounds. Nanoscale drug delivery systems (NDDSs), which incorporate functional nanocarriers, offer multifaceted therapeutic benefits, including anti-inflammatory effects, reactive oxygen species (ROS) scavenging, local blood glucose regulation, and clearance of senescent cells. These features have stimulated significant interest in their clinical application for managing diabetic wounds [5-7].
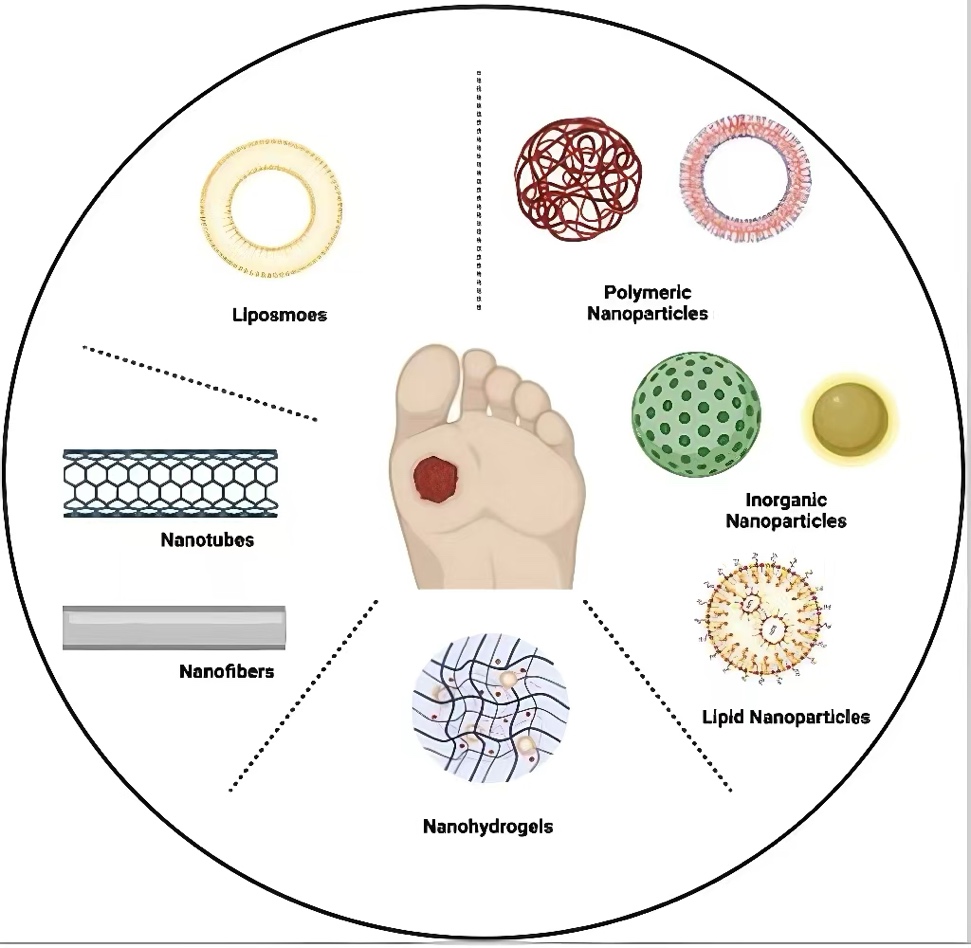
NDDSs are engineered constructs with particle diameters typically ranging from 1 to 100 nanometers. Their key advantages include enhanced drug stability, controlled and sustained drug release, and precise targeting capabilities, made possible through various biomaterial platforms [8]. Over the past decade, there has been a marked increase in the development of NDDSs specifically for diabetic wound care. These systems can be broadly categorized into six major classes: liposomes, polymeric nanoparticles (NPs), inorganic NPs, nanostructured lipid carrier (NLC), nanofibers, and nanohydrogels. Each type employs distinct mechanisms to accelerate the wound healing process. This functional diversity supports the rational selection of nanotherapeutics tailored to specific pathophysiological characteristics of diabetic wounds.
2. Physiology in wound healing process
2.1. Normal wound healing
Wound healing is a complicated physiology process which requires the interplay of mediators, extracellular matrix (ECM) components, growth factors, and proteinases [9]. As showed in Figure 1, that experiences well-ordered phases: hemostasis, inflammation proliferation and remodeling.
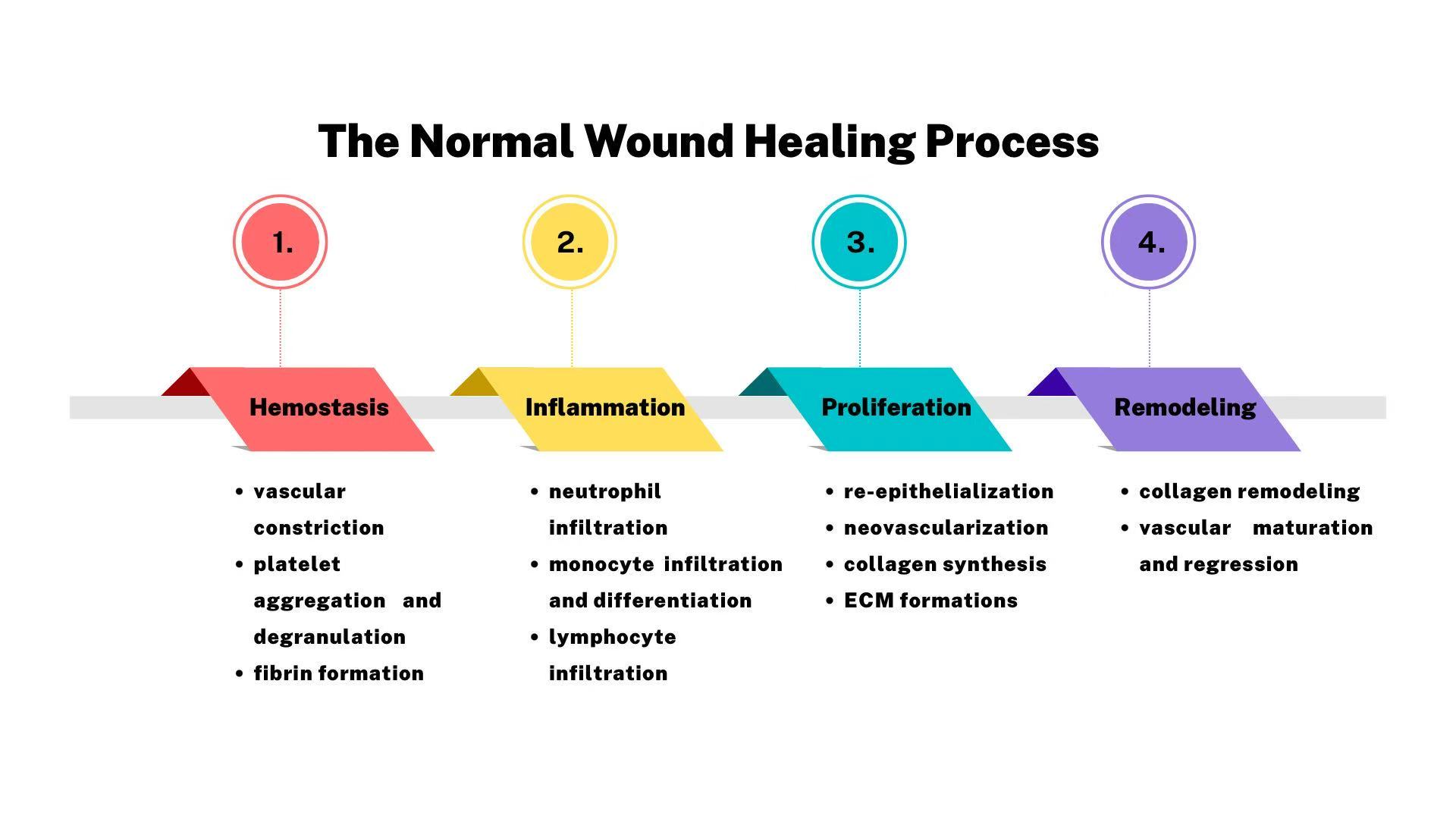
In the first stage of hemostasis, the capillaries around wound contract so that the volume of blood transported decreases, leading to decrease of blood loss and stimulation of aggregating platelets, which release growth factors such as epidermal growth factor (EGF), insulin-like growth factor 1 (IGF-1), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), transforming growth factor (TGF-α and TGF-β) , to form a clot and stop bleeding [11]. After these proinflammation mediators releasing, the neutrophils and monocytes are attracted to the wound where they exert supports on the stage of inflammation.
The normal inflammation takes place quickly after injury and lasts four to six days [10]. Neutrophils are infiltered to debride the wound via releasing cytokines, GFs and other mediators [12]. The monocytes is converted to macrophages which propel the phagocytosis to scavenge the bacteria around the wound and promote to contract wound before the formation of granulation [13].
It basically takes 3 days to 2 weeks during proliferative phase after injury to fill and cover the wound. The new capillaries are shaped under the proangiogenic factors such as PDGF released by platelets and inflammatory cells around wound area. In the addition, it’s necessary to form granulation by proliferation of fibroblasts, endothelial cells and keratinocytes, contributing to wound contraction [11]. Integrins, cells, cytokines and matrix metalloprotein (MMP) stimulates ECM production and cell migration [10].
In the phase of remodeling and re-epithelialization, when ECM reshaping to a structure that approaches normal tissue, the most critical alternation is the conversion from collagen collagen III to collagen I, playing an essential role in the revolution of the newborn collagen fibers to a more organized lattice structure which increase the tensile strength and flexibility of healed tissue [11].
2.2. Diabetic wound healing
Unlike normal wound healing, diabetic wounds are less likely to progress through the well-organized healing phases due to the interplay between intrinsic abnormalities and environmental disturbances, resulting in chronic wounds. A key factor is the toxicity of hyperglycemia, which leads to the accumulation of Advanced Glycation End Products (AGEs) in blood vessels and tissues, causing collagen to harden [14]. This process reduces the elasticity of blood vessels and narrows them, decreasing both blood flow and the rate of substance exchange. Consequently, reduced oxygen availability impairs aerobic respiration in tissue cells and reduces ATP production, weakening the proliferation and migration of repair cells (e.g., fibroblasts, keratinocytes) and inhibiting collagen synthesis [15]. Hypoxia also promotes cell death, and necrotic tissue releases pro-inflammatory cytokines (e.g., TNF-α, IL-6) [16], leading to chronic inflammation that further impedes healing. Macrophages shift from a pro-healing (M2) to a pro-inflammatory (M1) phenotype, sustaining the inflammatory state [17]. These downstream effects are primarily driven by persistent hyperglycemia.
Furthermore, diabetic neuropathy (DN), a common complication of diabetes, significantly impairs wound healing. Due to reduced pain sensation, patients may overlook minor injuries, increasing the risk of infection [18]. Unconscious behaviors, such as walking on an injured foot, can exacerbate the damage. Additionally, foot deformities (e.g., Charcot foot) increase localized pressure, contributing to the development of chronic ulcers [19].
3. Current treatment
3.1. Debridement
Diabetic wound ulceration is a common and serious complication in diabetic patients, primarily caused by neuropathy and circulatory disorders resulting from chronic hyperglycemia. Debridement is a standard clinical approach for treating such wounds; it aims to remove necrotic tissue and sources of infection while promoting the regeneration of healthy tissue.
Studies have shown that surgical debridement can effectively reduce bacterial load and accelerate wound healing. Moreover, combining negative pressure wound therapy after debridement can further enhance healing outcomes [20]. Therefore, debridement is not only forms the basis to diabetic wound management but also establishes favorable conditions for subsequent treatment. However, patients might feel unacceptable pain during debridement.
3.2. Revascularization
The most momentous ramification of toxicity of hyperglycemia contributing to diabetes is the accumulation of Advanced Glycation End Products (AGEs) in the wall of blood vessels and tissue which hardens collagen, leading to reduction to elasticity of blood vessels and narrow. Hince, a clinical pathway to address the problems is revascularization which is the restoration of blood flow to certain parts of blood vessels which are blocked or narrowed so that is universal used in treating diabetic wound from pharmaceutical to surgical technologies (angioplasty, endarterectomy, grafting or bypass).
However, there are some limitations about the revascularization: after surgical process, people are likely to undertake the risks of artery restenosis or even stent thrombosis, which depends on the health conditions of patients themselves [21].
3.3. Skin transplantation
Skin grafting is also a mainstream for treating diabetic wounds, which manifests a comprehensible mechanism that lies in covering the wound with transplanted healthy skin tissues which may be self, non-self or artificial, contributing to efficacy of accelerating healing and reducing the risks of infection [22]. although skin grafting exerts a certain curative effect on hard-to-heal wounds in diabetic patients, in clinical practice, the success rate of transplantation still depends on selecting an appropriate grafting method considering wound situations and strict postoperative management [23].
3.4. Pressure off-loading
After clinical cures, the pressure off-loading braces provide an accessorily supports to address the problem of long-term pressure or repeated friction caused by DFU when walking [24], which reduce local pressure and create a favorable environment for wound healing. Pressure off-loading braces can disperse pressure to avoid continuous stress on the wound area so that the blood circulation could be promoted, leading to effectiveness of oxygen and nutrients exchange around the wound and acceleration of tissue repair [25]. Additionally, the braces can isolate the wound from external friction, reduce the risk of infection, and maintain appropriate moisture in the wound, which is beneficial for the growth of granulation tissue.
Despite their significant effectiveness, the issue of adaptability has a relatively significant impact, as it can lead to potential discomfort. It needs to be combined with treatments such as debridement and cannot function effectively on its own [26].
3.5. Wound dressings
Following surgical interventions, these wounds remain highly vulnerable to infection so that wound dressings play a critical role in postoperative management by modulating the wound environment. By maintaining optimal moisture levels, they facilitate cellular migration and collagen organization which are vital for tissue regeneration [27]. For instance, some antimicrobial formulations such as silver-incorporated dressings could account for providing continuous protection against pathogens while mitigating persistent inflammation [28].
Since some drugs may be delivered to other health tissues rather than wound area, it is hard to attain and maintain a high level of drug concentration, leading to optimal effectiveness of drugs. In this way, it may require more doses of drugs so that high concentration of drugs like silver ions not only scavenge the bacteria around the wounds but also damage parts of heal tissues.
3.6. General measurements
Apart from clinical and further medications, normalization of blood glucose is critical for wound healing in diabetic patients. Hince anormal values of blood glucose is the fundamental factor to hyperglycemia, leading to diabetes and its complications, people with diabetes are required to raise the awareness of glycemic control which permeates in a myriad of segments of people’s lives from normalization of blood indicators, the management of blood fat, drinking and smoking cessation to diet control [29]. Those implements bring about boost in immunity and reduction in the risks of infection [30]. In short, blood glucose regulation is a prerequisite for wound healing in diabetic patients. Without stable blood glucose levels, the efficiency of essential measures such as debridement, revascularization and wound dressings will be greatly suppressed.
3.7. Limitations of current treatments
In general, there are still plenty of inevitable shortcomings from conventional approaches to address diabetic wound: debridement always results in unacceptable pain, leading to terror and fear; revascularization may make the artery be narrow if the patient with poor physical status; the curative rate of diabetic wound is also unstable and unconvincing under skin transplantation; the pressure off-loading leads to strong discomfort to patients; the wound dressings serve as accessory tools, which have the likelihood of health tissue injuries……
4. Nanoscales drug delivery system
Compared to conventional methods, a novel approach, called NDDSs, impedes the likelihood of the limitations from old one and even bring about a myriad of benefits, which employs tiny particles with nanoscale to achieve medication effectiveness, owing to the features of enhance drug solubility, protecting therapeutic agents from premature degradation and release sustainedly. As showed in Figure 2, there are considerable nano carriers delivering formulations to treat diabetic wounds, including liposomes, polymeric NPs, inorganic NPs, NLC, nanofibers and nanohydrogels. Table 1 shows some common applications of drug loading with nanocarriers.
|
Formulation |
Drug |
Administration |
Outcome |
Refs. |
|
Liposomes |
Phage Cocktail (MR-5+MR-10,1:1) |
Local injection |
Reduced significant number of bacteria around wound, decreasing inflammation and promoting diabetic wound contraction. |
[32] |
|
Hemoglobin (h-LEH, P50O2 = 10 mm Hg) |
Intravenous injection |
Increased Ki67 expression in immunohistochemistry, mitigated pro-inflammatory cytokines, considerably reduced inflammation and prompted proliferation, leading to diabetic wound contraction. |
[33] |
|
|
Polymeric NPs |
Asiaticoside |
Topical treatment |
Brought out an upregulated col-1 protein level and increased expression of α-SMA, resulted in sustained release, higher intra-cellular uptake and promotion of proliferation and migration of fibroblasts, leading to better efficacy of treating diabetic wound. |
[34] |
|
Ferulic acid |
Oral and topical administration |
Increased hydroxyproline and gained faster epithelialization. |
[35] |
|
|
Inorganic nanocarriers |
Insulin, Ag+ |
Subcutaneous injection |
Enhanced wound healing activity and regulated the balance between IL-6 and IL-10, accelerated wound healing |
[36] |
|
Fresh blood, Ag+ |
Intravenous injection |
Have a good effect on antibacterial and hemostasis, raise the wound closure rate |
[37] |
|
|
Lipid nanoparticles |
rhTM |
Oral and topical administration |
Promote angiogenesis in granulation tissue at the wound site, and improve the healing of chronic wounds |
[38] |
|
20(S)-protopanaxadiol |
Intradermal injection |
The wounds are completely closed without scars, promoting wound recovery |
[39] |
|
|
Nanofibers |
Insulin |
Subcutaneous injection |
Increase the content of transforming growth factor in the body and promote wound recovery |
[40] |
|
Curcumin |
Topical administration |
achieve the sustained release of curcumin, have the antioxidant and anti-inflammatory properties |
[41] |
|
|
Nanohydrogels |
Exosomes from HUVECs |
Applied topically |
Accelerated the proliferation, migration and angiogenesis of HUVECs, rendering better efficacy in diabetic wound restoration. |
[42] |
|
Curcumin |
Applied topically |
Increased the VEGF, prompted collagen synthesis, and deterred the bacterial infection, leading to a prominent preclinically curative rates of nearly 99.76% with only 18 days. |
[43] |
4.1. Liposomes
Liposomes are colloidal, vesicular structures, which have similar structures to the cell membrane and are composed of bilayers of cholesterol and phospholipid. The drugs are encapsuled in the hollow center of liposomes, leading to shielding effect of external environments exerted on the drugs, which ensures the high activity of drugs when reaching the destination [10]. During conveyance of drugs into the targeted cells, the liposomes would fuse with the cell membrane and release the drugs into the internal of cells through endocytosis, prompting the efficiency of medicines delivery. Due to a myriad of strengths of liposomes like nontoxicity, biocompatibility, biodegradation, the liposomes serve as a universal nano-vehicles which cure diabetic wound significantly.
Sanjay Chhibber et al. [32] evaluated the competence of a liposome encapsuled phage cocktail in diabetic wound healing, which proofed whether liposome encapsuled phage cocktail (MR-5+MR-10,1:1) mitigate the mortality or scavenge the infection. As a result, the bacteria amount for groups of liposomes encapsuled cocktail of phages (LCP) treated was neglect on day 7 and the wound area completely healed on day 9 while the free cocktail phages treated (FCP) cleared off bacteria completely on day 10. In addition, the wound area in the cases of LCP treated experienced a dramatic decline by day 3 and almost closure by day 9. However, the mice in FCP group persisted the wound with size of 5mm by day 3. So, the liposome facilitated the stability and persistence of phages to achieve better efficiency of phages, leading to decline of inflammation and promotion of diabetic wound contraction.
Tsuyoshi Fukui et al. [33] prepared a liposome-encapsuled hemoglobin with high oxygen affinity (h-LEH, P50O2 = 10 mm Hg) to facilitate the diabetic wound healing process. In this way, the experiments were carried out on dB/dB mice with retarded wound healing which imitated the human diabetic wound. As a result, by day 7 wound area was reduced to 47±8% of original size in h-LEH group while 68±18% in saline group and manifested an increase of Ki67 expression in immunohistochemistry in h-LEH group. According to the measurements in plasma, pro-inflammatory cytokines was mitigated via h-LEH treatment. So, it delineates that the h-LEH considerably reduces inflammation and prompts proliferation, leading to a boost of diabetic wound healing.
Despite the developed exploration of liposome-based nano drug delivery, the demerit is that it is hard to penetrate the therapeutic agents through deep layers of skin [31]. In addition, the liposomes have the likelihood of drug leakage before arriving targeted sites, resulting in the destroy of the healthy organs.
4.2. Polymeric NPs
Polymeric NPs are biocompatible colloidal system, which not only increase the total surface area of attaining drugs but also enhances the penetration. When embedment and conjugation are exerted on polymeric vehicles, the drugs could be conserved by deterring the degradation from surroundings before reaching targeted sites, mitigating the frequency of administrating drugs and fulfilling sustained release. The material of making polymeric NPs is primarily stem from polylactic-co-glycolic acid (PLGA), polyglycolic acid and other synthetic polymers, as well as alginate, gelatin, chitosan and other natural polymers.
The asiaticoside is a natural phytoconstituent which enhances the collagen biosynthesis but the curative effects of asiaticoside on diabetic wound is rather reduced, owing to high molecular weight, poor water solubility and poor permeability. So Saibhargav Narisepalli et al. [34] constructed asiaticoside polymeric nanoparticles (AST PNP). As a result, AST PNP not only illustrated sustained release profile up to 24 h while the free AST thoroughly released for 6 h but also manifested a boost of intra-cellular uptake in fibroblast within 3 h compared to the control groups. Additionally, AST PNP brought out an upregulated COL-1 protein level (∼1.85 fold vs free AST) and increased expression of α-SMA compared to free AST. In a short, it is persuasive that PNP are capable of better efficacy of treating diabetic wound using AST, which results in sustained release, higher intra-cellular uptake, and promotion of proliferation and migration of fibroblasts.
Ferulic acid (FA) was universally recognized in antidiabetic and antioxidant properties, due to scavenge of free radical and ROS, inhibition of inflammation and so on, but the poor solubility of FA served as an obstacle to achieve optimal utilization in diabetic wound therapy so that Ujjawal Bairagi et al. [35] prepared FA-PLGA NPs by nano precipitation method, followed by mixing FA-PLGA NPs into hydrogel for topical treatment. As a result, compared to control group, the mice treated with FA-PLGA NPs gained faster epithelialization, especially the hydroxyproline which is the key to collagen synthesis soared. It is convincing that FA-PLGA NPs tackles the delivery limitations, owing to low solubility of FA, but also surges the healing process of diabetes.
4.3. Inorganic NPs
Inorganic nanocarriers are mainly composed of metal nanoparticles like silver, gold and zinc, during preparation, which are shaped variously such as spherical, rod-shaped, flaky, and tubular.
Pawandeep Kuar et al. [36] encapsulated insulin in silver nanoparticles and added a 2nm-thick coating around the NPs to enable interaction between insulin and silver nanoparticles. Compared with the control group, the silver nanoparticles enhanced wound healing activity and regulated the balance between IL-6 and IL-10, accelerated wound healing.
Meenakshi Choudhary et al. [37] made a silver NPs with fresh blood served as the nutrient supply which makes contribution to antibacterial and hemostasis. On the 15th day of the experiment, the wound closure rate was 50% higher than that of the control group, indicating that the NPs with fresh blood enhanced the ability of diabetic wound healing.
However, some of their materials have potential cytotoxicity so that their safety needs to be concerned in long-term [44], triggering immune responses . In addition, the large-scale production might be limited, due to high cost.
4.4. Nanostructured lipid carriers
Nanostructured lipid carriers are, with sizes from 50 to 500 nanometers, constituted of natural or synthetic lipids, including phospholipids, cholesterol, triglycerides, etc. they are basically in the structures of sphere or quasi-sphere which have a hydrophilic core inside (which can encapsulate water-soluble drugs) and a lipid bilayer on the outer layer (which can load fat-soluble substances).The components of lipid NPs are similar to those of human cell membranes, which reduces the risks of immune rejection and toxicity. Moreover, the drug release rate relies on adjusting the lipid composition or surface modification, which means the loads can accurately be conveyed to the wounds.
Since recombinant human thrombomodulin (rhTM) can stimulate cell migration, Yuan-Shuo Hsueh et al. [38] used NLC to encapsulate rhTM to promote chronic wound healing. As a result, the NLC released rhTM continuously for more than 72 hours compared to control group, maintaining its stability for up to 12 weeks. So, it is compelling that NLC promotes angiogenesis in granulation tissue and improve the healing of chronic wounds.
Di Sun et al. [39] constructed a nano-lipid carrier loaded with 20(S)-protopanaxadiol (PPD). These carriers had excellent anti-inflammatory ability and enhanced vascular activity. When applying them to the wounds of diabetic mice, the complex moderated collagen accumulation and inhibited inflammation, resulting in a wound contraction rate of 98% and thorough wound recovery without scars.
Whereas NLC have poor stability easily affecting by temperature and pH, leading to high level for storage conditions. Furthermore, their drug-loading capacity is relatively low so that delivery efficiency is limited.
4.5. Nanofibers
Nanofibers are a membranous scaffold materials which are fluffy and porous, making them to efficiently load drugs, growth factors, or antibacterial agents [45]. Meanwhile, nanofibers have similar natural fibrous structure of human tissues, providing a suitable environment for cell migration and proliferation. In addition, nanofiber membranes are breathable and waterproof, which can not only effectively isolate external contamination but also allow the exchange of nutrients, thereby maintaining the microenvironment required for wound healing. They have an extremely high specific surface area which further improves the loading efficiency.
Chen-Hung Lee et al. [40] loaded insulin into nanofibers and found that the hydrophilicity and water-holding capacity of the nanofiber scaffolds were significantly lower than those of nanofibers loaded with and insulin. When treating diabetic mice, the nanofibers could continuously release insulin for four weeks, which significantly increased the content of transforming growth factor in the body and promoted wound recovery.
Yashi Agarwal et al. [41] fabricated a curcumin and polycaprolactone carried by nanofiber mats made in silk fibroin maintaining moisture around the wound. These nanofiber mats achieved the sustained release of curcumin and fulfill the antioxidant and anti-inflammatory, contributing to an outstanding therapeutic effect in diabetic wound healing.
4.6. Nanohydrogels
Nanohydrogels are also well-used in wound treatment, which are the polymer network system, with three-dimensional nanosized structures, leading to retain loads of water while maintaining the structural integrity so that provide a favorably moist environment where it enhances wound therapy [46]. Besides, the interplay between composition and structure endows the nanohydrogels with non-adhesion which allows oxygen penetrating in accessible way and conserves the wound surface [11]. Moreover, it is eminent for nanohydrogels to accommodate a variety of guest molecules, resulting in wider utilization in wound treatment.
Yiyao Zhang et al. [42] refined the exosomes from human umbilical cord mesenchymal stem cells (HUCMSCs) and embedded it into polyvinyl alcohol (PVA)/alginate (Alg) nanohydrogels, creating a combination (exo@H) for facilitating diabetic wound healing. The researchers carried out experiments on diabetic rats with control, exosomes, nanohydrogels, and exo@H treatment, followed by making comparisons of wound closure rates in the four groups. It is clear that the wound closure rates increased significantly in the exosomes and exo@H treated groups while other two groups experienced a slow wound closure rate as the time went. Unlike the control and nanohydrogels groups, the rats with exosomes and exo@H completed re-epithelialization. Particularly exo@H group had a smoother and more regular epithelium and the hair follicles in it began to form around wounds compared to exosomes group. So, it illustrated that the nanohydrogels contributed to better efficacy of exosomes in diabetic wound restoration, which accelerated the proliferation, migration and angiogenesis of HUVECs.
A curcumin-based nanohydrogel was created by K. Renuka et al. [43] to facilitate the diabetic wound healing. Curcumin is well-recognized for its forceful competences of anti-inflammatory, antioxidant, and antimicrobial which make curcumin be potential and prospective in treating DFU. Whereas the obstacle is due to the poor solubility and bioavailability so that the curcumin is hard to permeate into deeper layers of skin and accumulate around wound, leading to the low effectiveness of curcumin in healing diabetic wound. As a result, these nanohydrogels increased the vascular endothelial growth factor (VEGE), prompted collagen synthesis, and deterred the bacterial infection, leading to a prominent preclinically curative rates of nearly 99.76% with only 18 days via the treatment of the complex of the curcumin-loaded nano-vehicles and nanohydrogels. The nanohydrogels played a critical role in many segments of diabetic healing from the moist wound environment, compatibility to sustained release of drugs which were all the key factors to surge the diabetic curing rate.
5. Conclusion
Unlike normal wound, it is difficult for diabetic wound to heal naturally, due to intrinsic vicissitudes of the diabetic patients and the unmanageable wound environment. As so far, there have already emerged some methods to treat diabetic wound, including preliminary clinical debridement, skin transplantation and revascularization with something attached like wound dressings, pressure off-loading, and glycemic control. But the conventional tactics have some problems that might mitigate the curative effects and experienced trauma.
Hince, there is an emerged pathway triggering new insights in diabetic wound healing---the nanoscale drug delivery systems, which could combine with various formulations to augment the efficiency of the certain agents when treating the diabetic wounds, due to the functions of NDDSs: sustained release, protection of encapsuled agents and smart targeting and so on. Currently, a range of nano-vehicles have been already introduced and fulfill prominent achievements in diabetic wound healing, from liposomes, polymeric NPs, inorganic NPs, lipid NPs, nanofibers to nanohydrogels, which manifest eminent therapeutic capability and promising prospects. Whereas the shortcomings of NDDSs are still inevitable such as toxicity of nanomaterials, issues with metabolic efficiency as well as lack of human clinical evidence. However, researchers will persist to explore more about NDDSs in diabetic wound healing instead of being locked in the adversities. So, the NDDSs are expected to exert optimistic and authentic benefits in people’ daily life in the future.
References
[1]. Y. Liu, S. Zhou, Y. Gao, and Y. Zhai, "Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer, " Asian Journal of Pharmaceutical Sciences, vol. 14, no. 2, pp. 130-143, 2019.
[2]. D. Madhukiran, A. Jha, M. Kumar, G. Ajmal, G. V. Bonde, and B. Mishra, "Electrospun nanofiber-based drug delivery platform: advances in diabetic foot ulcer management, " Expert Opinion on Drug Delivery, vol. 18, no. 1, pp. 25-42, 2021.
[3]. S. Patel, S. Srivastava, M. R. Singh, and D. Singh, "Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing, " Biomedicine & Pharmacotherapy, vol. 112, p. 108615, 2019.
[4]. S. A. Shah et al., "Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review, " International journal of biological macromolecules, vol. 139, pp. 975-993, 2019.
[5]. H. Bai et al., "Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers, " Journal of tissue engineering, vol. 11, p. 2041731420947242, 2020.
[6]. C. Tu et al., "Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties, " Biomaterials, vol. 286, p. 121597, 2022.
[7]. M. E. Cam et al., "Accelerated diabetic wound healing by topical application of combination oral antidiabetic agents-loaded nanofibrous scaffolds: An in vitro and in vivo evaluation study, " Materials Science and Engineering: C, vol. 119, p. 111586, 2021.
[8]. Z. Qamar et al., "Nano-based drug delivery system: recent strategies for the treatment of ocular disease and future perspective, " Recent Patents on Drug Delivery & Formulation, vol. 13, no. 4, pp. 246-254, 2019.
[9]. G. Han and R. Ceilley, "Chronic wound healing: a review of current management and treatments, " Advances in therapy, vol. 34, no. 3, pp. 599-610, 2017.
[10]. M. Liu et al., "Recent advances in nano-drug delivery systems for the treatment of diabetic wound healing, " International journal of nanomedicine, pp. 1537-1560, 2023.
[11]. W. Wang, K.-j. Lu, C.-h. Yu, Q.-l. Huang, and Y.-Z. Du, "Nano-drug delivery systems in wound treatment and skin regeneration, " Journal of nanobiotechnology, vol. 17, no. 1, p. 82, 2019.
[12]. J. M. Daley et al., "Modulation of macrophage phenotype by soluble product (s) released from neutrophils, " The Journal of Immunology, vol. 174, no. 4, pp. 2265-2272, 2005.
[13]. T. J. Koh and L. A. DiPietro, "Inflammation and wound healing: the role of the macrophage, " Expert reviews in molecular medicine, vol. 13, p. e23, 2011.
[14]. V. Jakuš and N. Rietbrock, "Advanced glycation end-products and the progress of diabetic vascular complications, " Physiol Res, vol. 53, no. 2, pp. 131-142, 2004.
[15]. I. Aschermann, S. Noor, S. Venturelli, T. Sinnberg, C. Busch, and C. D. Mnich, "Extracorporal shock waves activate migration, proliferation and inflammatory pathways in fibroblasts and keratinocytes, and improve wound healing in an open-label, single-arm study in patients with therapy-refractory chronic leg ulcers, " Cellular Physiology and Biochemistry, vol. 41, no. 3, pp. 890-906, 2017.
[16]. M. I. Malkov, C. T. Lee, and C. T. Taylor, "Regulation of the hypoxia-inducible factor (HIF) by pro-inflammatory cytokines, " Cells, vol. 10, no. 9, p. 2340, 2021.
[17]. P. Krzyszczyk, R. Schloss, A. Palmer, and F. Berthiaume, "The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes, " Frontiers in physiology, vol. 9, p. 419, 2018.
[18]. B. Uzuner, S. Ketenci, and E. Salbaş, "General approach to diabetic neuropathy, " Acta Medica Alanya, vol. 4, no. 3, pp. 296-308, 2020.
[19]. A. I. Vinik, "Management of neuropathy and foot problems in diabetic patients, " Clinical cornerstone, vol. 5, no. 2, pp. 38-55, 2003.
[20]. B. Babamiri, F. Nikkhah, N. Faraji, R. Goli, N. V. Moghaddam, and K. Rahimi, "Diabetic foot ulcer: successful healing with combination therapy, including surgical debridement, maggot therapy, and negative pressure wound therapy, " International journal of surgery case reports, vol. 110, p. 108695, 2023.
[21]. M. Zeng, X. Yan, and W. Wu, "Risk factors for revascularization and in-stent restenosis in patients with triple-vessel disease after second-generation drug-eluting stent implantation: a retrospective analysis, " BMC cardiovascular disorders, vol. 21, no. 1, p. 446, 2021.
[22]. T. B. Santema, P. P. Poyck, and D. T. Ubbink, "Skin grafting and tissue replacement for treating foot ulcers in people with diabetes, " Cochrane database of systematic reviews, no. 2, 2016.
[23]. J. Holl et al., "Chronic diabetic wounds and their treatment with skin substitutes, " Cells, vol. 10, no. 3, p. 655, 2021.
[24]. S. L. Carter, J. H. Law, N. Seyler, Z. Tian, M. Widjajana, and D. Schoen, "Removable and Nonremovable Off-Loading Devices: A Plantar Pressure Analysis, " Journal of the American Podiatric Medical Association, vol. 115, no. 3, 2025.
[25]. E. Everett and N. Mathioudakis, "Update on management of diabetic foot ulcers, " Annals of the New York Academy of Sciences, vol. 1411, no. 1, pp. 153-165, 2018.
[26]. U. Majid and C. Argáez, "Off-Loading devices for people with diabetic neuropathic foot ulcers: a rapid qualitative review, " 2020.
[27]. B. S. Atiyeh, J. Ioannovich, C. A. Al-Amm, and K. A. El-Musa, "Management of acute and chronic open wounds: the importance of moist environment in optimal wound healing, " Current pharmaceutical biotechnology, vol. 3, no. 3, pp. 179-195, 2002.
[28]. D. J. Leaper, "Silver dressings: their role in wound management, " International wound journal, vol. 3, no. 4, pp. 282-294, 2006.
[29]. D. L. Steed et al., "Guidelines for the treatment of diabetic ulcers, " 2006.
[30]. N. Collins and Y. Belkaid, "Control of immunity via nutritional interventions, " Immunity, vol. 55, no. 2, pp. 210-223, 2022.
[31]. M. Liu et al., "Recent Advances in Nano-Drug Delivery Systems for the Treatment of Diabetic Wound Healing, " International Journal of Nanomedicine, vol. 18, no. null, pp. 1537-1560, 2023/12/31 2023, doi: 10.2147/IJN.S395438.
[32]. S. Chhibber, J. Kaur, and S. Kaur, "Liposome entrapment of bacteriophages improves wound healing in a diabetic mouse MRSA infection, " Frontiers in microbiology, vol. 9, p. 561, 2018.
[33]. T. Fukui, A. T. Kawaguchi, S. Takekoshi, M. Miyasaka, H. Sumiyoshi, and R. Tanaka, "Liposome‐encapsulated hemoglobin accelerates skin wound healing in diabetic dB/dB mice, " Artificial organs, vol. 41, no. 4, pp. 319-326, 2017.
[34]. S. Narisepalli, S. A. Salunkhe, D. Chitkara, and A. Mittal, "Asiaticoside polymeric nanoparticles for effective diabetic wound healing through increased collagen biosynthesis: In-vitro and in-vivo evaluation, " International Journal of Pharmaceutics, vol. 631, p. 122508, 2023.
[35]. U. Bairagi, P. Mittal, J. Singh, and B. Mishra, "Preparation, characterization, and in vivo evaluation of nano formulations of ferulic acid in diabetic wound healing, " Drug Development and Industrial Pharmacy, vol. 44, no. 11, pp. 1783-1796, 2018/11/02 2018, doi: 10.1080/03639045.2018.1496448.
[36]. P. Kaur et al., "Novel nano-insulin formulation modulates cytokine secretion and remodeling to accelerate diabetic wound healing, " Nanomedicine: Nanotechnology, Biology and Medicine, vol. 15, no. 1, pp. 47-57, 2019.
[37]. M. Choudhary, P. Chhabra, A. Tyagi, and H. Singh, "Scar free healing of full thickness diabetic wounds: a unique combination of silver nanoparticles as antimicrobial agent, calcium alginate nanoparticles as hemostatic agent, fresh blood as nutrient/growth factor supplier and chitosan as base matrix, " International Journal of Biological Macromolecules, vol. 178, pp. 41-52, 2021.
[38]. Y.-S. Hsueh et al., "Nanostructured lipid carrier gel formulation of recombinant human thrombomodulin improve diabetic wound healing by topical administration, " Pharmaceutics, vol. 13, no. 9, p. 1386, 2021.
[39]. D. Sun et al., "Silicone elastomer gel impregnated with 20 (S)-protopanaxadiol-loaded nanostructured lipid carriers for ordered diabetic ulcer recovery, " Acta Pharmacologica Sinica, vol. 41, no. 1, pp. 119-128, 2020.
[40]. C.-H. Lee et al., "Core-shell insulin-loaded nanofibrous scaffolds for repairing diabetic wounds, " Nanomedicine: Nanotechnology, Biology and Medicine, vol. 24, p. 102123, 2020.
[41]. Y. Agarwal et al., "Curcumin loaded polycaprolactone-/polyvinyl alcohol-silk fibroin based electrospun nanofibrous mat for rapid healing of diabetic wound: An in-vitro and in-vivo studies, " International Journal of Biological Macromolecules, vol. 176, pp. 376-386, 2021.
[42]. Y. Zhang, P. Zhang, X. Gao, L. Chang, Z. Chen, and X. Mei, "Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis, " Materials Science and Engineering: C, vol. 120, p. 111671, 2021.
[43]. K. Renuka, S. Priyanka, and J. Raphel Rose, "Curcumin-Powered Nanohydrogel Systems for Diabetic Foot Ulcers: Next-Generation Phytomedicine Approaches in Wound Care, " Biomedical Materials & Devices, pp. 1-15, 2025.
[44]. L. Accomasso, C. Cristallini, and C. Giachino, "Risk assessment and risk minimization in nanomedicine: a need for predictive, alternative, and 3Rs strategies, " Frontiers in Pharmacology, vol. 9, p. 228, 2018.
[45]. Z. Jiang et al., "Nanofiber scaffolds as drug delivery systems promoting wound healing, " Pharmaceutics, vol. 15, no. 7, p. 1829, 2023.
[46]. S. S. Makhathini et al., "Biomedicine innovations and its nanohydrogel classifications, " Pharmaceutics, vol. 14, no. 12, p. 2839, 2022.
Cite this article
Chen,S.;Feng,Z. (2025). Nanoscale Drug Delivery System for Diabetic Wound Treatment. Theoretical and Natural Science,137,66-78.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Y. Liu, S. Zhou, Y. Gao, and Y. Zhai, "Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer, " Asian Journal of Pharmaceutical Sciences, vol. 14, no. 2, pp. 130-143, 2019.
[2]. D. Madhukiran, A. Jha, M. Kumar, G. Ajmal, G. V. Bonde, and B. Mishra, "Electrospun nanofiber-based drug delivery platform: advances in diabetic foot ulcer management, " Expert Opinion on Drug Delivery, vol. 18, no. 1, pp. 25-42, 2021.
[3]. S. Patel, S. Srivastava, M. R. Singh, and D. Singh, "Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing, " Biomedicine & Pharmacotherapy, vol. 112, p. 108615, 2019.
[4]. S. A. Shah et al., "Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review, " International journal of biological macromolecules, vol. 139, pp. 975-993, 2019.
[5]. H. Bai et al., "Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers, " Journal of tissue engineering, vol. 11, p. 2041731420947242, 2020.
[6]. C. Tu et al., "Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties, " Biomaterials, vol. 286, p. 121597, 2022.
[7]. M. E. Cam et al., "Accelerated diabetic wound healing by topical application of combination oral antidiabetic agents-loaded nanofibrous scaffolds: An in vitro and in vivo evaluation study, " Materials Science and Engineering: C, vol. 119, p. 111586, 2021.
[8]. Z. Qamar et al., "Nano-based drug delivery system: recent strategies for the treatment of ocular disease and future perspective, " Recent Patents on Drug Delivery & Formulation, vol. 13, no. 4, pp. 246-254, 2019.
[9]. G. Han and R. Ceilley, "Chronic wound healing: a review of current management and treatments, " Advances in therapy, vol. 34, no. 3, pp. 599-610, 2017.
[10]. M. Liu et al., "Recent advances in nano-drug delivery systems for the treatment of diabetic wound healing, " International journal of nanomedicine, pp. 1537-1560, 2023.
[11]. W. Wang, K.-j. Lu, C.-h. Yu, Q.-l. Huang, and Y.-Z. Du, "Nano-drug delivery systems in wound treatment and skin regeneration, " Journal of nanobiotechnology, vol. 17, no. 1, p. 82, 2019.
[12]. J. M. Daley et al., "Modulation of macrophage phenotype by soluble product (s) released from neutrophils, " The Journal of Immunology, vol. 174, no. 4, pp. 2265-2272, 2005.
[13]. T. J. Koh and L. A. DiPietro, "Inflammation and wound healing: the role of the macrophage, " Expert reviews in molecular medicine, vol. 13, p. e23, 2011.
[14]. V. Jakuš and N. Rietbrock, "Advanced glycation end-products and the progress of diabetic vascular complications, " Physiol Res, vol. 53, no. 2, pp. 131-142, 2004.
[15]. I. Aschermann, S. Noor, S. Venturelli, T. Sinnberg, C. Busch, and C. D. Mnich, "Extracorporal shock waves activate migration, proliferation and inflammatory pathways in fibroblasts and keratinocytes, and improve wound healing in an open-label, single-arm study in patients with therapy-refractory chronic leg ulcers, " Cellular Physiology and Biochemistry, vol. 41, no. 3, pp. 890-906, 2017.
[16]. M. I. Malkov, C. T. Lee, and C. T. Taylor, "Regulation of the hypoxia-inducible factor (HIF) by pro-inflammatory cytokines, " Cells, vol. 10, no. 9, p. 2340, 2021.
[17]. P. Krzyszczyk, R. Schloss, A. Palmer, and F. Berthiaume, "The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes, " Frontiers in physiology, vol. 9, p. 419, 2018.
[18]. B. Uzuner, S. Ketenci, and E. Salbaş, "General approach to diabetic neuropathy, " Acta Medica Alanya, vol. 4, no. 3, pp. 296-308, 2020.
[19]. A. I. Vinik, "Management of neuropathy and foot problems in diabetic patients, " Clinical cornerstone, vol. 5, no. 2, pp. 38-55, 2003.
[20]. B. Babamiri, F. Nikkhah, N. Faraji, R. Goli, N. V. Moghaddam, and K. Rahimi, "Diabetic foot ulcer: successful healing with combination therapy, including surgical debridement, maggot therapy, and negative pressure wound therapy, " International journal of surgery case reports, vol. 110, p. 108695, 2023.
[21]. M. Zeng, X. Yan, and W. Wu, "Risk factors for revascularization and in-stent restenosis in patients with triple-vessel disease after second-generation drug-eluting stent implantation: a retrospective analysis, " BMC cardiovascular disorders, vol. 21, no. 1, p. 446, 2021.
[22]. T. B. Santema, P. P. Poyck, and D. T. Ubbink, "Skin grafting and tissue replacement for treating foot ulcers in people with diabetes, " Cochrane database of systematic reviews, no. 2, 2016.
[23]. J. Holl et al., "Chronic diabetic wounds and their treatment with skin substitutes, " Cells, vol. 10, no. 3, p. 655, 2021.
[24]. S. L. Carter, J. H. Law, N. Seyler, Z. Tian, M. Widjajana, and D. Schoen, "Removable and Nonremovable Off-Loading Devices: A Plantar Pressure Analysis, " Journal of the American Podiatric Medical Association, vol. 115, no. 3, 2025.
[25]. E. Everett and N. Mathioudakis, "Update on management of diabetic foot ulcers, " Annals of the New York Academy of Sciences, vol. 1411, no. 1, pp. 153-165, 2018.
[26]. U. Majid and C. Argáez, "Off-Loading devices for people with diabetic neuropathic foot ulcers: a rapid qualitative review, " 2020.
[27]. B. S. Atiyeh, J. Ioannovich, C. A. Al-Amm, and K. A. El-Musa, "Management of acute and chronic open wounds: the importance of moist environment in optimal wound healing, " Current pharmaceutical biotechnology, vol. 3, no. 3, pp. 179-195, 2002.
[28]. D. J. Leaper, "Silver dressings: their role in wound management, " International wound journal, vol. 3, no. 4, pp. 282-294, 2006.
[29]. D. L. Steed et al., "Guidelines for the treatment of diabetic ulcers, " 2006.
[30]. N. Collins and Y. Belkaid, "Control of immunity via nutritional interventions, " Immunity, vol. 55, no. 2, pp. 210-223, 2022.
[31]. M. Liu et al., "Recent Advances in Nano-Drug Delivery Systems for the Treatment of Diabetic Wound Healing, " International Journal of Nanomedicine, vol. 18, no. null, pp. 1537-1560, 2023/12/31 2023, doi: 10.2147/IJN.S395438.
[32]. S. Chhibber, J. Kaur, and S. Kaur, "Liposome entrapment of bacteriophages improves wound healing in a diabetic mouse MRSA infection, " Frontiers in microbiology, vol. 9, p. 561, 2018.
[33]. T. Fukui, A. T. Kawaguchi, S. Takekoshi, M. Miyasaka, H. Sumiyoshi, and R. Tanaka, "Liposome‐encapsulated hemoglobin accelerates skin wound healing in diabetic dB/dB mice, " Artificial organs, vol. 41, no. 4, pp. 319-326, 2017.
[34]. S. Narisepalli, S. A. Salunkhe, D. Chitkara, and A. Mittal, "Asiaticoside polymeric nanoparticles for effective diabetic wound healing through increased collagen biosynthesis: In-vitro and in-vivo evaluation, " International Journal of Pharmaceutics, vol. 631, p. 122508, 2023.
[35]. U. Bairagi, P. Mittal, J. Singh, and B. Mishra, "Preparation, characterization, and in vivo evaluation of nano formulations of ferulic acid in diabetic wound healing, " Drug Development and Industrial Pharmacy, vol. 44, no. 11, pp. 1783-1796, 2018/11/02 2018, doi: 10.1080/03639045.2018.1496448.
[36]. P. Kaur et al., "Novel nano-insulin formulation modulates cytokine secretion and remodeling to accelerate diabetic wound healing, " Nanomedicine: Nanotechnology, Biology and Medicine, vol. 15, no. 1, pp. 47-57, 2019.
[37]. M. Choudhary, P. Chhabra, A. Tyagi, and H. Singh, "Scar free healing of full thickness diabetic wounds: a unique combination of silver nanoparticles as antimicrobial agent, calcium alginate nanoparticles as hemostatic agent, fresh blood as nutrient/growth factor supplier and chitosan as base matrix, " International Journal of Biological Macromolecules, vol. 178, pp. 41-52, 2021.
[38]. Y.-S. Hsueh et al., "Nanostructured lipid carrier gel formulation of recombinant human thrombomodulin improve diabetic wound healing by topical administration, " Pharmaceutics, vol. 13, no. 9, p. 1386, 2021.
[39]. D. Sun et al., "Silicone elastomer gel impregnated with 20 (S)-protopanaxadiol-loaded nanostructured lipid carriers for ordered diabetic ulcer recovery, " Acta Pharmacologica Sinica, vol. 41, no. 1, pp. 119-128, 2020.
[40]. C.-H. Lee et al., "Core-shell insulin-loaded nanofibrous scaffolds for repairing diabetic wounds, " Nanomedicine: Nanotechnology, Biology and Medicine, vol. 24, p. 102123, 2020.
[41]. Y. Agarwal et al., "Curcumin loaded polycaprolactone-/polyvinyl alcohol-silk fibroin based electrospun nanofibrous mat for rapid healing of diabetic wound: An in-vitro and in-vivo studies, " International Journal of Biological Macromolecules, vol. 176, pp. 376-386, 2021.
[42]. Y. Zhang, P. Zhang, X. Gao, L. Chang, Z. Chen, and X. Mei, "Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis, " Materials Science and Engineering: C, vol. 120, p. 111671, 2021.
[43]. K. Renuka, S. Priyanka, and J. Raphel Rose, "Curcumin-Powered Nanohydrogel Systems for Diabetic Foot Ulcers: Next-Generation Phytomedicine Approaches in Wound Care, " Biomedical Materials & Devices, pp. 1-15, 2025.
[44]. L. Accomasso, C. Cristallini, and C. Giachino, "Risk assessment and risk minimization in nanomedicine: a need for predictive, alternative, and 3Rs strategies, " Frontiers in Pharmacology, vol. 9, p. 228, 2018.
[45]. Z. Jiang et al., "Nanofiber scaffolds as drug delivery systems promoting wound healing, " Pharmaceutics, vol. 15, no. 7, p. 1829, 2023.
[46]. S. S. Makhathini et al., "Biomedicine innovations and its nanohydrogel classifications, " Pharmaceutics, vol. 14, no. 12, p. 2839, 2022.









