1. Introduction
Meditative practices have garnered increasing scientific attention for their potential to modulate brain activity and promote mental well-being. Numerous neurophysiological studies suggest that meditation may alter resting-state brain dynamics, particularly in oscillatory activity across frequency bands such as theta (4–8 Hz), alpha (8–12 Hz), and gamma (30–45 Hz) [1-3]. However, findings remain heterogeneous across populations, meditation styles, and analytic approaches. While some studies report increased alpha or theta power during meditation, others emphasize gamma enhancement associated with focused attention or sustained awareness [1,4,5]. This variability highlights a need for more granular and temporally sensitive investigations of meditation-related EEG patterns. Resting-state EEG studies often average brain activity across extended periods, potentially obscuring dynamic fluctuations that unfold over time. Temporal segmentation—especially when anchored to self-reported mental states—offers a promising approach to capturing state-dependent neural changes during meditation. Moreover, individual differences such as age, gender, and meditation experience may further modulate oscillatory patterns, yet these variables are frequently treated as covariates or overlooked entirely [6-8].
The present study aimed to address these gaps by analyzing resting-state EEG activity collected during a meditation session with periodic self-report probes. We examined spectral power across canonical frequency bands and explored how it varied over five time segments surrounding internal concentration reports. In addition to comparing high vs. low concentration states (reported separately), we focused here on segment-based dynamics independent of reported mental state, offering an unbiased view of resting oscillatory evolution. Importantly, we stratified our analysis by gender, age group, and meditation experience to assess whether resting-state EEG power exhibits population-specific patterns. A 64-channel EEG system was used to collect high-resolution neural data from 24 participants (12 experienced meditators and 12 novices). Spectral power was computed using Welch’s method, and statistical analyses included independent t-tests, regression models, and segment-wise one-sample t-tests. Visualizations included scalp topographies, correlation matrices, and heatmaps of statistical significance across channels and frequency bands. Our results revealed consistent gamma band enhancement across most subgroups and segments, particularly in central and parietal regions, suggesting a stable marker of meditative rest. Beta power was selectively increased in male participants, while delta activity was more pronounced in females and older adults during early and late segments. Minimal differences were observed based on meditation experience, and traditional theta/alpha enhancements were limited to specific subgroups. These findings contribute to a nuanced understanding of meditation-related brain dynamics, emphasizing the temporal and demographic modulation of resting-state EEG oscillations. By integrating segment-wise analyses with individual difference factors, this study offers novel insight into the intrinsic neural architecture of meditation beyond static group-level comparisons.
2. Methodology
2.1. Participants and experimental design
Twenty-four participants took part in this study, including 12 experienced meditation practitioners (6 males, 6 females; mean age = 39.8 years, SD = 12.4) and 12 meditation novices (4 males, 8 females; mean age = 46.2 years, SD = 14.6). One novice participant did not report their age. All participants gave written informed consent prior to participation. The study was approved by the relevant ethics committee.
Participants engaged in a meditation task during which they were periodically interrupted approximately every two minutes by a prompt to report their mental state. The prompt consisted of three self-report questions regarding their subjective concentration, mind-wandering, and emotional valence (exact wording not specified). Participants responded via button presses. These prompts served as temporal anchors for extracting EEG segments corresponding to different mental states.
2.2. EEG data acquisition and preprocessing
EEG data were collected using a 64-channel Biosemi ActiveTwo system arranged according to the international 10–20 system. Signals were initially recorded at a sampling rate of 2048 Hz and subsequently downsampled to 256 Hz using the Biosemi BDF decimator tool (https://www.biosemi.com/download.htm). All EEG channels were re-referenced offline to the average of all scalp electrodes. Although additional physiological signals (e.g., EOG, EMG, GSR, respiration) were recorded, only the EEG data were analyzed in this study. Data preprocessing and analysis were conducted in Python using Jupyter Notebook, primarily utilizing the MNE, NumPy, pandas, and pingouin packages.
EEG preprocessing was performed using the MNE-Python library. The main steps included downsampling to 256 Hz (if not already applied prior to analysis), bandpass filtering between 1–45 Hz using a zero-phase FIR filter, line noise removal via a 50 Hz notch filter, re-referencing to the average of all EEG channels, epoch rejection based on amplitude thresholds and abnormal signal trends, and optional Independent Component Analysis (ICA) to identify and remove components related to ocular and muscular artifacts.
2.3. Event definition and epoching
Event markers were extracted from the BIDS-compliant.tsv event files. The primary event of interest was the onset of the first self-report question, corresponding to event code 128 (labeled as “stimulus”). Participant responses (event codes 2, 4, and 8) represented answers to three probe questions, but only responses to Question 1 (concentration level, code 2) were used to label EEG epochs. Events labeled as 16 (involuntary responses) were excluded.
Epochs were extracted for the 10 seconds immediately preceding each question onset (stimulus). The epoch window ranged from -10 to 0 seconds relative to the onset of the question, and baseline correction was either not applied or applied using the first 500 ms, depending on the analysis variant. Each epoch was labeled as “high concentration” or “low concentration” using a within-subject median split of the self-reported concentration scores.
2.4. Topographic, and statistical analyses
Power spectral density (PSD) was computed for each 10-second epoch using Welch’s method with 2-second Hamming windows and 50% overlap. PSD values were averaged within canonical frequency bands: Delta 1–4 Hz; Theta 4–8 Hz; Alpha 8–12 Hz; Beta 13–30 Hz; and Gamma 30–45 Hz.
Band power was computed for each channel and then averaged across epochs within each condition (“high concentration” vs. “low concentration”). Topographical visualizations were generated using MNE’s plot_topomap function for spatial distribution of band power across the scalp.
All statistical analyses were conducted in Python using the pingouin and statsmodels libraries. A multi-step inferential framework was employed to evaluate EEG spectral power differences across demographic and experimental factors.
First, independent samples t-tests were conducted to compare EEG band power across three between-subject factors: gender (male vs. female), age group (<30 vs. ≥30 years), and meditation experience (experienced vs. novice). These comparisons were performed separately for each of the five canonical frequency bands (delta, theta, alpha, beta, gamma). Effect sizes were computed using Cohen’s d, and p-values were Bonferroni-corrected for multiple comparisons.
Second, multiple linear regression analyses were conducted for each frequency band to assess the combined predictive influence of gender, age (as a continuous variable), and meditation experience on EEG power. Model fit was evaluated using R², and individual predictor significance was assessed using p-values and standardized beta coefficients.
Third, one-sample t-tests were performed on segment-wise EEG band power to determine whether spectral power significantly differed from zero across five temporal segments (Segment 0–4). These analyses were repeated within subgroups defined by gender, age group, and meditation experience. Results were visualized using heatmaps of p-values (channel × frequency band) and bar plots with standard deviation for significant effects.
Finally, inter-channel correlation matrices were computed using Pearson’s r to explore spatial synchrony across EEG electrodes during the meditation condition. The matrices were visualized using color-coded heatmaps to highlight patterns of local and long-range coherence.
A significance threshold of p < .05 (corrected where necessary) was adopted throughout unless otherwise noted.
3. Results
3.1. Group-level effects
To explore whether demographic and experiential variables were associated with differences in EEG activity, independent samples t-tests were conducted to compare EEG band power across gender, age group, and experience level for each canonical frequency band (delta, theta, alpha, beta, gamma). Corresponding effect sizes were estimated using Cohen’s d. Results are summarized below and visualized in Figure 1-3.
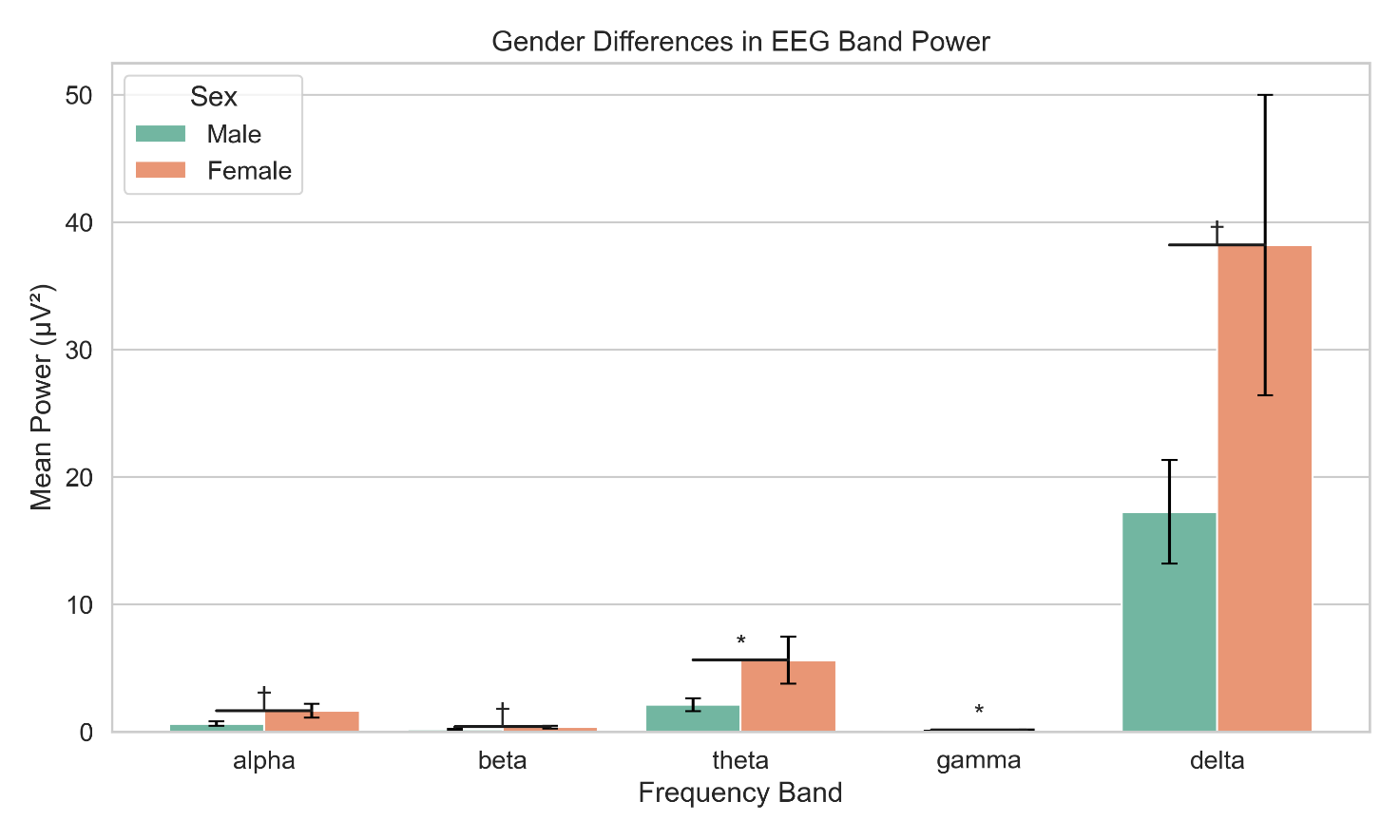
Bar graphs depict mean power (±SE) across five canonical frequency bands (delta, theta, alpha, beta, gamma) for male and female participants
Asterisks indicate statistically significant differences (p < .05)
Power values are averaged across all electrodes in the eyes-closed resting state
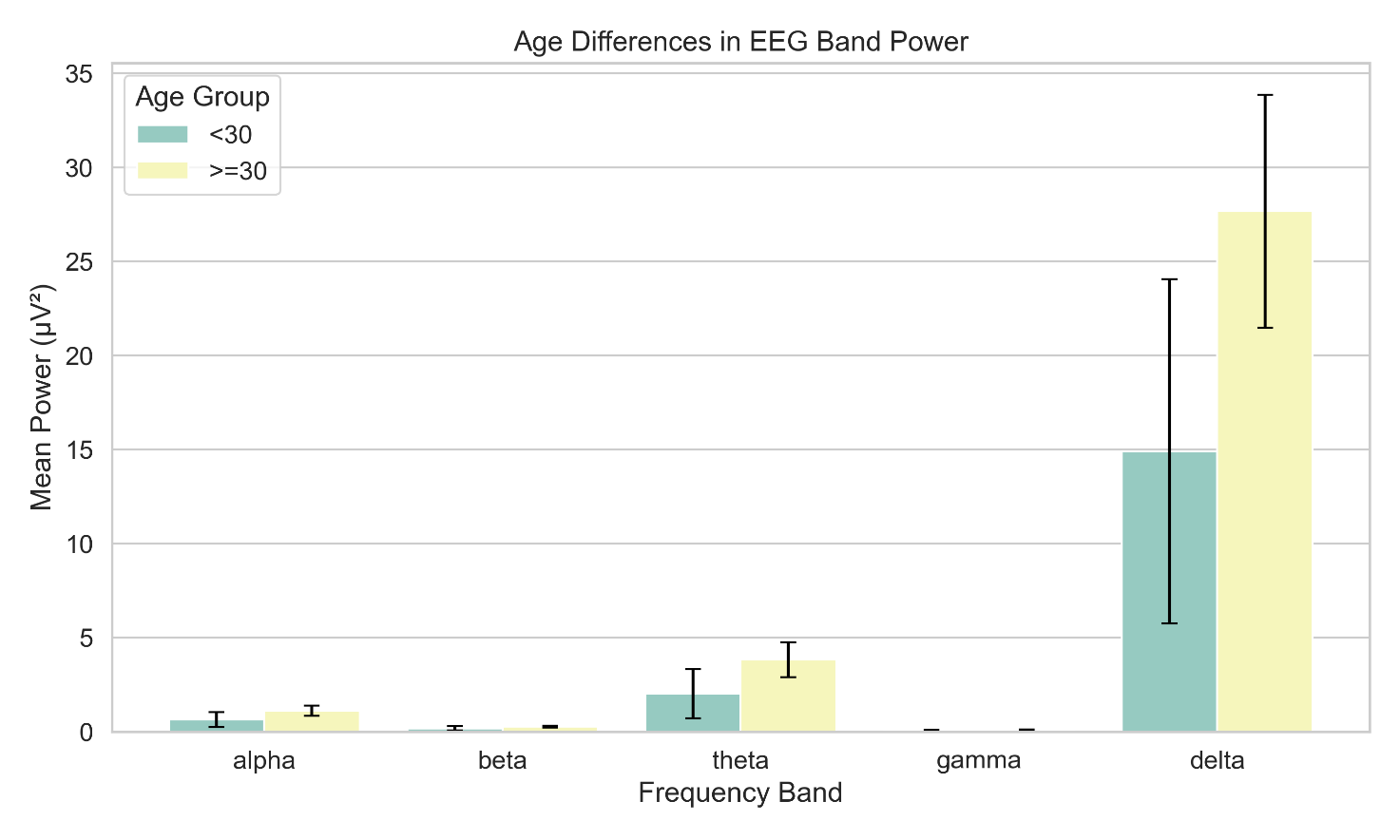
Power values (±SE) for each frequency band are plotted for participants grouped by age (median split)
Significant differences (p < .05) are marked with asterisks
Data are based on whole-brain average power during eyes-closed rest
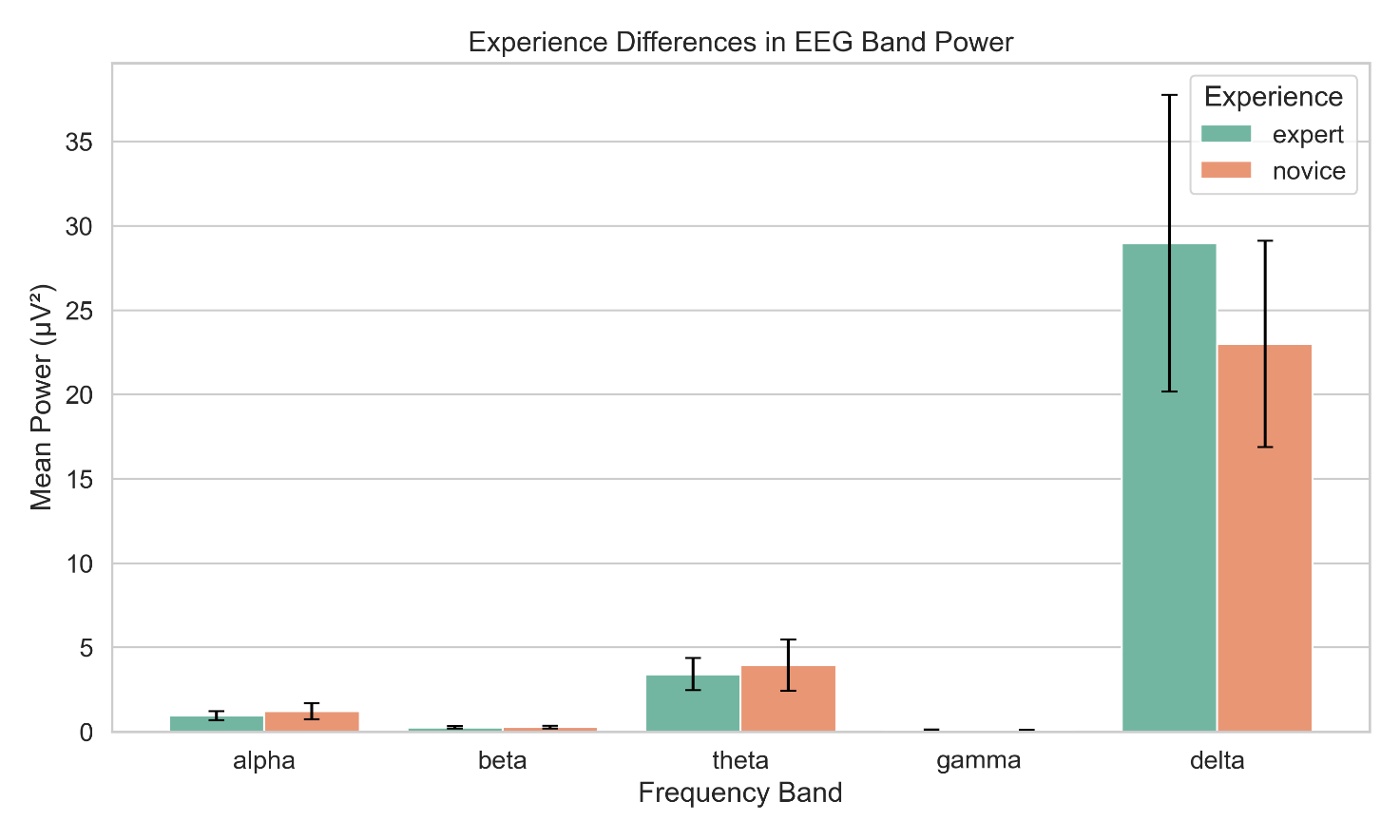
Participants were categorized into low- and high-experience groups
Mean power (±SE) for each frequency band is shown, with significant group differences (p < .05) indicated
Data reflect global scalp averages during resting state
There were no statistically significant gender differences in EEG band power after correction for multiple comparisons. However, theta (t(df) = –2.021, p = .043, d = –0.019) and gamma (t = –1.998, p = .046, d = –0.019) approached conventional significance levels, suggesting potentially greater theta and gamma power in female participants. Alpha and beta bands also showed marginal trends (p = .051 and .059, respectively), but effect sizes were consistently small (|d| < .02).
Participants were divided into two age groups (<30 and ≥30 years). No significant differences in EEG band power were observed across age groups for any frequency band (all p > .49). Effect sizes were negligible across all bands (Cohen’s d < .02), suggesting minimal impact of age within the sample range on spectral power.
When comparing experienced versus novice meditators, no significant differences in EEG band power were found (all p > .59). Effect sizes were close to zero (|d| < .005), indicating that prior meditation experience did not influence resting-state spectral power in the current dataset.
In addition to the independent t-tests, multiple linear regression analyses were conducted to examine the combined influence of gender, age, and meditation experience on EEG band power. Across all frequency bands, the overall explanatory power of the models was minimal (all R² ≈ 0.002–0.003), suggesting that the predictors explained very little variance in EEG power.
Despite the low model fit, gender consistently emerged as a significant predictor across multiple frequency bands: it was significantly associated with beta (p = .047), theta (p = .045), and gamma power (p = .013), and showed a trend-level effect in alpha (p = .088) and delta (p = .007). In contrast, age and experience were not significant predictors in any frequency band, although meditation experience approached significance in the delta band (p = .060). These findings further support the role of gender-related differences in EEG spectral power.
3.2. Spatial patterns of EEG activity
To further explore the spatial synchronization of brain activity during meditation, an inter-channel correlation matrix was computed across all EEG channels. As shown in Figure 4, this matrix revealed several important patterns:
High Inter-Hemispheric Correlation: notably, symmetrical channel pairs such as C3–C4 (central region) and F3–F4 (frontal region) exhibited strong positive correlations (r ≈ 0.8–0.9), suggesting synchronized activity across hemispheres in regions associated with attention and sensorimotor processes.
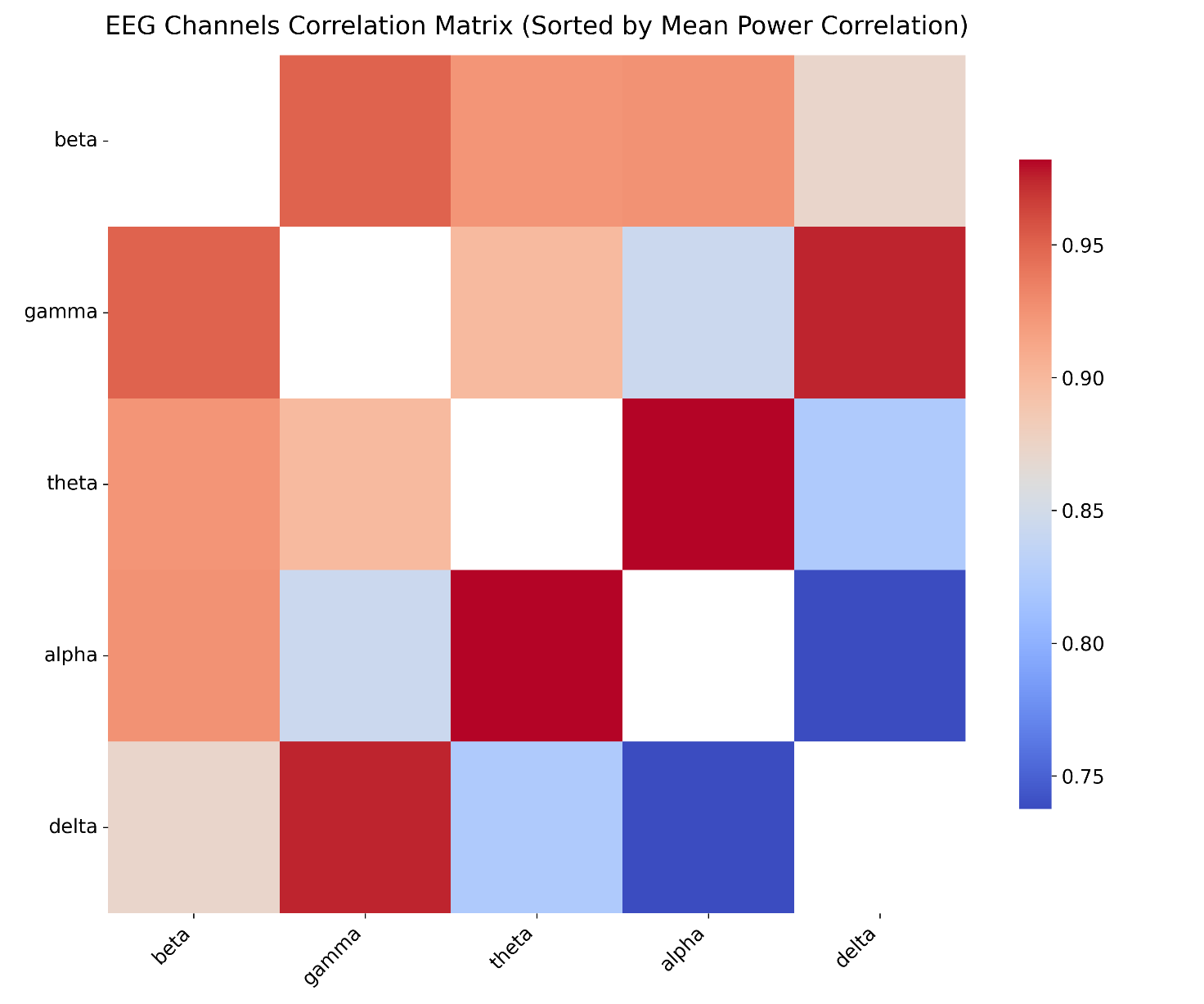
Red indicates high positive correlation, blue indicates negative correlation, and white indicates no correlation
The matrix reflects synchronous activity between homologous and neighboring channels during meditation
Low Cross-Region Correlation: channel pairs from distant regions (e.g., frontal F3 vs. occipital O1) showed low correlations (r ≈ 0), reflecting functional independence during meditation. This supports the notion that while some regions are functionally coupled, others may operate more independently depending on cognitive demands.
Limited Negative Correlations: a few channel pairs showed mild negative correlations (r ≈ -0.2 to -0.4), possibly indicating antagonistic activation patterns between certain areas.
Topographical Insight: the highest correlations were typically observed between neighboring or homologous electrodes, which may reflect both anatomical proximity and functional co-activation during meditative states. Conversely, low correlations between distant channels suggest that not all brain regions are engaged equally.
Overall, the correlation matrix reveals a characteristic pattern of localized synchrony and distal independence during meditation, especially in frontal and central regions. These findings complement the band power results by highlighting spatially coordinated neural activity beyond amplitude differences alone.
Topographical scalp maps (Figure 5) were used to visualize the spatial distribution of EEG power across five frequency bands during the meditation condition. All maps represent the voltage distribution at 0.000 seconds (in microvolts, μV). Alpha (8–12 Hz): The power was primarily distributed in the central and frontal regions, with a large range from -8,000,000 to 8,000,000 μV. The pronounced frontal-central dominance may reflect the role of alpha oscillations in relaxation and attentional disengagement during meditation. Theta (4–7 Hz): Theta activity also showed strong frontal-central dominance, although its amplitude range was much smaller (–75,000 to 75,000 μV). This focused distribution suggests stable engagement of internalized attention and introspective processes often associated with meditative states. Delta (1–4 Hz): The power distribution was relatively uniform across the scalp, with a range between –1,000,000 and 1,000,000 μV. This widespread pattern may reflect a generalized state of deep relaxation. Beta (13–30 Hz): Like alpha and theta, beta activity was mainly observed in the frontal and central regions (–800,000 to 800,000 μV), possibly reflecting a residual level of alertness or executive monitoring during the meditative state. Gamma (31–45 Hz): Gamma power was evenly distributed, with moderate amplitudes (–1,000,000 to 1,000,000 μV). This may indicate diffuse cognitive processing, such as subtle internal imagery or processing of verbal meditation cues.
Overall, alpha, theta, and beta bands showed spatially overlapping frontal-central activation, while delta and gamma bands appeared more diffusely distributed, reflecting multiple aspects of the meditative state, including relaxation, introspection, and attention modulation.
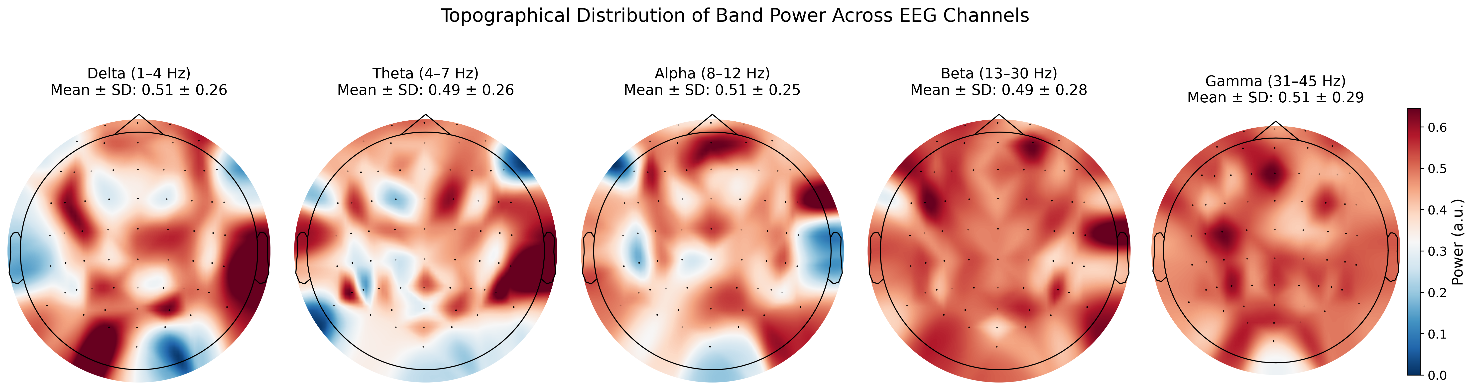
The maps reflect average power across all subjects, plotted at 0.000 seconds, using a common color scale (μV) Frontal-central dominance is particularly notable in Alpha, Theta, and Beta bands
3.3. Temporal dynamics of EEG activity
To examine resting-state EEG dynamics across different temporal segments, one-sample t-tests were conducted separately for each of the five segmented periods (Segments 0–4), testing whether spectral power in each frequency band and channel significantly differed from zero. Analyses were further stratified by gender, age group, and meditation experience. For each subgroup and segment, two types of visualizations were generated: (1) heatmaps showing p-values across all channels and frequency bands, and (2) bar plots for significant channels with error bars representing standard deviation. Segment 0: No significant channels were observed when analyzing all participants, males, experienced meditators, novice meditators, or younger participants (<35 years). Notably, female participants showed significant delta band increases in multiple bilateral channels (e.g., A28, B29, B9, A7, B3), indicating increased low-frequency power in frontal and parietal areas. Participants aged ≥35 years demonstrated strong gamma band increases in frontal-central channels (e.g., A24, A10, A6, A5, A11), along with marginal theta band effects, suggesting elevated high-frequency activity in older adults at rest.
Across the four temporal segments, a remarkably consistent pattern of neural activity emerged, dominated by gamma band enhancements. In Segment 1, widespread significant activation was observed across the entire sample, with gamma power particularly elevated in central and parietal regions (e.g., A12, A11, A32, B24, B15; p < 1e–7). Gender differences began to surface—males exhibited more beta-band activity (notably in channels like A18, A13, A12, B23), while females showed stronger gamma effects, especially in frontal sites (e.g., B2, B4, A1, A3). Both experienced and novice meditators demonstrated robust gamma activation, though with slight topographic variations, hinting at shared underlying mechanisms during resting meditation. Age-related effects were also apparent: younger participants showed increases not only in gamma but also delta and alpha bands, whereas older adults displayed primarily gamma and delta enhancements.
Segment 2 largely reinforced these findings. Gamma activity remained prominent across the sample, now with a slightly more bilateral parietal-temporal distribution (e.g., A12, A13, A32, B24, A31). The male group continued to show distinct beta activity, while females again exhibited gamma-dominant responses in frontal regions. Within the experienced group, significant gamma and delta activity emerged across frontal and central electrodes (e.g., A11, A5, B5), suggesting a deepening of meditative states. Novices, as well as both age groups, retained strong gamma signatures, pointing to the temporal stability of high-frequency activation.
By Segment 3, these spectral dynamics had largely stabilized. Gamma power remained dominant in frontal and parietal areas across the full cohort. Gender-specific patterns persisted: males maintained elevated beta activity, while females continued to show gamma-dominant responses. Experienced meditators, in particular, showed marked gamma increases (e.g., A2, A20, B15), while novices demonstrated bilateral gamma activation with some frontal spread. Age-based comparisons again revealed consistent gamma elevation, with minor delta or theta activity surfacing in a few sites.
In Segment 4, the final phase of the resting period, these trends remained evident. Gamma enhancements were now concentrated more toward parietal-occipital regions (e.g., A12, A11, A13, B16), with males continuing to show beta increases and females maintaining pronounced gamma responses (e.g., A5, A1, B14, B1). Among experienced meditators, dense gamma activity was observed across central and posterior channels, while novices also exhibited significant gamma power alongside emerging theta activity in posterior sites. Interestingly, older adults in this segment showed increased delta activity in bilateral parietal areas (B13, B14), along with continued gamma elevation—suggesting subtle temporal shifts in lower-frequency dynamics with age.
3.4. Summary and interpretation
Across all five segments, a consistent pattern emerged: gamma power significantly exceeded zero in nearly every group and segment (Segments 1–4), especially in central-parietal and fronto-temporal areas; beta activity was specifically enhanced among male participants, suggesting possible gender-based modulation of alertness or cognitive engagement at rest; delta power increases were mostly restricted to female and older participants in Segments 0 and 4, possibly reflecting deeper relaxation or age-related shifts in cortical dynamics; and theta and alpha bands showed fewer significant changes overall, with isolated effects in female and older subgroups.
These findings suggest a temporally stable yet spatially nuanced enhancement of high-frequency EEG activity (especially gamma) during resting-state periods, modulated subtly by gender, age, and experience. The results support the view that even during rest, intrinsic brain activity is dynamically organized across time and population subgroups.
4. Discussion
This study investigated spectral and spatial characteristics of resting-state EEG during meditation, with particular attention to how gender, age, and meditation experience modulate neural dynamics over time. Through rigorous statistical analysis, several key findings emerged: (1) consistent gamma-band enhancement across multiple segments and participant groups; (2) gender-based differences, with males showing increased beta activity and females exhibiting stronger gamma and delta effects; and (3) age-related elevation of high- and low-frequency power, particularly among older participants.
4.1. Gamma activity as a robust resting-state marker
Perhaps the most striking and temporally stable finding was the persistent enhancement of gamma-band power (30–45 Hz) across Segments 1–4 in nearly all subgroups. This supports a growing body of literature suggesting that gamma oscillations may serve as a neurophysiological marker of sustained internal attention and sensory integration during meditative and resting states [1,9]. The widespread topography of gamma activity—particularly in parietal and fronto-central regions—suggests involvement of large-scale neural assemblies supporting self-monitoring, spontaneous cognition, and possibly non-verbal imagery [10].
While gamma-band enhancements are often associated with task engagement, our results imply that such activity may persist even during non-task resting periods in meditators and non-meditators alike. This challenges traditional assumptions that resting-state EEG is predominantly low-frequency and may reflect intrinsic mental effort or readiness for awareness-based processing [5,11,12].
4.2. Gender differences in beta and gamma power
Gender-based distinctions in spectral profiles were observed both in group-level comparisons and across temporal segments. Males demonstrated greater beta activity (13–30 Hz), particularly in frontal and central regions, which could be interpreted as elevated alertness or executive monitoring, possibly linked to differential cognitive strategies during rest. In contrast, females exhibited consistently stronger gamma power across multiple scalp regions [13,14]. This aligns with prior evidence of gender-specific modulation of neural oscillations, potentially due to hormonal, structural, or attentional style differences [15].
Interestingly, delta increases in females during Segment 0 may reflect a more rapid engagement with relaxation or internally-directed mental states at the onset of the resting block. These findings warrant deeper investigation into gender-based neural strategies during undirected states.
4.3. Age effects and frontal gamma enhancement
Participants aged ≥35 years consistently exhibited stronger gamma power in frontal and central regions, with additional delta and theta enhancement in late segments. While age is often associated with reduced high-frequency activity, our findings suggest that in meditative or restful conditions, older adults may display compensatory increases in gamma oscillations. This may reflect age-related shifts toward more effortful maintenance of attention or engagement with internal imagery and self-referential thought [16,17].
Alternatively, the results may reflect cohort effects—such as greater familiarity with introspection or relaxation techniques in older participants—that were not fully captured by binary experience categorization [17,18].
4.4. Limited impact of meditation experience
Contrary to expectations, meditation experience did not significantly modulate spectral power in between-subject comparisons or regression models. Both novice and experienced meditators demonstrated similar patterns of gamma enhancement, suggesting that resting-state neural dynamics may reflect more general traits (e.g., attentional style or mental noise suppression) rather than specific training effects—at least within the short epochs analyzed here [19].
Nonetheless, segment-wise analyses did show topographic differences between groups in gamma distribution, hinting at subtle trait-level modulation that might be detected more robustly with longitudinal or task-embedded designs[19,20].
4.5. Temporal stability and segment dynamics
The use of temporally segmented t-tests provided nuanced insights into the dynamics of resting-state EEG. While Segment 0 showed sparse significance, likely reflecting transition into rest, subsequent segments revealed temporally stable gamma activity, suggesting rapid neural adaptation to internal states. This temporal pattern is consistent with prior findings that resting EEG stabilizes after initial adaptation [21,22].
Moreover, the persistence of spectral signatures across segments reinforces the value of time-resolved analysis in EEG, especially in naturalistic or semi-structured paradigms [23,24].
4.6. Limitations and future directions
Several limitations should be noted. First, the sample size, while balanced across key demographics, remains modest and may underpower subgroup comparisons, especially interaction effects [25-27]. Second, the self-report probes—used to anchor epochs—did not directly feed into the segment-wise analyses and may be further integrated in future models [28,29]. Third, although ICA was applied, residual artifacts may still influence spectral results, particularly in high-frequency bands [30-32].
Future work should consider longitudinal designs, connectivity-based metrics (e.g., phase coherence or graph metrics), and larger, age-diverse samples. The integration of behavioral or phenomenological reports could also clarify the cognitive correlates of observed spectral patterns. Furthermore, differentiating between spontaneous cognitive content (e.g., inner speech, visual imagery) during meditation could enrich interpretations of frequency-specific activity.
5. Conclusion
This study reveals a consistent pattern of gamma-band enhancement during resting-state meditation, modulated subtly by gender and age, but not strongly by meditation experience. These findings emphasize the richness and temporal stability of intrinsic neural oscillations and support the view that even during rest, the brain maintains dynamic, subgroup-specific patterns of cortical engagement. Future work may further clarify the cognitive and clinical implications of these patterns in meditative and psychiatric populations.
References
[1]. Cahn, B. R., & Polich, J. (2006). Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin, 132(2), 180–211. https: //doi.org/10.1037/0033-2909.132.2.180
[2]. Lutz, A., Greischar, L. L., Rawlings, N. B., Ricard, M., & Davidson, R. J. (2004). Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the National Academy of Sciences, 101(46), 16369–16373. https: //doi.org/10.1073/pnas.0407401101
[3]. Travis, F., & Shear, J. (2010). Focused attention, open monitoring and automatic self-transcending: Categories to organize meditations from Vedic, Buddhist and Chinese traditions. Consciousness and Cognition, 19(4), 1110–1118. https: //doi.org/10.1016/j.concog.2010.01.007
[4]. Lomas, T., Ivtzan, I., & Fu, C. H. Y. (2015). A systematic review of the neurophysiology of mindfulness on EEG oscillations. Neuroscience and Biobehavioral Reviews, 57, 401–410. https: //doi.org/10.1016/j.neubiorev.2015.09.018
[5]. Braboszcz, C., Cahn, B. R., Levy, J., Fernandez, M., & Delorme, A. (2017). Increased Gamma Brainwave Amplitude Compared to Control in Three Different Meditation Traditions. PloS One, 12(1), e0170647. https: //doi.org/10.1371/journal.pone.0170647
[6]. Berger, A. M., & Davelaar, E. J. (2018). Frontal Alpha Oscillations and Attentional Control: A Virtual Reality Neurofeedback Study. Neuroscience, 378, 189–197. https: //doi.org/10.1016/j.neuroscience.2017.06.007
[7]. Cahn, B. R., Delorme, A., & Polich, J. (2010). Occipital gamma activation during Vipassana meditation. Cognitive Processing, 11(1), 39–56. https: //doi.org/10.1007/s10339-009-0352-1
[8]. State-trait influences of Vipassana meditation practice on P3 EEG dynamics. (2019). In Progress in Brain Research (Vol. 244, pp. 115–136). Elsevier. https: //doi.org/10.1016/bs.pbr.2018.10.027
[9]. From alpha to gamma: Electrophysiological correlates of meditation-related states of consciousness. (2010). Medical Hypotheses, 75(2), 218–224. https: //doi.org/10.1016/j.mehy.2010.02.025
[10]. Assem, M., Hart, M. G., Coelho, P., Romero-Garcia, R., McDonald, A., Woodberry, E., Morris, R. C., Price, S. J., Suckling, J., Santarius, T., Duncan, J., & Erez, Y. (2023). High gamma activity distinguishes frontal cognitive control regions from adjacent cortical networks. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 159, 286–298. https: //doi.org/10.1016/j.cortex.2022.12.007
[11]. McQueen, B., Murphy, O. W., Fitzgerald, P. B., & Bailey, N. W. (2024). The Mindful Brain at Rest: Neural Oscillations and Aperiodic Activity in Experienced Meditators. Mindfulness, 15(10), 2484–2502. https: //doi.org/10.1007/s12671-024-02461-z
[12]. Berkovich-Ohana, A., Glicksohn, J., & Goldstein, A. (2012). Mindfulness-induced changes in gamma band activity – Implications for the default mode network, self-reference and attention. Clinical Neurophysiology, 123(4), 700–710. https: //doi.org/10.1016/j.clinph.2011.07.048
[13]. Jaušovec, N., & Jaušovec, K. (2010). Resting brain activity: Differences between genders. Neuropsychologia, 48(13), 3918–3925. https: //doi.org/10.1016/j.neuropsychologia.2010.09.020
[14]. Gender Differences in Spontaneous and Evoked Activities of the Human Brain | Human Physiology. (n.d.). Retrieved August 11, 2025, from https: //link.springer.com/article/10.1134/S0362119717040041
[15]. Thériault, R.-K., & Perreault, M. L. (2019). Hormonal regulation of circuit function: Sex, systems and depression. Biology of Sex Differences, 10(1), 12. https: //doi.org/10.1186/s13293-019-0226-x
[16]. Lutz, A., Greischar, L. L., Rawlings, N. B., Ricard, M., & Davidson, R. J. (2004). Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the National Academy of Sciences, 101(46), 16369–16373. https: //doi.org/10.1073/pnas.0407401101
[17]. Banks, J. C., Hariri, S., Kveraga, K., Ouyang, A., Gallagher, K., Quadri, S. A., Tesh, R. A., Reed, P. U., Thomas, R. J., Westover, M. B., Sun, H., & Subramaniam, B. (2025). Sleep-Based Brain Age Is Reduced in Advanced Inner Engineering Meditators. Mindfulness, 16(6), 1675–1692. https: //doi.org/10.1007/s12671-025-02583-y
[18]. Bakhtiari, A., Petersen, J., Urdanibia-Centelles, O., Ghazi, M. M., Fagerlund, B., Mortensen, E. L., Osler, M., Lauritzen, M., & Benedek, K. (2023). Power and distribution of evoked gamma oscillations in brain aging and cognitive performance. GeroScience, 45(3), 1523–1538. https: //doi.org/10.1007/s11357-023-00749-x
[19]. Duda, A. T., Clarke, A. R., & Barry, R. J. (2024). Mindfulness meditation alters neural oscillations independently of arousal. International Journal of Psychophysiology, 205, 112439. https: //doi.org/10.1016/j.ijpsycho.2024.112439
[20]. Lieberman, J. M., McConnell, P. A., Estarellas, M., & Sacchet, M. D. (2025). Neurophysiological mechanisms of focused attention meditation: A scoping systematic review. Imaging Neuroscience, 3, IMAG.a.14. https: //doi.org/10.1162/IMAG.a.14
[21]. Temporal segmentation of EEG based on functional connectivity network structure | Scientific Reports. (n.d.). Retrieved August 11, 2025, from https: //www.nature.com/articles/s41598-023-49891-8
[22]. Wiesman, A. I., da Silva Castanheira, J., & Baillet, S. (2022). Stability of spectral estimates in resting-state magnetoencephalography: Recommendations for minimal data duration with neuroanatomical specificity. NeuroImage, 247, 118823. https: //doi.org/10.1016/j.neuroimage.2021.118823
[23]. Michel, C. M., & Koenig, T. (2018). EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: A review. NeuroImage, 180, 577–593. https: //doi.org/10.1016/j.neuroimage.2017.11.062
[24]. Morales, S., & Bowers, M. E. (2022). Time-frequency analysis methods and their application in developmental EEG data. Developmental Cognitive Neuroscience, 54, 101067. https: //doi.org/10.1016/j.dcn.2022.101067
[25]. Deolindo, C. S., Ribeiro, M. W., Aratanha, M. A., Afonso, R. F., Irrmischer, M., & Kozasa, E. H. (2020). A Critical Analysis on Characterizing the Meditation Experience Through the Electroencephalogram. Frontiers in Systems Neuroscience, 14, 53. https: //doi.org/10.3389/fnsys.2020.00053
[26]. Vozzi, A., Ronca, V., Aricò, P., Borghini, G., Sciaraffa, N., Cherubino, P., Trettel, A., Babiloni, F., & Di Flumeri, G. (2021). The Sample Size Matters: To What Extent the Participant Reduction Affects the Outcomes of a Neuroscientific Research. A Case-Study in Neuromarketing Field. Sensors, 21(18), 6088. https: //doi.org/10.3390/s21186088
[27]. Osborn, M., Shankar, S., Szymanski, O., Gunningham, K., Caldwell, B., Perera, M. P. N., Michael, J., Wang, M., Fitzgerald, P. B., & Bailey, N. W. (2022). Meta-analysis Provides Weak Evidence for an Effect of Mindfulness on Neural Activity Related to Error-Processing in Healthy Individuals Only. Mindfulness, 13(12), 2907–2931. https: //doi.org/10.1007/s12671-022-02009-z
[28]. Rodriguez-Larios, J., Oca, E. A. B. M. de, & Alaerts, K. (2021). The EEG spectral fingerprints of meditation and mind wandering differ between experienced meditators and novices (p. 2021.07.06.451305). bioRxiv. https: //doi.org/10.1101/2021.07.06.451305
[29]. Frontiers | Prediction of Mind-Wandering with Electroencephalogram and Non-linear Regression Modeling. (n.d.). Retrieved August 11, 2025, from https: //www.frontiersin.org/journals/human-neuroscience/articles/10.3389/fnhum.2017.00365/full
[30]. Removal of Artifacts from EEG Signals: A Review—PMC. (n.d.). Retrieved August 11, 2025, from https: //pmc.ncbi.nlm.nih.gov/articles/PMC6427454/
[31]. Muthukumaraswamy, S. D. (2013). High-frequency brain activity and muscle artifacts in MEG/EEG: A review and recommendations. Frontiers in Human Neuroscience, 7, 138. https: //doi.org/10.3389/fnhum.2013.00138
[32]. Optimizing EEG ICA decomposition with data cleaning in stationary and mobile experiments | Scientific Reports. (n.d.). Retrieved August 11, 2025, from https: //www.nature.com/articles/s41598-024-64919-3
Cite this article
Song,Y. (2025). Resting-State EEG Dynamics During Mindfulness Meditation: Gamma-Band Enhancement and Subgroup Differences. Theoretical and Natural Science,138,1-12.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: Computational Modelling and Simulation for Biology and Medicine
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Cahn, B. R., & Polich, J. (2006). Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin, 132(2), 180–211. https: //doi.org/10.1037/0033-2909.132.2.180
[2]. Lutz, A., Greischar, L. L., Rawlings, N. B., Ricard, M., & Davidson, R. J. (2004). Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the National Academy of Sciences, 101(46), 16369–16373. https: //doi.org/10.1073/pnas.0407401101
[3]. Travis, F., & Shear, J. (2010). Focused attention, open monitoring and automatic self-transcending: Categories to organize meditations from Vedic, Buddhist and Chinese traditions. Consciousness and Cognition, 19(4), 1110–1118. https: //doi.org/10.1016/j.concog.2010.01.007
[4]. Lomas, T., Ivtzan, I., & Fu, C. H. Y. (2015). A systematic review of the neurophysiology of mindfulness on EEG oscillations. Neuroscience and Biobehavioral Reviews, 57, 401–410. https: //doi.org/10.1016/j.neubiorev.2015.09.018
[5]. Braboszcz, C., Cahn, B. R., Levy, J., Fernandez, M., & Delorme, A. (2017). Increased Gamma Brainwave Amplitude Compared to Control in Three Different Meditation Traditions. PloS One, 12(1), e0170647. https: //doi.org/10.1371/journal.pone.0170647
[6]. Berger, A. M., & Davelaar, E. J. (2018). Frontal Alpha Oscillations and Attentional Control: A Virtual Reality Neurofeedback Study. Neuroscience, 378, 189–197. https: //doi.org/10.1016/j.neuroscience.2017.06.007
[7]. Cahn, B. R., Delorme, A., & Polich, J. (2010). Occipital gamma activation during Vipassana meditation. Cognitive Processing, 11(1), 39–56. https: //doi.org/10.1007/s10339-009-0352-1
[8]. State-trait influences of Vipassana meditation practice on P3 EEG dynamics. (2019). In Progress in Brain Research (Vol. 244, pp. 115–136). Elsevier. https: //doi.org/10.1016/bs.pbr.2018.10.027
[9]. From alpha to gamma: Electrophysiological correlates of meditation-related states of consciousness. (2010). Medical Hypotheses, 75(2), 218–224. https: //doi.org/10.1016/j.mehy.2010.02.025
[10]. Assem, M., Hart, M. G., Coelho, P., Romero-Garcia, R., McDonald, A., Woodberry, E., Morris, R. C., Price, S. J., Suckling, J., Santarius, T., Duncan, J., & Erez, Y. (2023). High gamma activity distinguishes frontal cognitive control regions from adjacent cortical networks. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 159, 286–298. https: //doi.org/10.1016/j.cortex.2022.12.007
[11]. McQueen, B., Murphy, O. W., Fitzgerald, P. B., & Bailey, N. W. (2024). The Mindful Brain at Rest: Neural Oscillations and Aperiodic Activity in Experienced Meditators. Mindfulness, 15(10), 2484–2502. https: //doi.org/10.1007/s12671-024-02461-z
[12]. Berkovich-Ohana, A., Glicksohn, J., & Goldstein, A. (2012). Mindfulness-induced changes in gamma band activity – Implications for the default mode network, self-reference and attention. Clinical Neurophysiology, 123(4), 700–710. https: //doi.org/10.1016/j.clinph.2011.07.048
[13]. Jaušovec, N., & Jaušovec, K. (2010). Resting brain activity: Differences between genders. Neuropsychologia, 48(13), 3918–3925. https: //doi.org/10.1016/j.neuropsychologia.2010.09.020
[14]. Gender Differences in Spontaneous and Evoked Activities of the Human Brain | Human Physiology. (n.d.). Retrieved August 11, 2025, from https: //link.springer.com/article/10.1134/S0362119717040041
[15]. Thériault, R.-K., & Perreault, M. L. (2019). Hormonal regulation of circuit function: Sex, systems and depression. Biology of Sex Differences, 10(1), 12. https: //doi.org/10.1186/s13293-019-0226-x
[16]. Lutz, A., Greischar, L. L., Rawlings, N. B., Ricard, M., & Davidson, R. J. (2004). Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the National Academy of Sciences, 101(46), 16369–16373. https: //doi.org/10.1073/pnas.0407401101
[17]. Banks, J. C., Hariri, S., Kveraga, K., Ouyang, A., Gallagher, K., Quadri, S. A., Tesh, R. A., Reed, P. U., Thomas, R. J., Westover, M. B., Sun, H., & Subramaniam, B. (2025). Sleep-Based Brain Age Is Reduced in Advanced Inner Engineering Meditators. Mindfulness, 16(6), 1675–1692. https: //doi.org/10.1007/s12671-025-02583-y
[18]. Bakhtiari, A., Petersen, J., Urdanibia-Centelles, O., Ghazi, M. M., Fagerlund, B., Mortensen, E. L., Osler, M., Lauritzen, M., & Benedek, K. (2023). Power and distribution of evoked gamma oscillations in brain aging and cognitive performance. GeroScience, 45(3), 1523–1538. https: //doi.org/10.1007/s11357-023-00749-x
[19]. Duda, A. T., Clarke, A. R., & Barry, R. J. (2024). Mindfulness meditation alters neural oscillations independently of arousal. International Journal of Psychophysiology, 205, 112439. https: //doi.org/10.1016/j.ijpsycho.2024.112439
[20]. Lieberman, J. M., McConnell, P. A., Estarellas, M., & Sacchet, M. D. (2025). Neurophysiological mechanisms of focused attention meditation: A scoping systematic review. Imaging Neuroscience, 3, IMAG.a.14. https: //doi.org/10.1162/IMAG.a.14
[21]. Temporal segmentation of EEG based on functional connectivity network structure | Scientific Reports. (n.d.). Retrieved August 11, 2025, from https: //www.nature.com/articles/s41598-023-49891-8
[22]. Wiesman, A. I., da Silva Castanheira, J., & Baillet, S. (2022). Stability of spectral estimates in resting-state magnetoencephalography: Recommendations for minimal data duration with neuroanatomical specificity. NeuroImage, 247, 118823. https: //doi.org/10.1016/j.neuroimage.2021.118823
[23]. Michel, C. M., & Koenig, T. (2018). EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: A review. NeuroImage, 180, 577–593. https: //doi.org/10.1016/j.neuroimage.2017.11.062
[24]. Morales, S., & Bowers, M. E. (2022). Time-frequency analysis methods and their application in developmental EEG data. Developmental Cognitive Neuroscience, 54, 101067. https: //doi.org/10.1016/j.dcn.2022.101067
[25]. Deolindo, C. S., Ribeiro, M. W., Aratanha, M. A., Afonso, R. F., Irrmischer, M., & Kozasa, E. H. (2020). A Critical Analysis on Characterizing the Meditation Experience Through the Electroencephalogram. Frontiers in Systems Neuroscience, 14, 53. https: //doi.org/10.3389/fnsys.2020.00053
[26]. Vozzi, A., Ronca, V., Aricò, P., Borghini, G., Sciaraffa, N., Cherubino, P., Trettel, A., Babiloni, F., & Di Flumeri, G. (2021). The Sample Size Matters: To What Extent the Participant Reduction Affects the Outcomes of a Neuroscientific Research. A Case-Study in Neuromarketing Field. Sensors, 21(18), 6088. https: //doi.org/10.3390/s21186088
[27]. Osborn, M., Shankar, S., Szymanski, O., Gunningham, K., Caldwell, B., Perera, M. P. N., Michael, J., Wang, M., Fitzgerald, P. B., & Bailey, N. W. (2022). Meta-analysis Provides Weak Evidence for an Effect of Mindfulness on Neural Activity Related to Error-Processing in Healthy Individuals Only. Mindfulness, 13(12), 2907–2931. https: //doi.org/10.1007/s12671-022-02009-z
[28]. Rodriguez-Larios, J., Oca, E. A. B. M. de, & Alaerts, K. (2021). The EEG spectral fingerprints of meditation and mind wandering differ between experienced meditators and novices (p. 2021.07.06.451305). bioRxiv. https: //doi.org/10.1101/2021.07.06.451305
[29]. Frontiers | Prediction of Mind-Wandering with Electroencephalogram and Non-linear Regression Modeling. (n.d.). Retrieved August 11, 2025, from https: //www.frontiersin.org/journals/human-neuroscience/articles/10.3389/fnhum.2017.00365/full
[30]. Removal of Artifacts from EEG Signals: A Review—PMC. (n.d.). Retrieved August 11, 2025, from https: //pmc.ncbi.nlm.nih.gov/articles/PMC6427454/
[31]. Muthukumaraswamy, S. D. (2013). High-frequency brain activity and muscle artifacts in MEG/EEG: A review and recommendations. Frontiers in Human Neuroscience, 7, 138. https: //doi.org/10.3389/fnhum.2013.00138
[32]. Optimizing EEG ICA decomposition with data cleaning in stationary and mobile experiments | Scientific Reports. (n.d.). Retrieved August 11, 2025, from https: //www.nature.com/articles/s41598-024-64919-3









