1. Introduction
Huntington's disease is a monogenic dominant neurodegenerative disorder caused by the accumulation of mutant huntingtin protein. Patients with this disease exhibit symptoms such as neuronal damage, movement disorders (dystonia), and cognitive decline. The majority of patients die within 10 to 25 years after the onset of symptoms. The genetic defect kills the neurons in the brain, and death is not the end of Huntington's chorea. One or more of the patient's descendants will inevitably develop the disease. Huntington's chorea most commonly occurs between the ages of 30 and 50. The accumulation of proteins has the most significant impact on neurons in the striatum and cortex, which control human activities, cognition, and spirit. The symptoms of the disease revolve around these three parts, but they are not so obvious in the early stages. In 50% of cases, emotional instability, anxiety, and mild depression are the first symptoms. During the early stage of the disease, a large accumulation of huntingtin protein leads to damage to neurons in the striatum and cortex, which control human activities, cognition, and spirit. Later symptoms also revolve around these three parts. At the beginning, involuntary twitching of the face, fingers, and toes occurs. Due to the relatively small number of damaged neurons at the beginning, these movements are not obvious, and patients often do not notice their abnormalities. In the middle stage of the disease, patients develop dystonia, causing twisting and repetitive movements throughout the body, resembling dancing. This is also the origin of the name "dancing disease." The neurons in the brain are also severely damaged, which leads to the inability of patients to think, plan, and engage in complex interactions at this stage. In the late stage, muscle spasms and twitches have spread to the mouth and throat, and the patient's respiratory function is severely affected. The patient gradually loses the ability to eat and drink, and this severe damage increases the risks of malnutrition and aspiration pneumonia and may lead to asphyxiation and death at any time. Current medical treatments include symptomatic drug therapy and non-drug intervention. Antidopamine drugs in drug therapy can alleviate chorea, but they may aggravate cognitive impairment. Antidepressants can manage mental symptoms, but they cannot improve the disease progression. Non-drug intervention includes deep brain stimulation therapy, which can improve motor function temporarily but has a gradually declining long-term efficacy. So far, all treatment methods cannot reduce the load of huntingtin protein and neuronal death. Based on this, this article will focus on summarizing the targeted therapies for mHTT clearance in the new era, which represents a disruptive direction for the treatment of Huntington's disease.
2. The key target of ccRCC and traditional therapy
In the urinary system, RCC is the most lethal malignancy. With the development and wide use of imaging technologies, many more RCC cases are detected in asymptomatic patients. Discovery of many ccRCC cases shows significant geographical variation globally and occurs more frequently in males and older individuals. Smoking, obesity, hypertension, and chronic kidney disease are considered major risk factor. Due to the lack of specific symptoms in the early stages, effective screening and early diagnosis remain challenging, which highlight the importance of identifying biomarkers and understanding the underlying molecular mechanisms.
ccRCC primarily originates from the proximal tubular epithelial cell of the nephron. Its tumorigenesis and progression are heavily dependent on a series of genetic and epigenetic alternations, with VHL tumor suppressor gene acting as the central initial event. A specific region of VHL gene encodes pVHL, which is responsible for the degradation of hypoxi-inducible factors (HIF-a subunits). Under normoxic conditions, HIF-a proteins enable identification and tagging with ubiquitin by pVHL, resulting in their subsequent degradation. In contrast, under hypoxia conditions or when VHL is mutated or deleted, HIF-a subunits escape degradation and accumulate in the nucleus and activate transcription of various target genes. Nearly all ccRCC associated VHL mutations disrupt this regulatory mechanism, resulting in dysregulated HIF signalling.
There are two types of HIF-a, which are HIF-1a and HIF-2a. HIF-1a suppresses the growth of tumor and is subject to homozygous loss or aberrant splicing in some cases of ccRCC, leading to loss of function. While HIF-2a functions as an oncogenic driver in ccRCC. The aberrant stabilization of HIF-2a activates the expression of multiple oncogenic genes, such as VEDF, MET, AXL, and c-KIT. These downstream signaling pathways promote tumor angiogenesis, stimulation of stromal cells, and enhanced tumor cell migration, thereby shaping a tumor-supportive microenvironment. In turn, this microenvironment facilitates upregulation of downstream factors, thereby activating immune checkpoint pathways that enable the tumor to evade immune surveillance.
Current treatment of ccRCC mainly follows two major approaches: TKIs and ICIs. TKIs block tumor growth and angiogenesis by inhibiting receptor tyrosine kinases such as VEGFR, PDGFR, MET, and AXL. ICIs can block inhibitory signals to enhance and recover T-cell's activity. As a result, the immune system is able to eliminate cancer cells.
Although TKIs and ICIs have given substantial benefits to patients with ccRCC, clinical data reveal that TKIs commonly cause adverse effects such as hypoxia,nausea, and fatigue [1]. ICIs carry a higher risk of toxicity, including immune-related adverse events such as pneumonitis and colitis [2]. As a result, recent research has increasingly focused on new therapeutic targets, particularly strategies that directly target the VHL–HIF pathway. One of the most promising advances in this area is Belzutifan, which is specifically designed to target tumorigenic mechanisms in the context of VHL loss.
3. The therapeutic mechanism of Belzutifan
To get to know how Belzutifan work, the structure of the HIF-2a is important to learn firstly (Figure 1). Under normal oxygenated conditions, which is called normoxia. HIF-2α is first hydroxylated by (PHD). The hydroxylated HIF-2α is then targeted by VHL, ubiquitinated, and subsequently degraded by the proteasome. At this stage, HIF-2α cannot bind to ARNT via its PAS-B domain, and the entire process functions normally. In patients with ccRCC, as the VHL gene is accidentally lost or mutated, the protein becomes inactivated. In the end, even after hydroxylation, HIF-2α cannot be correctly recognized and bound by VHL, leading to sustained activation of the HIF pathway. This causes pathological accumulation of HIF-2α in the cell—a condition known as pseudo-hypoxia because it mimics the outcome of true hypoxia. Under these conditions, ARNT binds to an area of HIF-2α, which is called PAS-B domain, to generate a heterodimer that stimulates transcription of the tumor-promoting genes. Belzutifan acts by binding to the site which the ARNT binds, and competitively inhibiting its binding with ARNT, eventually suppressing the tumor-promoting effects of excessive HIF-2α.
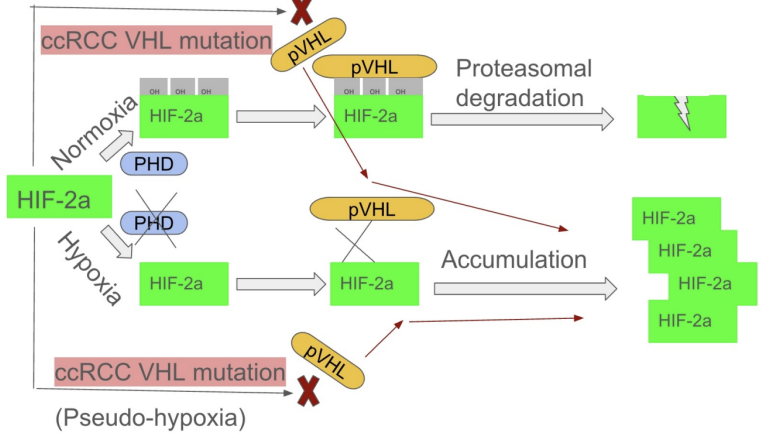
In normoxia, PHD enzymes hydroxylate HIF-2α, enabling VHL to bind and trigger its degradation. In hypoxia, PHDs are inactive, allowing HIF-2α to accumulate, bind ARNT via its PAS-B domain, and activate gene transcription. In VHL-deficient ccRCC, HIF-2α is not degraded even when hydroxylated, leading to its abnormal buildup and continuous activation of the HIF pathway.
4. Discussion
Belzutifan is a small molecule medicine that can specifically target HIF-2α. Belzutifan can be taken orally. As a second-generation HIF-2α–targeted agent, Belzutifan exhibits high selectivity and high potency, with higher AUC and Cmax values and lower variability in drug concentrations in clinical studies [4], which allows for significant antitumor effects at lower doses. In addition, unlike conventional agents such as TKIs and ICIs, which act on downstream signaling molecules of the HIF-2α pathway or function as indirect inhibitors, Belzutifan directly targets the transcription factor and blocks the activity of HIF-2α in the situation of VHL loss. Our preclinical trail reveals the Belzutifan’s efficacy and its drug properties through three aspects, pharmacology, tolerability, and clinical readiness. Belzutifan interferes with the HIF-2a and ARNT dimer formation. It is demonstrated that Belzutifan suppresses HIF-2α–dependent transcriptional activity with an IC50 of about 17 nM. In VHL-deficient cell lines [5], Belzutifan dose-dependently regulates the genes which are targeted by HIF-2a(e.g., VEGFA, EPO, Cyclin D1). As a highly selective and orally bioavailable small molecular HIF-2a inhibitor, Belzutifan demonstrates a nanomolar-level binding affinity and produces significant antitumor activity with sustained downstream target gene suppression in VHL-deficient renal cancer lines and animal models. These consistent pharmacodynamic and pharmacokinetic characteristics have laid a foundation for its entry into clinical research, driving a series of phase I, II, and III trails. These cynical tails make an evaluation of its efficacy and safety both as a monotherapy and a combination regimen across diverse patient populations.
The phase I trial research of Belzutifan is examining the patients who have advanced solid tumors and those tumors have been treated with multiple regimens. To treat these patients, researchers use a method that splits people in groups and administers the drug at a low dose firstly, then gradually increases. With each group of patients, monitoring for the adverse effects and tolerability. Another method is also useful to test for the phase I trail. More patients are enrolled and treated with the recommended dose to evaluate the drug’s efficacy in specific tumor types or patient populations. This trial shows an objective response rate of ccRCC 25%. Through this trial, Belzutifan has no dose-limiting toxicity. According to Jonasch et al., the long follow-up of the phase I trail shows an ORR of 25%, which includes the complete response of 1.8% and the partial response of 23.6%.This drug’s therapeutic response is durable, with the majority of responding patients still benefiting. In conclusion, the phase I trial research of the Belzutifan shows its durable anti-tumor activity as a HIF-2a, and it is significant that this is the first-in-human data to confirm the safety of the new small molecular drug [6]. Confirming the favorable safety and efficacy of Belzutifan, the phase II trial is needed now to further evaluate the Belzutifan’s anti-tumor activity and safety in a larger scale of patients population. In the open-label phase II trial, patients received an oral dose of 120mg per day. This trial enrolled patients with VHL-related RCC. The objective response rate of ccRCC is 49%, which includes a complete response of 0% and the partial response of 49%. Same as the phase I trial, The phase II trial also claims that the drug’s therapeutic response is durable, with the majority of responding patients still benefiting [7]. For the test of safety of Belzutifan in phase II trial, the side effects reported are fatigue, nausea, headache, and dizziness. These reported adverse effects are acceptable and manageable, most of whom are grade 1 or 2. There were some more serious side effects, but only 33% of patients. The good news is that no treatment-related death occurred in this research [7]. According to Jonasch st al.’s report, from the follow-up research of phase I trial, Belzutifan maintained good tolerability and with no serious side effects and new issues observed [6]. That means the overall safety is favorable for the following research for the combination of the Belzutifan and other drugs.
After knowing that the Belzutifan has a great efficacy and a favorable tolerability. The research is then focused on how it works and performs in combination with other drugs. The phase III clinical trial is about the combination of Belzutifan and Cabozantinib. This trial was targeted on the patients with advanced ccRCC that was previously untreated or pretreated with immunotherapy. The study gave patients with Belzutifan of 120mg orally per day and Cabozantinib of 60mg per day until an unacceptable adverse effect occurred. The results show that the objective response rate is 70%, which includes the complete response of 8% and the partial response of 62% [8]. For this trial, when Belzutifan is combined with Cabozantinib, the adverse events included hypertension, which is grade 3-4, fatigue, and anemia. There is no treatment- related death observed in this trial and 14% of patients had serious adverse events [8].
From all the phase I, II, and II trials, the Belzutifan shows favorable tolerability. Also the adverse effects are largely under control. The grade >=3 adverse events are infrequent. Dose-limiting and dose-related toxicity are rare and there are no treatment-related deaths observed. Forethermore, there are no significant off-target effects observed due to Belzutifan’s high selectivity of HIF-2aPAS-B domain.
Studying the ccRCC’s mechanism and the methodology of Belzutifan. It reveals that Belzutifan has many advantages, for example it has high selectivity of HIF-2a, which makes it only target on the specific transcription factor, not to mistakenly target on other molecules, this maintains that there are no off-target effects in clinical research. The other good thing about it is that Belzutifan has better safety than other current drugs that treat ccRCC. Its adverse effects are manageable and mostly grade <=3. Unlike other drugs for example TKIs, which have side effects include cardiovascular and congestive heart failure, Cardiac damage from TKIs is also common [9]. Another example is ICIs. ICIs side effects are mostly immune-related and high-grade [2]. Belzutifan is well tolerated in both monotherapy and in combination with Cabozantinib.
5. Conclusion
Belzutifan has emerged as a highly effective and clinically feasible inhibitor of HIF-2α, demonstrating significant promise in the treatment of clear cell renal cell carcinoma (ccRCC). However, recent clinical observations have identified the emergence of resistance mutations, specifically the G323E mutation in HIF-2α and the F446L mutation in ARNT, following prolonged administration of Belzutifan. Structural insights obtained through X-ray crystallography reveal that these mutations induce conformational changes in the PAS-B domain, resulting in a partially disordered and open configuration that reduces drug binding affinity. These findings underscore the dynamic evolutionary capacity of HIF-2α under therapeutic pressure and highlight a critical challenge in sustaining long-term efficacy.
This evolving resistance mechanism reinforces the necessity for continued innovation in the structural-based drug design of HIF-2α inhibitors. Future research should prioritize the development of next-generation compounds capable of effectively targeting these mutated forms, particularly by enhancing inhibitory effects on the protein–protein interaction between HIF-2α and ARNT. Additionally, achieving greater selectivity to minimize off-target effects on other HIF subtypes, such as HIF-1α, will be essential to improving therapeutic safety and efficacy.
Further structural and biophysical studies focusing on the PAS-B domain in its mutated state will be invaluable for designing molecules that can adapt to the altered binding environment. Concurrently, efforts should be directed toward optimizing treatment regimens, identifying predictive biomarkers for patient response, and elucidating mechanisms of resistance to guide clinical decision-making. Ultimately, the development of novel inhibitors that effectively engage the mutated PAS-B pocket represents a strategic imperative for advancing the treatment landscape of ccRCC and maximizing the clinical benefit of HIF-2α–targeted therapies.
References
[1]. Atkins, M. B., & Tannir, N. M. (2018). Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer treatment reviews, 70, 127–137.
[2]. Fiorentino, V., Tralongo, P., Larocca, L. M., Pizzimenti, C., Martini, M., & Pierconti, F. (2023). First-line ICIs in renal cell carcinoma. Human vaccines & immunotherapeutics, 19(2), 2225386.
[3]. Choi, W. W., Boland, J. L., Kalola, A., & Lin, J. (2023). Belzutifan (MK-6482): Biology and Clinical Development in Solid Tumors. Current oncology reports, 25(2), 123–129..
[4]. Suárez, C., Vieito, M., Valdivia, A., González, M., & Carles, J. (2023). Selective HIF2A Inhibitors in the Management of Clear Cell Renal Cancer and Von Hippel-Lindau-Disease-Associated Tumors. Medical sciences (Basel, Switzerland), 11(3), 46.
[5]. Ren, X., Diao, X., Zhuang, J., & Wu, D. (2022). Structural basis for the allosteric inhibition of hypoxia-inducible factor (HIF)-2 by belzutifan. Molecular pharmacology, 102(6), MOLPHARM-AR-2022-000525.
[6]. Jonasch, E., Bauer, T. M., Papadopoulos, K. P., Plimack, E. R., Merchan, J. R., McDermott, D. F., Dror Michaelson, M., Appleman, L. J., Roy, A., Perini, R. F., Liu, Y., & Choueiri, T. K. (2024). Phase I LITESPARK-001 study of belzutifan for advanced solid tumors: Extended 41-month follow-up in the clear cell renal cell carcinoma cohort. European journal of cancer (Oxford, England : 1990), 196, 113434.
[7]. Jonasch, E., Donskov, F., Iliopoulos, O., Rathmell, W. K., Narayan, V. K., Maughan, B. L., Oudard, S., Else, T., Maranchie, J. K., Welsh, S. J., Thamake, S., Park, E. K., Perini, R. F., Linehan, W. M., Srinivasan, R., & MK-6482-004 Investigators (2021). Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease. The New England journal of medicine, 385(22), 2036–2046.
[8]. Choueiri, T. K., Merchan, J. R., Figlin, R., McDermott, D. F., Arrowsmith, E., Michaelson, M. D., Tykodi, S. S., Heath, E. I., Spigel, D. R., D'Souza, A., Kassalow, L., Perini, R. F., Vickery, D., & Bauer, T. M. (2025). Belzutifan plus cabozantinib as first-line treatment for patients with advanced clear-cell renal cell carcinoma (LITESPARK-003): an open-label, single-arm, phase 2 study. The Lancet. Oncology, 26(1), 64–73. https: //doi.org/10.1016/S1470-2045(24)00649-1
[9]. Lai, Y., Zhao, Z., Zeng, T., Liang, X., Chen, D., Duan, X., Zeng, G., & Wu, W. (2018). Crosstalk between VEGFR and other receptor tyrosine kinases for TKI therapy of metastatic renal cell carcinoma. Cancer cell international, 18, 31.
Cite this article
Li,Y. (2025). The Target Area of Belzutifan in Towards Clear Cell Renal Cell Carcinoma (ccRCC). Theoretical and Natural Science,137,163-168.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Atkins, M. B., & Tannir, N. M. (2018). Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer treatment reviews, 70, 127–137.
[2]. Fiorentino, V., Tralongo, P., Larocca, L. M., Pizzimenti, C., Martini, M., & Pierconti, F. (2023). First-line ICIs in renal cell carcinoma. Human vaccines & immunotherapeutics, 19(2), 2225386.
[3]. Choi, W. W., Boland, J. L., Kalola, A., & Lin, J. (2023). Belzutifan (MK-6482): Biology and Clinical Development in Solid Tumors. Current oncology reports, 25(2), 123–129..
[4]. Suárez, C., Vieito, M., Valdivia, A., González, M., & Carles, J. (2023). Selective HIF2A Inhibitors in the Management of Clear Cell Renal Cancer and Von Hippel-Lindau-Disease-Associated Tumors. Medical sciences (Basel, Switzerland), 11(3), 46.
[5]. Ren, X., Diao, X., Zhuang, J., & Wu, D. (2022). Structural basis for the allosteric inhibition of hypoxia-inducible factor (HIF)-2 by belzutifan. Molecular pharmacology, 102(6), MOLPHARM-AR-2022-000525.
[6]. Jonasch, E., Bauer, T. M., Papadopoulos, K. P., Plimack, E. R., Merchan, J. R., McDermott, D. F., Dror Michaelson, M., Appleman, L. J., Roy, A., Perini, R. F., Liu, Y., & Choueiri, T. K. (2024). Phase I LITESPARK-001 study of belzutifan for advanced solid tumors: Extended 41-month follow-up in the clear cell renal cell carcinoma cohort. European journal of cancer (Oxford, England : 1990), 196, 113434.
[7]. Jonasch, E., Donskov, F., Iliopoulos, O., Rathmell, W. K., Narayan, V. K., Maughan, B. L., Oudard, S., Else, T., Maranchie, J. K., Welsh, S. J., Thamake, S., Park, E. K., Perini, R. F., Linehan, W. M., Srinivasan, R., & MK-6482-004 Investigators (2021). Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease. The New England journal of medicine, 385(22), 2036–2046.
[8]. Choueiri, T. K., Merchan, J. R., Figlin, R., McDermott, D. F., Arrowsmith, E., Michaelson, M. D., Tykodi, S. S., Heath, E. I., Spigel, D. R., D'Souza, A., Kassalow, L., Perini, R. F., Vickery, D., & Bauer, T. M. (2025). Belzutifan plus cabozantinib as first-line treatment for patients with advanced clear-cell renal cell carcinoma (LITESPARK-003): an open-label, single-arm, phase 2 study. The Lancet. Oncology, 26(1), 64–73. https: //doi.org/10.1016/S1470-2045(24)00649-1
[9]. Lai, Y., Zhao, Z., Zeng, T., Liang, X., Chen, D., Duan, X., Zeng, G., & Wu, W. (2018). Crosstalk between VEGFR and other receptor tyrosine kinases for TKI therapy of metastatic renal cell carcinoma. Cancer cell international, 18, 31.









