1. Introduction
It is well known that anxiety and depression are prevalent mental disorders around the world. According to the World Health Organization, an estimated 3.8% of the population (including about 280 million adults) experiences depression, and 300 million individuals suffer from anxiety. More than 720,000 people die due to suicide every year. These disorders impair quality of life and daily functioning, leading to increased risks of self-harm, and can ultimately lead to suicide. As a result, these disorders impose a heavy economic burden due to reduced productivity and increased healthcare costs. Thus, there is an urgent need for effective treatments, highlighting a critical public health concern. However, existing medications fail to provide sufficient relief for all patients. For instance, aselective serotonin reuptake inhibitors (SSRIs) are widely used nowadays [1]. SSRIs functions by blocking the reuptake of serotonin in the synaptic gap, which makes serotonin remains between neurons for a longer time to promote transmission of the signals. This increases serotonin availability and improves mood in depressed patients. However, there is usually a delay of 2 - 6 weeks before clinical effects appear, which is why many patients choose to discontinue treatment. Besides, up to 30-40% of patients show only partial or no response. Moreover, there are several side effects such as weight gain, sexual dysfunction, and sleep disturbances [2]. Such adverse effects often lead to poor treatment adherence. Psychological interventions, such as cognitive behavioural therapy (CBT), are effective but still have limitations. They are usually expensive and require long-term commitment. These challenges emphasise the need for new therapeutic strategies. To address this challenge, researchers have shifted their focus toward the neurobiological mechanisms underlying mood regulation. They have found that disabilities in emotional regulation are closely associated with neuroplasticity, especially in the hippocampus. It is widely recognised for its role in memory formation, spatial navigation, and emotional processes. Within the hippocampus, radial neural stem cells (rNSCs) continue to generate new neurons in the dentate gyrus (DG). The supramammillary nucleus (SuM) projects to the DG as well as to the medial septum and lateral hypothalamus, which makes the SuM significant in linking arousal, reward, and memory circuits. This process is known as adult hippocampal neurogenesis (AHN). An increasing number of studies have demonstrated that AHN plays an important role in cognitive functions and emotional regulation [3,4]. In particular, stress resilience and mood regulation can be improved by enhancing AHN. However, the underlying neuromodulators that regulate AHN are not fully understood. Although in previous studies, they have shown that signallings sent from the SuM neurons to DG in the hippocampus can activate rNSCs and promote their proliferation, which is driven by glutamate, not GABA, neuropeptides like substance P (SP) have gained attention as potential regulators of hippocampal plasticity [5]. While glutamatergic inputs can enhance AHN, SP may act as an opposing signal [6]. It is being released from the SuM and functioning via neurokinin-1 receptor (NK1-R), modulating stress, pain and emotions [7,8]. Although the involvement of SP in emotional regulation has already been recognized [9,10], there remains a knowledge gap as to whether SP influences anxiety and depression-like behaviour by modulating AHN. Therefore, discovering the mechanism by which SP modulates AHN and its effects on anxiety and depression may deepen our understanding of the pathophysiology of emotional disorders and pave the way for new therapeutic targets.
2. Experimental design
This experiment was designed to investigate the role of SP in regulating AHN through the SuM-DG pathway and its effects on depression and anxiety-like behaviour. Nestin-GFP mice were randomly divided into three groups. The control group was established for better comparison and received vehicle injection and saline treatment; the over-expression (OE) group was established to examine the effect of increased SP signalling and received stereotaxic injection of AAV-hSyn-SP. AAV is commonly used for efficient gene delivery into neurons. It has low immunogenicity and long-term expression, making it ideal for in vivo studies. The hSyn promoter can ensure neuron-specific expression, avoiding non-specific effects in glial cells. Spread of virus can be tracked by co-expressing a fluorescent marker like GFP. While the receptor antagonist (RA) group was included to evaluate the effect of blocking the receptor of SP, NK1-R. L822429 is a selective antagonist of NK1-R and it has been shown to reduce anxiety and depression-like behaviour. Repeated daily injections allowed for short-term, reversible blockade of SP signalling. Then, behaviour tests were carried out to test for behaviour of the mice. In open field test (OFT), total distance travelled by mice was measured to reflect their locomotor activity and reduced centre exploration may result from anxiety or less motivation. In elevated plus maze (EPM), number of entries in open arm was used to evaluate level of anxiety. In sucrose preference test (SPT), total fluid intake was measured to calculate sucrose preference of the mice. In forced swim test (FST), immobility time was analysed the level of depression.
2.1. Animal model
Nestin-GFPA mice were chosen as the experimental model to visualise rNSCs and neural progenitors in the DG, which allowed tracking of neurogenesis and cell proliferation via fluorescence imaging. 8-10-week-old male mice were selected to reduce hormonal variability.
2.2. Viral injection
For the OE group, as shown in Figure 1a, AAV-hSyn-SP was stereotaxically injected into the SuM region under anaesthesia [11]. The injection was performed at a rate of 30-50 nl/min with a volume of 0.3-0.5μl per site to avoid diffusion. Then allowed 3-4 weeks recovery for stable viral expression. All operations were carried out under sterile conditions to minimise the risk of infection.
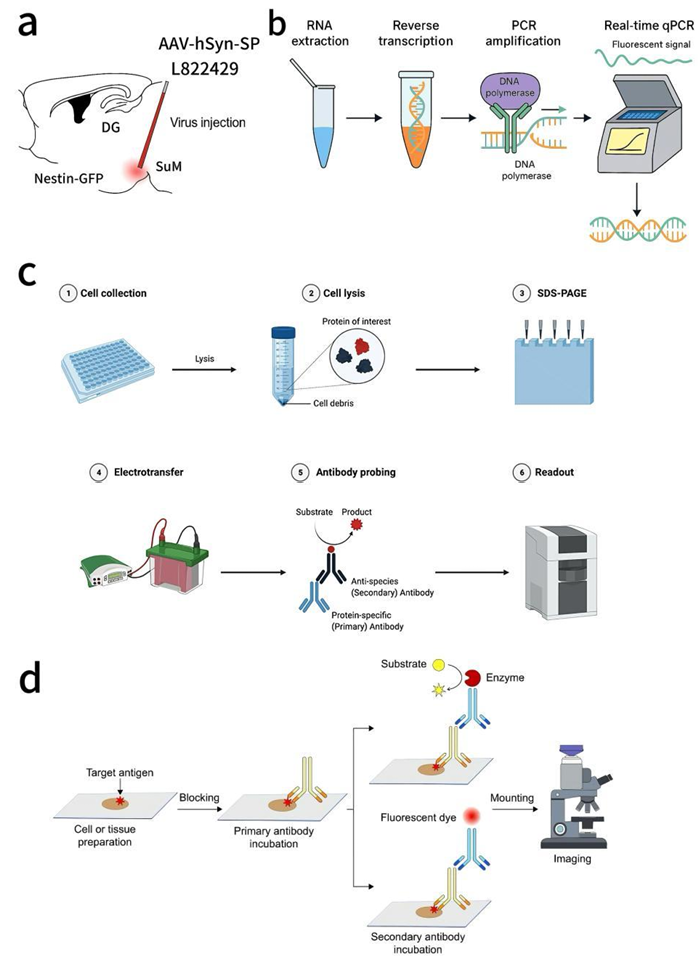
Diagram of AAV-hSyn-SP and L822429 injected stereotaxically into the SuM region. (b-c) Testings for level of mRNA (b) RT-qPCR and expressed SP (c) Western blot. (d) Procedure of immunostaining.
2.3. Drug administration
For the RA group, in Figure 1a, L822429 was injected into the SuM region once per day after the viral recovery period (Day 21-28). The control and OE group received an equal volume of saline during the same period to ensure that all groups undergo similar injection procedure and eliminate stress-related confounding factors.
2.4. RT-qPCR shown in Figure 1b
Step 1: Extract and purify RNA from tissues.
Step 2: RNA was converted into complementary DNA (cDNA) using reverse transcriptase.
Step 3: Perform PCR amplification using specific primers and fluorescent dyes.
Step 4: Monitor the amplification in real time via fluorescent signals. Fluorescence intensity correlated with the amount of DNA, which allowed us to quantify gene expression levels accurately.
2.5. Western blot shown in Figure 1c
Step 1: Harvest cells or tissues containing SP.
Step 2: Break open cells with lysis buffer to release proteins, debris was removed by centrifugation.
Step 3: Separate proteins by size using gel electrophoresis.
Step 4: Transfer separated proteins from the gel to a PVDF or nitrocellulose membrane.
Step 5: Block non-specific sites on the membrane. Add primary antibody to bind the target protein. Add enzyme-linked secondary antibody for detection.
Step 6: Visualise protein bands using a chemiluminescence imaging system.
2.6. Behaviour tests
Behaviour tests were conducted during Day 28-35. A 24-hour interval was maintained between each behaviour test to avoid fatigue. Mice were provided with adequate water and food except during the sucrose preference test.
2.6.1. Anxiety tests
In Figure 2a, open field test was carried out in a 50*50 cm square chamber, consisting a peripheral and central zone.The mice were placed in the centre of the chamber, and their free exploration was recorded for 5-10 minutes using a tracking system. Time spent in two zones reflects the level of anxiety.
In Figure 2b, the plus maze was consisted of two open arms and two closed arms (each 30*5 cm), elevated 50 cm above the ground. The mice were placed on the central platform facing an open arm and allowed to explore freely for 5 minutes. We recorded the time spent and number of entries into open and closed arms.
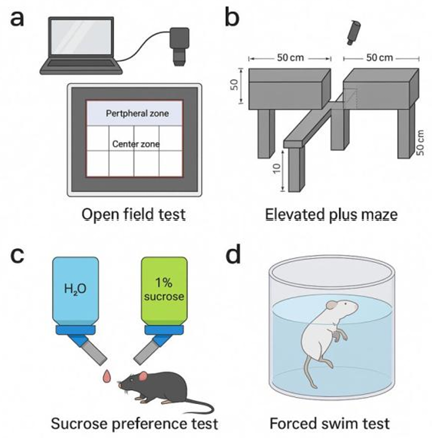
2.6.2. Depression tests
In sucrose preference test, 24 hours before official testing, two bottles were filled with water. This allowed the mice to become familiar with the setup and reduces novelty-induced stress. In Figure 2c, one of the bottles was switched into 1% of sucrose solution, switching positions daily to prevent side bias. Sucrose preference was calculated by using the formula sucrose intake/total fluid intake *100% [12].
In forced swim test, A transparent cylindrical tank with 20-25 cm diameter, 15-20 cm water depth, and 25±1°C water temperature. In Figure 2d, each mice was placed into the water tank individually for 6 minutes. The entire procedure was video-recorded, and immobility time during last 4 minutes was analysed [13].
2.7. AHN measurement
After behaviour tests were finished (Day 35), the mice were anesthetised and transcardially perfused with saline and 4% paraformaldehyde (PFA). In Figure 1d, Nestin⁺ (rNSCs), DCX⁺ (immature neurons), and Ki67⁺ (proliferating cells) were labelled by using immunostaining [14]. Images of the DG were captured under a con-focal microscope and cell densities were analysed.
3. Expected results
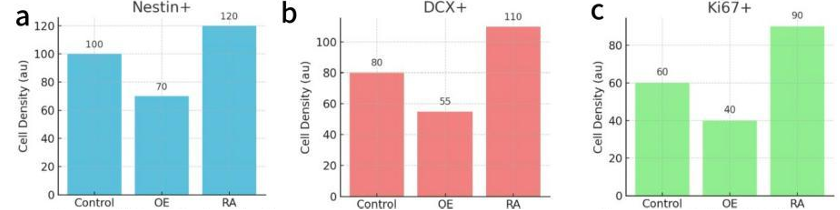
3.1. Possible result 1: SP reduced AHN, and this reduction was partially restored by NK1-R antagonism
In Figure 3, Overexpression of SP reduced cell densities of Nestin⁺, DCX⁺, and Ki67⁺ cells, while NK1-R antagonist treatment restored the levels partially and approach to the control group level, suggesting reversibility of SP-induced inhibition, which was consistent with the hypothesis that SP suppresses neurogenesis via NK1-R signaling.
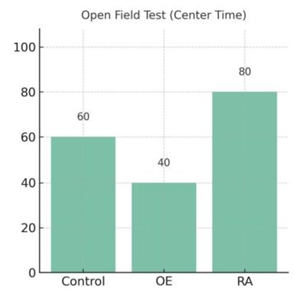
3.2. Possible result 2: SP overexpression reduced exploration and induced anxiety-like behaviour, whereas RA treatment enhanced locomotion and open-arm exploration
In open field test, SP overexpression group spent significantly less time in the central zone and showed reduced locomotion compared to the control group. RA treatment increased centre exploration and overall activity. Those data was shown in Figure 4.
In elevated plus maze test, as Figure 5 indicated, SP overexpression mice spent less time and made fewer entries into open arms, whereas RA mice entered open arms more frequently.
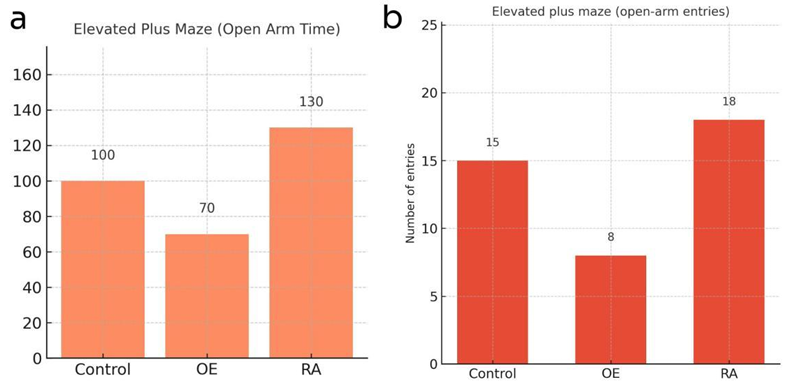
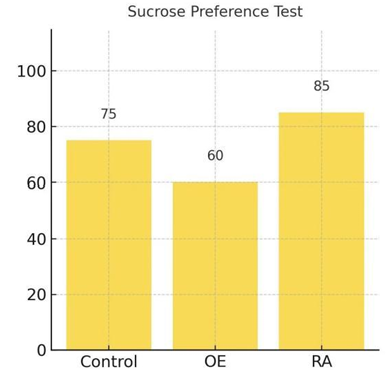
3.3. Possible result 3: SP overexpression induced anhedonia and behavioural despair, while RA treatment alleviated these effects by restoring sucrose preference and reducing immobility, indicating an antidepressant effect
In sucrose preference test, demonstrated in Figure 6, OE mice showed lower sucrose preference comparing to RA group. RA group restored preference closer to control level.
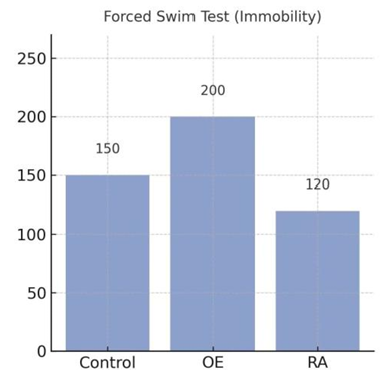
In forced swim test, OE mice showed longer immobility duration, whereas RA mice exhibited longer struggling time and reduced passive floating, shown in Figure 7. Changes in the results further supported the antidepressant-like effect of NK1-R antagonism.
4. Discussion
Our expected results indicated that SP over-expression significantly reduced the density of Nestin⁺, DCX⁺, and Ki67⁺ cells, while NK1-R antagonist had reverse effects. Behaviourally, SP over-expression induced depression and anxiety-like behaviour, as shown by performance in OFT, EPM, SPT, and FST. The reduced locomotion in central zone and open arms in OE group indicated increased anxiety, while low sucrose preference and longer immobility time suggested increased depression. These interpretations align with previous studies showing that stress-related substance P inhibits hippocampal plasticity and contributes to mood disorders. The reverse effects observed in RA group highlights the antidepressant effects of NK1-R antagonist. One possible mechanism is that SP activation of NK1-R increases the release of stress hormone via the HPA axis, thereby inhibiting rNSCs proliferation in the DG [15,16]. SP may also increase the expression of inflammatory cytokines. Neuroinflammation has been shown to suppress AHN [17]. Alternatively, AP might inhibit hippocampal brain-derived neurotrophic factor (BDNF), which affects neuroplasticity [18-20]. As previous papers have investigated that glutamatergic signalling from the SuM neurons can enhance AHN [5], the contribution of neuropeptides like SP may provide a complementary regulatory pathway in modulating the DG. Despite these insights, our study has several limitations that need to be addressed. Our interpretations are based on expected results only, lacking actual experimental data. Moreover, only short-term effects will be tested, long-term impacts remain unknown. AAV injection may cause local inflammation as well. The underlying mechanism is only partially understood, and the molecular pathway by which SP affects AHN is still unclear. To strengthen the evidence, further studies should perform molecular analyses to identify down stream targets of SP signalling and explore therapeutic potential of NK1-R antagonists in combination with classical antidepressants.
5. Conclusion
In summary, our study showed that SP-NK1-R signalling within the SuM-DG pathway played a crucial role in regulating AHN and modulating anxiety and depression-like behaviours. Based on our expected outcomes, SP overexpression suppressed the rNSC proliferation and reduced the density of immature and proliferating neurons in the DG, ultimately contributing to mood-related deficits. Conversely, NK1-R antagonist restored both neurogenic and behavioural parameters to control levels, highlighting its potential role as the therapeutic strategy for anxiety and depression-like behaviours. These findings emphasised the importance of neuropeptides, particularly SP in emotional regulation. Future research would aim to validate these effects on chronic stress models and further explore the long-term potential therapy through targeting the SP-NK1-R signalling.
Acknowledgment
I would like to express my sincere gratitude to my professor, Samuel Kunes, for his continuous support, insightful guidance and encouragement throughout the project. I am also deeply thankful to my teaching assistants, Lin Lu and Manhuan Xiao, for their patient assistance during experimental and writing stages. I would like to thank my group members for their inspiration and for the helpful discussions we shared. Finally, I would like to thank South China University of Technology, Guangzhou International Campus for providing the research space, and the Neuscholar platform for making this project possible.
References
[1]. Kovich H, Kim W, Quaste AM. Pharmacologic Treatment of Depression. Am Fam Physician. 2023 Feb; 107(2): 173-181. PMID: 36791444.
[2]. Fava M. Weight gain and antidepressants. J Clin Psychiatry. 2000; 61 Suppl 11: 37-41. PMID: 10926053.
[3]. Kempermann G, Song H, Gage FH. Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol. 2015 Jul 1; 7(9): a018812. doi: 10.1101/cshperspect.a018812. PMID: 26330519.
[4]. Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011 Aug 24; 476(7361): 458-61. doi: 10.1038/nature10287. PMID: 21814201.
[5]. Li YD, Luo YJ, Chen ZK, Quintanilla L, Cherasse Y, Zhang L, Lazarus M, Huang ZL, Song J. Hypothalamic modulation of adult hippocampal neurogenesis in mice confers activity-dependent regulation of memory and anxiety-like behavior. Nat Neurosci. 2022 May; 25(5): 630-645. doi: 10.1038/s41593-022-01065-x. Epub 2022 May 6. PMID: 35524139; PMCID: PMC9287980.
[6]. Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids. 2006 Sep; 31(3): 251-72. doi: 10.1007/s00726-006-0335-9. PMID: 16820980.
[7]. Iftikhar K, Siddiq A, Baig SG, Zehra S. Substance P: A neuropeptide involved in the psychopathology of anxiety disorders. Neuropeptides. 2020 Feb; 79: 101993. doi: 10.1016/j.npep.2019.101993. Epub 2019 Nov 11. PMID: 31735376.
[8]. Bassi GS, de Carvalho MC, Brandão ML. Effects of substance P and Sar-Met-SP, a NK1 agonist, in distinct amygdaloid nuclei on anxiety-like behaviour in rats. Neurosci Lett. 2014 May 21; 569: 121-5. doi: 10.1016/j.neulet.2014.03.065. Epub 2014 Apr 4. PMID: 24708929.
[9]. Kupcova, I., Danisovic, L., Grgac, I., & Harsanyi, S. (2022). Anxiety and Depression: What Do We Know of Neuropeptides? behavioral Sciences, 12(8), 262. https: //doi.org/10.3390/bs12080262
[10]. 14.McLean S. Do substance P and the NK1 receptor have a role in depression and anxiety? Curr Pharm Des. 2005; 11(12): 1529-47. doi: 10.2174/1381612053764779. PMID: 15892660.
[11]. Stephen Johnston, Sarah L Parylak, Stacy Kim, Nolan Mac, Christina Lim, Iryna Gallina, Cooper Bloyd, Alexander Newberry, Christian D Saavedra, Ondrej Novak, J Tiago Gonçalves, Fred H Gage, Matthew Shtrahman (2021) AAV ablates neurogenesis in the adult murine hippocampus eLife 10: e59291.
[12]. Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, Chen H, Zhu DY, Zhou QG. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 2018 Jul; 13(7): 1686-1698. doi: 10.1038/s41596-018-0011-z. PMID: 29988104.
[13]. Chen L, Faas GC, Ferando I, Mody I. Novel insights into the behavioral analysis of mice subjected to the forced-swim test. Transl Psychiatry. 2015 Apr 14; 5(4): e551. doi: 10.1038/tp.2015.44. PMID: 25871976; PMCID: PMC4462607.
[14]. 15.Park G, Kim SS, Shim J, Lee SV. Brief guide to immunostaining. Mol Cells. 2025 Jan; 48(1): 100157. doi: 10.1016/j.mocell.2024.100157. Epub 2024 Nov 19. PMID: 39571972; PMCID: PMC11699723
[15]. Sosulina L, Strippel C, Romo-Parra H, Walter AL, Kanyshkova T, Sartori SB, Lange MD, Singewald N, Pape HC. Substance P excites GABAergic neurons in the mouse central amygdala through neurokinin 1 receptor activation. J Neurophysiol. 2015 Oct; 114(4): 2500-8. doi: 10.1152/jn.00883.2014. Epub 2015 Sep 2. PMID: 26334021; PMCID: PMC4620133.
[16]. Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008 Sep; 31(9): 464-8. doi: 10.1016/j.tins.2008.06.006. PMID: 18675469.
[17]. Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016 Jan; 16(1): 22-34. doi: 10.1038/nri.2015.5. PMID: 26689365.
[18]. Duric V, McCarson KE. Hippocampal neurokinin-1 receptor and brain-derived neurotrophic factor gene expression is decreased in rat models of pain and stress. Neuroscience. 2005; 133(4): 999-1006. doi: 10.1016/j.neuroscience.2005.04.002. PMID: 15964488.
[19]. Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006 Jun 15; 59(12): 1116-27. doi: 10.1016/j.biopsych.2006.02.013. PMID: 16631126.
[20]. Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010 Apr; 70(5): 289-97. doi: 10.1002/dneu.20758. PMID: 20186711.
Cite this article
Li,J. (2025). Role of Supramammillary Nucleus Derived Substance P in Regulating Adult Hippocampal Neurogenesis and Depression and Anxiety-Like Behaviour. Theoretical and Natural Science,139,1-10.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Kovich H, Kim W, Quaste AM. Pharmacologic Treatment of Depression. Am Fam Physician. 2023 Feb; 107(2): 173-181. PMID: 36791444.
[2]. Fava M. Weight gain and antidepressants. J Clin Psychiatry. 2000; 61 Suppl 11: 37-41. PMID: 10926053.
[3]. Kempermann G, Song H, Gage FH. Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol. 2015 Jul 1; 7(9): a018812. doi: 10.1101/cshperspect.a018812. PMID: 26330519.
[4]. Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011 Aug 24; 476(7361): 458-61. doi: 10.1038/nature10287. PMID: 21814201.
[5]. Li YD, Luo YJ, Chen ZK, Quintanilla L, Cherasse Y, Zhang L, Lazarus M, Huang ZL, Song J. Hypothalamic modulation of adult hippocampal neurogenesis in mice confers activity-dependent regulation of memory and anxiety-like behavior. Nat Neurosci. 2022 May; 25(5): 630-645. doi: 10.1038/s41593-022-01065-x. Epub 2022 May 6. PMID: 35524139; PMCID: PMC9287980.
[6]. Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids. 2006 Sep; 31(3): 251-72. doi: 10.1007/s00726-006-0335-9. PMID: 16820980.
[7]. Iftikhar K, Siddiq A, Baig SG, Zehra S. Substance P: A neuropeptide involved in the psychopathology of anxiety disorders. Neuropeptides. 2020 Feb; 79: 101993. doi: 10.1016/j.npep.2019.101993. Epub 2019 Nov 11. PMID: 31735376.
[8]. Bassi GS, de Carvalho MC, Brandão ML. Effects of substance P and Sar-Met-SP, a NK1 agonist, in distinct amygdaloid nuclei on anxiety-like behaviour in rats. Neurosci Lett. 2014 May 21; 569: 121-5. doi: 10.1016/j.neulet.2014.03.065. Epub 2014 Apr 4. PMID: 24708929.
[9]. Kupcova, I., Danisovic, L., Grgac, I., & Harsanyi, S. (2022). Anxiety and Depression: What Do We Know of Neuropeptides? behavioral Sciences, 12(8), 262. https: //doi.org/10.3390/bs12080262
[10]. 14.McLean S. Do substance P and the NK1 receptor have a role in depression and anxiety? Curr Pharm Des. 2005; 11(12): 1529-47. doi: 10.2174/1381612053764779. PMID: 15892660.
[11]. Stephen Johnston, Sarah L Parylak, Stacy Kim, Nolan Mac, Christina Lim, Iryna Gallina, Cooper Bloyd, Alexander Newberry, Christian D Saavedra, Ondrej Novak, J Tiago Gonçalves, Fred H Gage, Matthew Shtrahman (2021) AAV ablates neurogenesis in the adult murine hippocampus eLife 10: e59291.
[12]. Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, Chen H, Zhu DY, Zhou QG. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 2018 Jul; 13(7): 1686-1698. doi: 10.1038/s41596-018-0011-z. PMID: 29988104.
[13]. Chen L, Faas GC, Ferando I, Mody I. Novel insights into the behavioral analysis of mice subjected to the forced-swim test. Transl Psychiatry. 2015 Apr 14; 5(4): e551. doi: 10.1038/tp.2015.44. PMID: 25871976; PMCID: PMC4462607.
[14]. 15.Park G, Kim SS, Shim J, Lee SV. Brief guide to immunostaining. Mol Cells. 2025 Jan; 48(1): 100157. doi: 10.1016/j.mocell.2024.100157. Epub 2024 Nov 19. PMID: 39571972; PMCID: PMC11699723
[15]. Sosulina L, Strippel C, Romo-Parra H, Walter AL, Kanyshkova T, Sartori SB, Lange MD, Singewald N, Pape HC. Substance P excites GABAergic neurons in the mouse central amygdala through neurokinin 1 receptor activation. J Neurophysiol. 2015 Oct; 114(4): 2500-8. doi: 10.1152/jn.00883.2014. Epub 2015 Sep 2. PMID: 26334021; PMCID: PMC4620133.
[16]. Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008 Sep; 31(9): 464-8. doi: 10.1016/j.tins.2008.06.006. PMID: 18675469.
[17]. Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016 Jan; 16(1): 22-34. doi: 10.1038/nri.2015.5. PMID: 26689365.
[18]. Duric V, McCarson KE. Hippocampal neurokinin-1 receptor and brain-derived neurotrophic factor gene expression is decreased in rat models of pain and stress. Neuroscience. 2005; 133(4): 999-1006. doi: 10.1016/j.neuroscience.2005.04.002. PMID: 15964488.
[19]. Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006 Jun 15; 59(12): 1116-27. doi: 10.1016/j.biopsych.2006.02.013. PMID: 16631126.
[20]. Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010 Apr; 70(5): 289-97. doi: 10.1002/dneu.20758. PMID: 20186711.









