1. Introduction
Skin regeneration is one of the important branches in the medicine area. Also, for skin regeneration material there is a huge market [1]. To repair the skin, the techniques that the doctors use is skin graft [2]. By transplanting the skin in good condition, for example, the skin may come from the other parts of the body or using fish skin transplanting to repair the injured area [3]. Although these techniques are the current mainstream for skin repair surgeries, several problems still need to be solved by using these techniques. First, the skin is a complex system, it has four different layers with different functions [4]. With different depths and areas of injuries, the functions that need to be rebuilt are different. Then, the skin transplanting treatment may bring second injuries to patients, due to the skin being transplanted from the other part of the body, surgery for grabbing the good skin is necessary. During the surgery process, new wounds are created, bringing more unexpected situations. Finally, the body rejection reaction [5] will lead to failure of the surgery and may cause irreversible results, these surgeries that are mentioned use materials that are different from the original skin, either the skin from the other part or fish skin, the immune system will alarm the body, leads to the death of the transplant tissue [6]. These issues will increase the cost of the treatment, decrease the total efficiency and may lead to medical malpractice. As traditional material and techniques cannot bring ideal treatment effect, new materials and techniques are introduced, 3D printing skin with Bio-Ink.
It seems that utilizing 3D printing skin with Bio-Ink has several reasons that this technique is better than the traditional one. First, the machine that controls 3D printing skin has much higher accuracy than the other techniques; by controlling the nozzle printing speed and nozzle injection size, a precise and accurate skin sample that fits the wound will be printed [7]. Secondly, the most essential aspect, the utilization of Bio-ink, the 3D printing biomaterial. By choosing different bio-ink, wound in different parts of the body will be healed. Thirdly, the different material processing methods can produce different physical properties products from one kind of bio-ink, it will shorten the surgery preparation period. Lastly, the cost of the bio-ink is more economical, two major categories of bio-ink are natural and synthetic. For natural materials, Gelatin, Collagen and Alginate are widely used in 3D printing skin, also for polyethylene glycol and hydroxyapatite are the common synthetic materials for 3D printing man-made skin. These materials can be mass produced from industry in comparison to the existing therapeutic materials [8].
In this review, by analyzing the references in recent 10 years, different materials used for 3D Bio-Ink will be discussed and analyzed their physical and chemical properties of these five kinds of materials. Then, another type of Bio-Ink, complex Bio-Ink will be introduced and by using this kind of structure, a future expectation will be raised.
2. Material use for 3D printing
2.1. Natural materials
2.1.1. Collagen
Collagen, the most abundant structural protein in the extracellular matrix, has long been regarded as a cornerstone material for tissue engineering and 3D bioprinting. Its biocompatibility and structural fidelity to native tissues make it an ideal bioink candidate. Collagen is primarily obtained from two sources: animal-derived tissues and recombinant production systems, each with distinct advantages and limitations.
Animal-derived collagen, commonly extracted from bovine or porcine skin, chicken cartilage, and rat tail tendons, is economical and widely available. However, risks of immunogenicity, pathogen transmission, and batch-to-batch variability limit its reproducibility and safety. Ethical concerns and insufficient mechanical strength further constrain its application. Chemical crosslinking can enhance stability but often at the expense of biocompatibility [9].
Recombinant collagen, produced via microbial or mammalian expression systems, overcomes many of these issues by ensuring high purity, batch consistency, and eliminating animal-borne pathogens. In vivo studies in rats have shown that 3D bioprinted GelMA/collagen scaffolds accelerate wound closure compared with controls, particularly within the first week (Figure 1) [10]. Quantitative data confirmed significantly higher healing rates (p < 0.05, p < 0.01), with performance attributed to the scaffold’s precisely engineered architecture that directs cell migration and proliferation. Despite these advantages, recombinant collagen remains limited by high production costs, low yields, and difficulty in replicating complex post-translational modifications. Nevertheless, its synergy with 3D bioprinting presents a promising avenue for advanced wound repair applications.
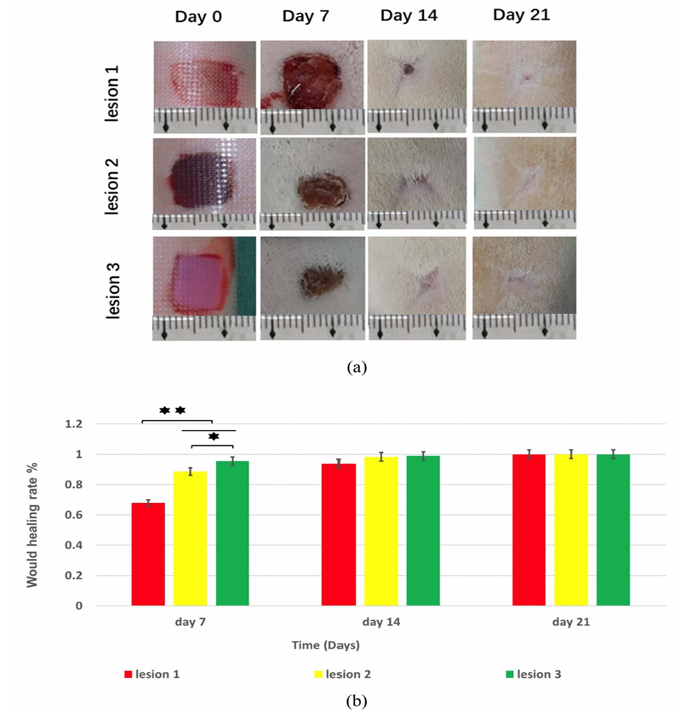
2.1.2. Gelatin
Gelatin is a widely used basic material in the field of biology nowadays, favored by the medical and biological communities for its low cost, easy availability, and high biocompatibility. Gelatin can be easily extracted from animal skin and bones and has the characteristic of stable integration with most other biomaterials, making it suitable for the production of many complex and multifunctional materials, including the 3D printing of human skin that we are discussing.
As a basic material, pure gelatin itself is obviously not strong enough, with a strength of only about 0.1 MPa, far lower than that of normal human skin, which is approximately 5 to 20 MPa [11] . Therefore, if gelatin is to be made into a material similar to human skin, it can rely on its advantage of high biocompatibility and be combined with other materials to create a stronger material.
The first material considered for combination is sodium alginate, a substance containing a high concentration of calcium ions. Its presence will significantly enhance the mechanical strength of the overall material and, through adjustment, bring it closer to the mechanical strength of human skin. First, gelatin and sodium alginate are placed together and subjected to a chemical cross-linking reaction using ultraviolet light (UV), which will greatly increase the strength and Young's modulus of the produced material.
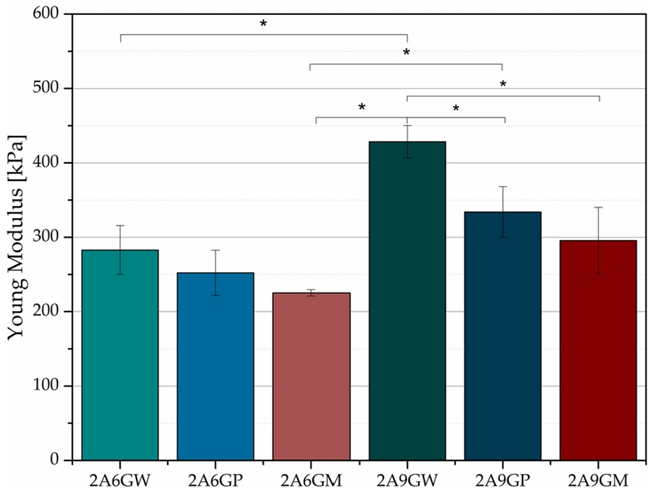
Second kind of material that could be used to combine with gelatin is silk fibroin, a kind of material with high modulus and tensile strength, the protein fiber structure could copy the muscular fiber structure easier, becoming more similar to the human skin. mixed gelatin with silk fibroin and stirred it to make the mixture even. Then add a natural crosslinker called Genipin. It reacts with amino groups in both gelatin and silk fibroin, linking them together and forming a stable three-dimensional network. After the reaction was complete, we washed the material several times with deionized water to remove any leftover Genipin and other small molecules. The finished material had a light blue color, which shows that the cross-linking worked. By adding silk fibroin makes the material stronger, more elastic, and more heat-resistant because silk fibroin has special β-sheet structures that act like a support frame inside the gel. The composite is also biocompatible since both gelatin and silk fibroin come from natural proteins that are friendly to cells. The way the material breaks down over time can be controlled by changing the amount of silk fibroin and how much cross-linking happens. This makes it useful for artificial skin, wound dressings, or soft tissue repair. However, there are some drawbacks. Silk fibroin can reduce the swelling ability of gelatin, which might slow down nutrient transport for cells. Also, too much Genipin or too much cross-linking can make the material too stiff, losing the softness that real skin has. This is why it is important to adjust the balance between gelatin, silk fibroin, and cross-linking carefully. Any leftover Genipin should be removed to avoid harming cells, and the sterilization method should not damage the structure of the material.
Gelatin is often used for making artificial skin because it is natural and safe for cells. But it has many problems. One big problem is it is too soft and weak, so it can break or tear easily. This makes it hard to use when the skin needs to handle stretching or pressure. Another problem is it changes when the temperature or water around it changes. In warm or wet places, it can get soft or even dissolve. It also breaks down fast in the body, so it cannot last long enough for some skin healing. Last but not least, Gelatin can also swell too much in water, which changes its shape and strength. And by itself, it does not feel or act like real skin. Because of these problems, people often mix gelatin with other materials to make it stronger and more stable.
2.1.3. Alginate
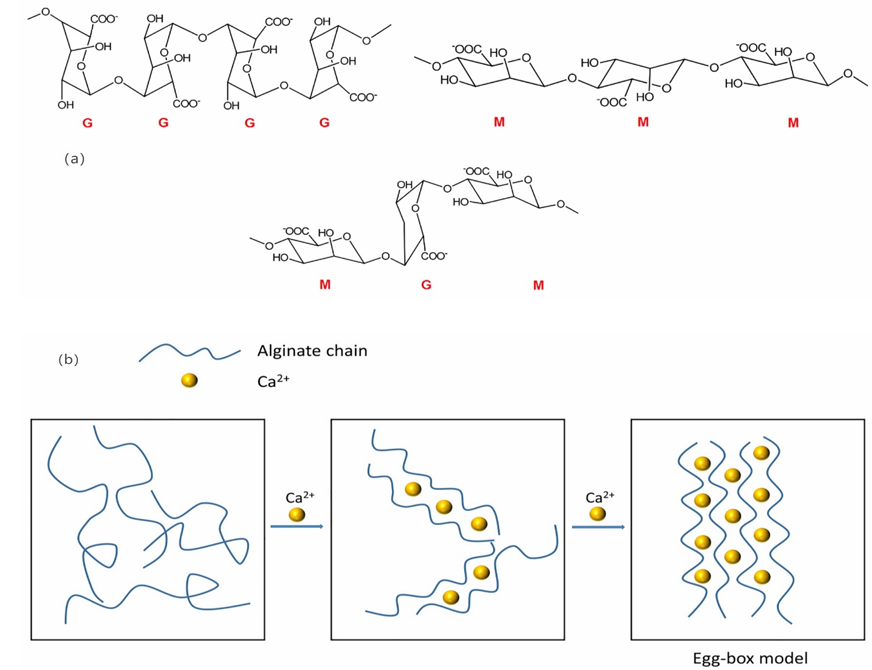
Alginate is one of the popular natural biomaterials in 3D bioprinting. Alginate utilizes seaweed as raw material, by simply processing and manufacturing, a gel like material can be produced. However, the raw Alginate cannot be 3D-printed because Alginate is not shear-thinning, as a result, the raw alginate cannot maintain its geometry after 3D printing.
How to make this kind of material be a 3D Bio-Ink. One of the solutions is to add Calcium ions into raw alginate [13]. Calcium ions act as a crosslinker that will organize the alginate chain and form hydrogel like material, with the increase in concentration of the calcium ions, the mechanical property of the alginate will be influenced [13]. With this method the properties of the alginate bio-ink can be controlled. The figure below shows the chemical structure and crosslinking process with Calcium ion.
The second method is to combine with other types of 3D bio-ink. As an example, Alginate can combine with Gelatin. Gelatin can be printed without any special preparation. The mixture of Gelatin and Alginate becomes a shear-thinning material that can be directly printed, as the needed printing skin is produced, transferring it into the Calcium ion solution to increase the cross-linking inside the 3D printing sample. This action will improve the mechanical properties of the sample and create a suitable environment for the cells to live. The figure below illustrates the application of the Alginate and Gelatin Mixed 3D bio-printing skin.
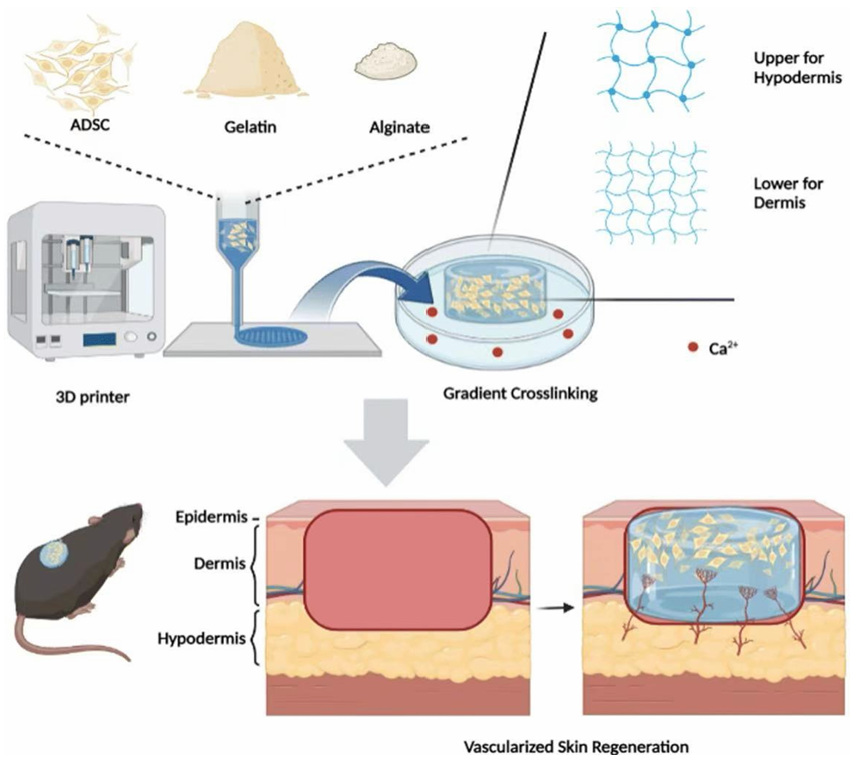
From Figure 4 [14], it shows the Alginate bio-ink real application and illustrates the steps to produce 3D printing skin by using Alginate based material. To be utilized as a Bio-Ink in 3D printing for skin regeneration, the rate of the wound healing rate should be considered. If this material is going to increase the wound healing rate and decrease the probability of the side effect, as a result, this kind of material should be considered as a perfect Bio-Ink material.
For the figure below it gives three groups of wound healing sample, it contains control group, and two experiment groups. With different recipes of the PCL/Alginate base material, they will show different performances. In this review, the control group and PCL30/Alginate experiment group are going to discuss, the third group has less correlation with this review.
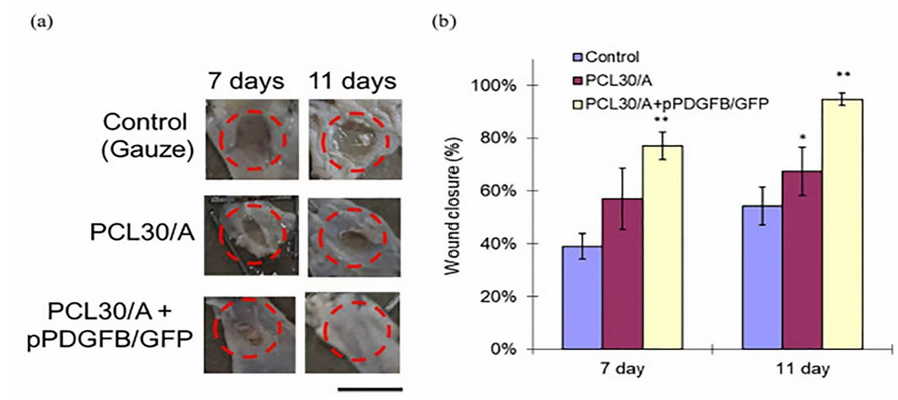
From figure 5.(a) [13], it is clear the wound using Alginate base materials have faster healing rate than the control group. This is an 11-day experiment, from figure (a), the wound area shrinking rate of PCL30/Alginate group is quicker than the control group, this can also be seen from Figure (b) [13]. As a result, the Alginate base Bio-Ink do have the ability to increase the wound healing rate.
Alginate is a good material to produce Bio-Ink due to several advantages of this kind of material. First, the biocompatibility of Alginate is better, with low probability of body rejection reaction [13]. Second, the cost of alginate base material is significantly lower than the traditional method, as Alginate is mainly made from seaweed [15]. Lower rejection reaction and cost will bring large market, more suitable for 3D-printing skin.
2.2. Synthetic materials
In the field of biological 3D printing, material selection plays a vital role in achieving both functional and structural fidelity. While natural biopolymers are known for their biocompatibility and chemical properties, they often suffer from poor mechanical strength, making them less ideal for supporting complex tissue structures. Recent advances in material science have led to the development of engineered biopolymers that combine biological safety with improved mechanical performance, making them more suitable for 3D bioprinting applications.
For optimal performance, bio-inks must exhibit specific physical and chemical characteristics. These characteristics include biocompatibility to support cell survival, adhesion, and proliferation, and sufficient mechanical properties, such as appropriate stiffness and elasticity, to mimic the natural extracellular matrix. On this basis, several synthetic materials, such as polyethylene glycol (PEG) and skin fiber (SF) have been developed to meet these stringent requirements.
2.2.1. PEG(Polyethylene Glycol)
PEG is a widely used water-soluble polymer synthesized from ethylene oxide. It is easily soluble in organic solvents such as water, ethanol, and acetone. PEG is favored in biomedical applications due to its low toxicity, low immunogenicity, and excellent biocompatibility. In addition, PEG can be functionalized by binding to proteins, drugs, or nanoparticles, enhancing its adaptability for targeted drug delivery or cell encapsulation.
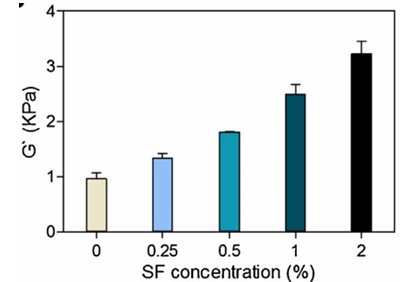
To enhance the mechanical strength of PEG, it can bind to silk fibroin. Silk fibroin (SF) is a natural protein with excellent mechanical properties and biological activity. The mechanical properties of PEG hydrogel can be significantly improved by combining SF with PEG. The SF molecular chain contains a large number of hydrophobic regions, which can form β-sheet crystals under appropriate conditions (such as ultrasound treatment, freezing thawing). These β- folded structures can serve as physical crosslinking points, forming interpenetrating networks (IPNs) with chemical crosslinking networks of PEG (such as photo crosslinking of PEG4A) to enhance overall rigidity. In addition, hydrogen bonds can be formed between the ether bond (- O -) of PEG and the amide bond (- CONH -) of SF, further enhancing the interfacial adhesion between the two phases. This interaction can reduce the slip of PEG chain and improve the elastic modulus of hydrogel. From the experimental results shown in figure 6 below, it can also be seen that the shear modulus increases with the increase of SF concentration, indicating an increase in material stiffness. As a type of skin material, it is more durable.
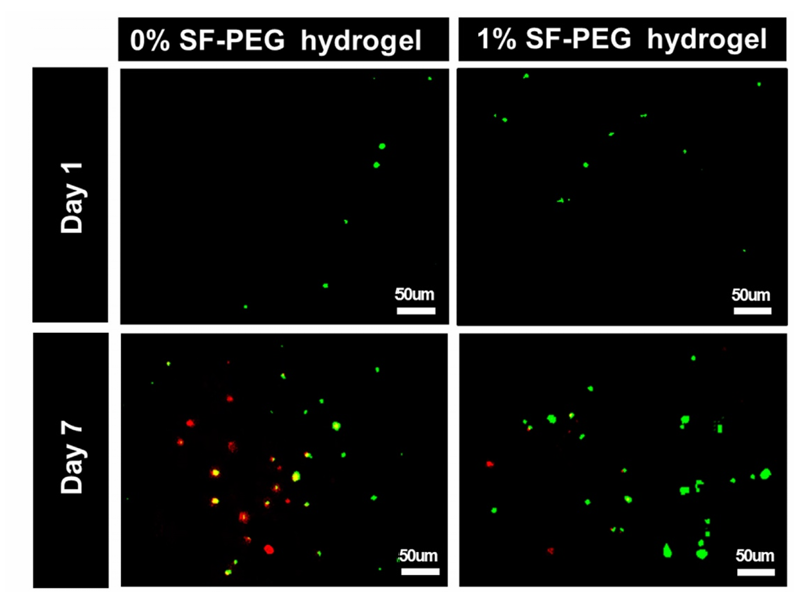
Meanwhile, silk fibroin (SF) contains a natural protein RGD(Arg Gly Asp, arginine glycine aspartic acid) sequence that can promote cell adhesion through integrin receptors on the cell surface, avoiding cell separation or death due to the lack of attachment points on smooth PEG surfaces. RGD sequence is a short peptide sequence widely present in extracellular matrix (ECM) proteins such as fibronectin, fibronectin, collagen, etc. It is the main recognition site of integrin receptors, directly mediating the adhesion of cells to ECM or biomaterials. After being recognized by integrin receptors on the cell membrane, RGD sequences trigger intracellular signaling pathways to regulate cell behavior, such as adhesion, spreading, survival, and proliferation [17]. Introducing RGD into PEG can simulate the microenvironment of natural ECM and enhance cell viability. The following figure 7 shows the relationship between cell survival rate and SF addition amount [16]. It can be observed that the number of cells significantly increased after the addition of silk fibroin protein. The addition of SF improved the cell survival environment and increased the cell survival rate.
PEG also has limitations. It exhibits poor oxidative stability and exposure to ultraviolet radiation during photo crosslinking may lead to cell death, limiting its use in light-based printing technology. However, PEG based materials are commonly used for applications such as antibacterial membranes and anti-adhesion membranes, especially in abdominal surgery [18].
2.2.2. PLGA (Poly lactic-co-glycolic Acid)
Poly Lactic-co-Glycolic Acid, PLGA, one kind of synthetic 3D bio-ink. The physical and chemical properties of PLGA are mainly dependent on the structure of the polymer chain--the ratio of Lactic acid (LA) and Glycolic acid (GA) in the chain. The figure below illustrates how the Ratio of GA and LA in the polymer chain will influence the properties.
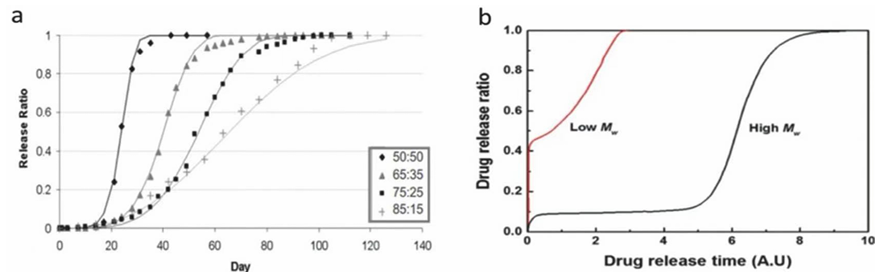
From Figure 8(a), the ratio of LA and GA will influence the time that the PLGA degradation. Also, from Figure 8(b) the molecular weight of the polymer chain will influence the degradation rate of the PLGA. However, according to the research, the PLGA is not suitable for being a bio-ink for 3D printing, the product of PLGA is tough not suitable for 3D printing skin. The reason for using PLGA is not to print the skin, but utilize it additional uses, the nanoparticles made by PLGA, the nanoparticles can be t he carrier of drugs, such as anti-bacterial drugs. Then combine the PLGA nanoparticles with drugs to the bio-ink to form a complex bio-ink to reach better performance of skin regeneration.
2.3. Complex Bio-Ink
These Bio-Ink materials that mentioned in previous text are the common materials for 3D printing skin for skin regeneration. Although these materials are suitable materials for 3D printing skin, still there are some issues with these materials. For example, the natural bio-ink collagen, normally the wound needs at least 21 days to heal [10], however, the collagen will degrade in less than 20 days, as a result, the collagen will degrade before the wound perfectly healed. Another example is the synthetic bio-ink PLGA, normally, the objects that are 3D-printed by PLGA are tough and indeformable, not similar to the properties of human skin. So, a new type of Bio-Ink is introduced, complex Bio-Ink.
Complex Bio-Ink, made by two or more kinds of Bio-Ink materials. Nowadays, according to the paper, one of the known complex Bio-Ink is Collagen with HA (Hyaluronic Acid) [20]. The combination of these two materials solves lots of problems. The mechanical properties of Bio-Ink will be improved, the addition of HA will increase the UTS (ultimate tensile strength) of material [20]. Another type of complex Bio-Ink is Alginate and Gelatin, the previous paragraphs mention that alginate cannot be shaped without Calcium ions, however after adding the Calcium ions, the viscosity and flowability will change, it is not suitable for the printer to work perfectly. So, Gelatin and Alginate complex Bio-Ink will solve the problem, as the needed geometry of the artificial skin is printed, transfer the sample to the Calcium ion solution to increase the crosslinking of the material. As a result, the final product will be suitable for wound healing.
From the previous paragraph, the advantages of Complex Bio-ink are more than single Bio-ink. So, new thinking comes out, by improving the performance of the 3D printing skin with Collagen Bio-Ink. Since Collagen Bio-Ink will degrade before the wound perfectly healed. It will increase the probability of wound infection, ascend the chances of getting serious diseases. As a result, to increase the speed of wound healing or decrease the rate of material degradation will deal with this issue.
According to the relevant references, found that using nanoparticles contain drugs with Collagen Bio-ink is a new technical route, which combine PLGA nanoparticles that contains the anti-bacterial drugs and drugs can increase the wound healing rate with the Collagen Bio-Ink to form a complex Bio-Ink, as the PLGA nanoparticles degrade [19], it will release the drug to the wound surface and increase the speed of wound healing. Which wound will heal before the Collagen completely degrade. This technical route will perfectly solve the degradation rate of Collagen quicker than wound healing rate.
3. Conclusion
All in all, 3D printing skin is a cutting-edge technology, which has lots of benefits than the traditional treatment method. What dominates the 3D printing skin technology is the printing material, the Bio-Ink, the physical and chemical properties of Bio-Ink will directly influence the performance of the Artificial 3D printing skins. Also, for different parts of skin on the body, the recipes of the Bio-Ink will be different to achieve the full function of the 3D printing skin. With the development of the nano-biomaterial, nanoparticles contain different drugs have been developed. With the addition of these nanoparticles, the performance of the Bio-ink will reach the next level, and the disadvantages of the old generation Bio-inks will be covered by the improvement of printing material.
Acknowledgement
Yuxuan Liu, Yuguan Zhou, and Zixuan Zhou contributed equally to this work and should be considered co-first authors.
References
[1]. S. N. Economidou and D. Douroumis, '3D printing as a transformative tool for microneedle systems: Recent advances, manufacturing considerations and market potential’, Jun. 01, 2021, Elsevier B.V. doi: 10.1016/j.addr.2021.03.007.
[2]. L. P. Kamolz, P. Kotzbeck, M. Schintler, and S. Spendel, 'Skin regeneration, repair, and reconstruction: present and future’, European Surgery - Acta Chirurgica Austriaca, vol. 54, no. 3, pp. 163–169, Jun. 2022, doi: 10.1007/s10353-022-00757-9.
[3]. H. Luze, S. P. Nischwitz, C. Smolle, R. Zrim, and L. P. Kamolz, 'The Use of Acellular Fish Skin Grafts in Burn Wound Management—A Systematic Review’, Jul. 01, 2022, MDPI. doi: 10.3390/medicina58070912.
[4]. A. Alsaif, M. Karam, A. Hayre, A. Abul, A. Aldubaikhi, and N. Kahlar, 'Full thickness skin graft versus split thickness skin graft in paediatric patients with hand burns: Systematic review and meta-analysis’, Burns, vol. 49, no. 5, pp. 1017–1027, Aug. 2023, doi: 10.1016/j.burns.2022.09.010.
[5]. F. Schlottmann, V. Bucan, P. M. Vogt, and N. Krezdorn, 'A short history of skin grafting in burns: From the gold standard of autologous skin grafting to the possibilities of allogeneic skin grafting with immunomodulatory approaches’, Mar. 01, 2021, MDPI AG. doi: 10.3390/medicina57030225.
[6]. J. Dean et al., 'Advancements in bioengineered and autologous skin grafting techniques for skin reconstruction: a comprehensive review’, Front Bioeng Biotechnol, vol. 12, 2024, doi: 10.3389/fbioe.2024.1461328.
[7]. S. Wang et al., 'A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future’, Feb. 01, 2023, MDPI. doi: 10.3390/pharmaceutics15020416.
[8]. S. Saleh Alghamdi, S. John, N. Roy Choudhury, and N. K. Dutta, 'polymers Additive Manufacturing of Polymer Materials: Progress, Promise and Challenges’, 2021, doi: 10.3390/polym13.
[9]. S. Debnath, A. Agrawal, N. Jain, K. Chatterjee, and D. J. Player, 'Collagen as a bio-ink for 3D printing: a critical review’, Jan. 08, 2025, Royal Society of Chemistry. doi: 10.1039/d4tb01060d.
[10]. Y. Shi et al., 'Tyrosinase-doped bioink for 3D bioprinting of living skin constructs’, Biomedical Materials (Bristol), vol. 13, no. 3, Mar. 2018, doi: 10.1088/1748-605X/aaa5b6.
[11]. K. Rosińska, M. Bartniak, A. Wierzbicka, A. Sobczyk-Guzenda, and D. Bociaga, 'Solvent types used for the preparation of hydrogels determine their mechanical properties and influence cell viability through gelatine and calcium ions release’, J Biomed Mater Res B Appl Biomater, vol. 111, no. 2, pp. 314–330, Feb. 2023, doi: 10.1002/jbm.b.35152.
[12]. W. Sun, T. Incitti, C. Migliaresi, A. Quattrone, S. Casarosa, and A. Motta, 'Genipin-crosslinked gelatin–silk fibroin hydrogels for modulating the behaviour of pluripotent cells’, J Tissue Eng Regen Med, vol. 10, no. 10, pp. 876–887, Oct. 2016, doi: 10.1002/term.1868.
[13]. N. Qosim, Y. Dai, G. R. Williams, and M. Edirisinghe, 'Structure, properties, forming, and applications of alginate fibers: A review’, International Materials Reviews, vol. 69, no. 5–6, pp. 309–333, Sep. 2024, doi: 10.1177/09506608241280419.
[14]. Y. Ma et al., '3D bioprinting of a gradient stiffened gelatin-alginate hydrogel with adipose-derived stem cells for full-thickness skin regeneration’, J Mater Chem B, vol. 11, no. 13, pp. 2989–3000, Feb. 2023, doi: 10.1039/d2tb02200a.
[15]. H. Bojorges, M. J. Fabra, A. López-Rubio, and A. Martínez-Abad, 'Alginate industrial waste streams as a promising source of value-added compounds valorization’, Science of the Total Environment, vol. 838, Sep. 2022, doi: 10.1016/j.scitotenv.2022.156394.
[16]. H. Kwak, S. Shin, H. Lee, and J. Hyun, 'Formation of a keratin layer with silk fibroin-polyethylene glycol composite hydrogel fabricated by digital light processing 3D printing’, Journal of Industrial and Engineering Chemistry, vol. 72, pp. 232–240, Apr. 2019, doi: 10.1016/j.jiec.2018.12.023.
[17]. J. M. Galindo, S. Merino, and M. A. Herrero, 'Advanced Hydrogels: Enhancing Tissue Bioengineering with RGD Peptides and Carbon Nanomaterials’, Feb. 01, 2025, John Wiley and Sons Ltd. doi: 10.1002/cmdc.202400587.
[18]. A. Toncheva et al., 'Antibacterial PLA/PEG electrospun fibers: Comparative study between grafting and blending PEG’, Eur Polym J, vol. 75, pp. 223–233, Feb. 2016, doi: 10.1016/j.eurpolymj.2015.12.019.
[19]. C. Martins, F. Sousa, F. Araújo, and B. Sarmento, 'Functionalizing PLGA and PLGA Derivatives for Drug Delivery and Tissue Regeneration Applications’, Jan. 10, 2018, Wiley-VCH Verlag. doi: 10.1002/adhm.201701035.
[20]. Y. Zhou et al., 'Sulfated hyaluronic acid/collagen-based biomimetic hybrid nanofiber skin for diabetic wound healing: Development and preliminary evaluation’, Carbohydr Polym, vol. 334, Jun. 2024, doi: 10.1016/j.carbpol.2024.122025.
Cite this article
Liu,Y.;Zhou,Y.;Zhou,Z. (2025). The Recipes for Different 3D Printing Bio-Ink. Theoretical and Natural Science,139,69-80.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. S. N. Economidou and D. Douroumis, '3D printing as a transformative tool for microneedle systems: Recent advances, manufacturing considerations and market potential’, Jun. 01, 2021, Elsevier B.V. doi: 10.1016/j.addr.2021.03.007.
[2]. L. P. Kamolz, P. Kotzbeck, M. Schintler, and S. Spendel, 'Skin regeneration, repair, and reconstruction: present and future’, European Surgery - Acta Chirurgica Austriaca, vol. 54, no. 3, pp. 163–169, Jun. 2022, doi: 10.1007/s10353-022-00757-9.
[3]. H. Luze, S. P. Nischwitz, C. Smolle, R. Zrim, and L. P. Kamolz, 'The Use of Acellular Fish Skin Grafts in Burn Wound Management—A Systematic Review’, Jul. 01, 2022, MDPI. doi: 10.3390/medicina58070912.
[4]. A. Alsaif, M. Karam, A. Hayre, A. Abul, A. Aldubaikhi, and N. Kahlar, 'Full thickness skin graft versus split thickness skin graft in paediatric patients with hand burns: Systematic review and meta-analysis’, Burns, vol. 49, no. 5, pp. 1017–1027, Aug. 2023, doi: 10.1016/j.burns.2022.09.010.
[5]. F. Schlottmann, V. Bucan, P. M. Vogt, and N. Krezdorn, 'A short history of skin grafting in burns: From the gold standard of autologous skin grafting to the possibilities of allogeneic skin grafting with immunomodulatory approaches’, Mar. 01, 2021, MDPI AG. doi: 10.3390/medicina57030225.
[6]. J. Dean et al., 'Advancements in bioengineered and autologous skin grafting techniques for skin reconstruction: a comprehensive review’, Front Bioeng Biotechnol, vol. 12, 2024, doi: 10.3389/fbioe.2024.1461328.
[7]. S. Wang et al., 'A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future’, Feb. 01, 2023, MDPI. doi: 10.3390/pharmaceutics15020416.
[8]. S. Saleh Alghamdi, S. John, N. Roy Choudhury, and N. K. Dutta, 'polymers Additive Manufacturing of Polymer Materials: Progress, Promise and Challenges’, 2021, doi: 10.3390/polym13.
[9]. S. Debnath, A. Agrawal, N. Jain, K. Chatterjee, and D. J. Player, 'Collagen as a bio-ink for 3D printing: a critical review’, Jan. 08, 2025, Royal Society of Chemistry. doi: 10.1039/d4tb01060d.
[10]. Y. Shi et al., 'Tyrosinase-doped bioink for 3D bioprinting of living skin constructs’, Biomedical Materials (Bristol), vol. 13, no. 3, Mar. 2018, doi: 10.1088/1748-605X/aaa5b6.
[11]. K. Rosińska, M. Bartniak, A. Wierzbicka, A. Sobczyk-Guzenda, and D. Bociaga, 'Solvent types used for the preparation of hydrogels determine their mechanical properties and influence cell viability through gelatine and calcium ions release’, J Biomed Mater Res B Appl Biomater, vol. 111, no. 2, pp. 314–330, Feb. 2023, doi: 10.1002/jbm.b.35152.
[12]. W. Sun, T. Incitti, C. Migliaresi, A. Quattrone, S. Casarosa, and A. Motta, 'Genipin-crosslinked gelatin–silk fibroin hydrogels for modulating the behaviour of pluripotent cells’, J Tissue Eng Regen Med, vol. 10, no. 10, pp. 876–887, Oct. 2016, doi: 10.1002/term.1868.
[13]. N. Qosim, Y. Dai, G. R. Williams, and M. Edirisinghe, 'Structure, properties, forming, and applications of alginate fibers: A review’, International Materials Reviews, vol. 69, no. 5–6, pp. 309–333, Sep. 2024, doi: 10.1177/09506608241280419.
[14]. Y. Ma et al., '3D bioprinting of a gradient stiffened gelatin-alginate hydrogel with adipose-derived stem cells for full-thickness skin regeneration’, J Mater Chem B, vol. 11, no. 13, pp. 2989–3000, Feb. 2023, doi: 10.1039/d2tb02200a.
[15]. H. Bojorges, M. J. Fabra, A. López-Rubio, and A. Martínez-Abad, 'Alginate industrial waste streams as a promising source of value-added compounds valorization’, Science of the Total Environment, vol. 838, Sep. 2022, doi: 10.1016/j.scitotenv.2022.156394.
[16]. H. Kwak, S. Shin, H. Lee, and J. Hyun, 'Formation of a keratin layer with silk fibroin-polyethylene glycol composite hydrogel fabricated by digital light processing 3D printing’, Journal of Industrial and Engineering Chemistry, vol. 72, pp. 232–240, Apr. 2019, doi: 10.1016/j.jiec.2018.12.023.
[17]. J. M. Galindo, S. Merino, and M. A. Herrero, 'Advanced Hydrogels: Enhancing Tissue Bioengineering with RGD Peptides and Carbon Nanomaterials’, Feb. 01, 2025, John Wiley and Sons Ltd. doi: 10.1002/cmdc.202400587.
[18]. A. Toncheva et al., 'Antibacterial PLA/PEG electrospun fibers: Comparative study between grafting and blending PEG’, Eur Polym J, vol. 75, pp. 223–233, Feb. 2016, doi: 10.1016/j.eurpolymj.2015.12.019.
[19]. C. Martins, F. Sousa, F. Araújo, and B. Sarmento, 'Functionalizing PLGA and PLGA Derivatives for Drug Delivery and Tissue Regeneration Applications’, Jan. 10, 2018, Wiley-VCH Verlag. doi: 10.1002/adhm.201701035.
[20]. Y. Zhou et al., 'Sulfated hyaluronic acid/collagen-based biomimetic hybrid nanofiber skin for diabetic wound healing: Development and preliminary evaluation’, Carbohydr Polym, vol. 334, Jun. 2024, doi: 10.1016/j.carbpol.2024.122025.









