1. Introduction
Diabetes mellitus is a prevalent metabolic disorder; however, if left untreated or managed inadequately, it can precipitate a spectrum of health complications, consequently elevating the death rate. Clinically, diabetes is categorized into two primary types: type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM). Fundamentally, T1DM arises from the destruction of insulin-producing beta cells within the pancreatic islets, resulting in absolute insulin deficiency and an inability to maintain healthy blood sugar levels [1]. Type 2 diabetes is characterized by insulin resistance and impaired insulin secretion, which consequently disrupts blood glucose metabolism [2]. The human pancreas contains five distinct types of hormone-secreting cells: α cells, β cells, δ cells, ϵ cells, and PP cells [3]. When β cells are damaged, their capacity for insulin secretion is compromised. Notably, human islets exhibit limited regenerative potential. Currently, none of the available therapeutic approaches for diabetes can achieve complete remission. Patients diagnosed with type 1 diabetes universally require medication for disease management.
However, recent studies have found that Procr+ cells in mouse islets can grow into islet organoids through in vitro culture and contain mature beta cells, and reverse mouse diabetes after transplantation [4]. Wang et al. determined that Procr+ cells in mouse islets are multi-potent islet progenitor cells that can differentiate into β, α, δ, and PP cells. At the same time, Procr+ islet cells are also islet progenitor cells of adult islets [4]. When a single Procr+ progenitor cell was cultured into an islet-like organoid, Wang et al. found that when growth factors including B27, ITS, EGF, heparin, and FGF2 were supplied to the culture medium, only 1 out of 15 Procr+ islet cells could form a colony and survive for no more than 7 days. However, when endothelial cells (ECs) were added to the culture medium, a quarter of the Procr+ islet cells showed colony-forming ability [5]. VEGF induces endothelial cells to release signals to promote their differentiation into functional β cells [6]. Endothelial cells have growth factors that promote cell β differentiation, but the specific mechanism by which Procr+ cell cultured with endothelial cells can promote cell colony formation still requires further research. Through this discovery, this article intends to explore the effect of circular RNA derived from endothelial cells on the in vitro colony-forming ability of procr+ cells. This will further help the proliferation of procr+ cells in the body and then make procr+ cells grow into pancreatic islet organoids, thereby reversing diabetes. This might be a method for treating diabetes in the future.
When procr+ cells are co-cultured with endothelial cells, the endothelial cells release extracellular vesicles (EVs) into the extracellular environment. EVs, once regarded as cell debris, are now considered important mediators for intercellular communication [7]. Circular RNAs not only remain within cells but can also be selectively packaged into extracellular vesicles (EVs), such as exosomes and microvesicles, and released into the extracellular space. This process may serve as a mechanism for circRNA clearance, preventing their excessive accumulation in cells. Given that EVs can be absorbed by recipient cells, the circular RNA secreted by them may also promote intercellular communication, potentially affecting the function of recipient cells [8]. Marvin et al. detected that Circular RNA usually shows tissue/developmental stage-specific expression, and it was also found that Circular RNA competes with other RNAs for the binding of RNA-binding proteins or miRNA [9]. Among them, CDR1as is the sponge of MiRNA-7. Expressing CDR1as in zebrafish can damage midbrain development, similar to knocking down miR-7, indicating that CDR1as is a miRNA antagonist [9]. Overexpression of MiRNA-7 in β cells in mice inhibits insulin secretion and affects the functional of β cells [10], but overexpression of CDRq1as in islet cells increases insulin content and secretion [11]. These two examples show that the role of Circular RNA varies in different tissues, even if it is the sponge of the same MiRNA. In recent studies, thousands of Circular RNAs have been discovered, Dialloc et al. detected circCARD6 in endothelial cells and found that it acts as a sponge for MiRNA-31 [12]. CircCARD6 derived from endothelial cells may contribute to the promotion of colony formation in procr+ cell cultures.
Therefore, this study aims to investigate the specific circular RNA derived from endothelial cells functioning as a microRNA sponge, to elucidate the novel mechanism by which circRNA promotes colony formation in procr+ cells and their subsequent growth into pancreatic organoids, thereby providing innovative strategies for the treatment of diabetes.
2. Construction and geometrical dimensions of specimens
The objective of this project is to investigate the functional roles of circular RNAs derived from endothelial cells by co-cultivating Procr+ cells with endothelial cells, thereby promoting the proliferation of Procr+ cells. I hypothesize that certain highly expressed circular RNA in Procr+ cells originate from endothelial cells. These circular RNA function as microRNA (miRNA) sponges, subsequently modulating miRNA expression to facilitate the colony formation of Procr+ cells (Figure 1).
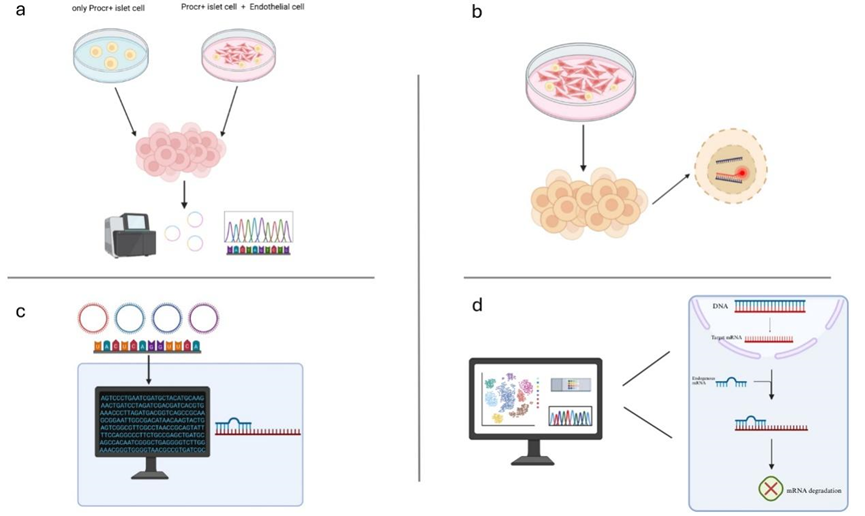
3. Test results and discussions
3.1. Objective1: upon co-culturing endothelial cells and Procr+ cells, the Circ RNA graph of Procr+ cells will be altered
Rationale: When endothelial cells (ECs) are co-cultured with Procr+ cells, ECs provide critical nutritional signals to support the proliferation, differentiation, and survival of stem cells. Our objective is to detect the absorption of circular RNA from extracellular vesicles (EVs) of endothelial cells by Procr+ cells, leading to an increased abundance of specific circular RNAs that were originally present in Procr+ cells. Two distinct in vitro experimental systems must be established. Specifically, when Procr+ cells and endothelial cells are co-cultured to form colonies, circular RNA sequencing should be performed for both experimental systems.
Method: To conduct the two in vitro experiments, Procr+ cells must first be isolated from the pancreas of mice. In the initial in vitro experimental medium, only Procr+ cells and growth factors are required. However, the second in vitro experimental medium necessitates the specific addition of endothelial cells and Procr+ cells for co-culture. During the isolation of Procr+ cells, Fluorescence-Activated Cell Sorting (FACS) is employed. FACS is a methodology that separates heterogeneous biological cell mixtures into two or more containers based on the distinct light scattering and fluorescence characteristics of each cell, processed individually [13]. To preserve islet integrity, it is imperative to meticulously control the digestion time, purify and perfuse the islets to ensure cell viability, followed by manual selection under an anatomical microscope. Subsequently, Procr+ cells are isolated using FACS. Concurrently, endothelial cells are also isolated from the fat pad in the groin or the dorsal skin, utilizing the FACS technique as well [5]. Upon co-culturing Procr+ cells with endothelial cells, wherein Procr+ cells form colonies, Circular RNAs are extracted from the entire cytoplasm of Procr+ cells across the two in vitro experiments. These circRNAs are then subjected to sequencing via RNA sequencing (RNA-seq). The process involves rRNA depletion followed by digestion of linear RNA with RNase R to enrich circular RNA [14]. Through RNA-seq analysis, I can compare the abundance of specific circRNAs in Procr+ cells between the two in vitro experiments, with circCARD6 potentially exhibiting increased abundance in the co-culture of Procr+ cells and endothelial cells.
Expected outcome: Through this experiment, I aim to predict that the abundance of certain circular RNAs within Procr+ cells increases following their co-culture with endothelial cells, with a specific focus on circCARD6 as a potential candidate.
3.2. Objective2: confirm that the highly expressed circular RNA in the co-culture of Procr+ cells and endothelial cells is derived from the extracellular vesicles of endothelial cells
Rationale: It is established that endothelial cells regulate Procr+ cells by releasing extracellular vesicles, and circular RNA carried by these vesicles can be delivered to recipient cells. In Objective 1, I predicted that the co-culture of Procr+ cells and endothelial cells would result in an elevated abundance of circCARD6 in Procr+ cells. I aimed to verify that after co-culture, the highly expressed circCARD6 in Procr+ cells originates from extracellular vesicles secreted by endothelial cells. I will employ fluorescence in situ hybridization (FISH) to track the localization of the target circCARD6.
Method: In Objective 2, the primary task is to ascertain whether the circCARD6 detected in Procr+ cells post co-culture originated from extracellular vesicles (EVs) derived from endothelial cells (ECs). This requires the re-establishment of two in vitro experimental media. Specifically, the co-culture of Procr+ cells and endothelial cells can be conducted using a transwell system, which facilitates the exchange of secretions between the two cell types without direct physical contact. Subsequently, fluorescence in situ hybridization (FISH) technology will be employed to localize circCARD6. FISH is a molecular cytogenetic technique that enables the detection and localization of specific DNA sequences on chromosomes by utilizing fluorescently labeled DNA probes that bind to complementary DNA sequences within cell chromosomes [15]. However, due to the unique structural characteristics of Circular RNA (circRNA), it is imperative to design probes that are specific to circular RNA. circFISH, targeting the back-splice junction (BSJ) probe, can directly localize circular RNA within cells [16]. The probe design will specifically target the BSJ sequence of corcCARD6.
Expected outcome: Target tracking by FISH demonstrates that circCARD6 is delivered to Procr+ cells via extracellular vesicles from endothelial cells, resulting in an increased abundance of circCARD6 in the cells when Procr+ cells form colonies.
3.3. Objective3: to investigate the impact of the microRNA sponge function of circCARD6 on the differentiation and proliferation of islet stem cells
Rationale: Circular RNA (circRNA) can function as a competitive endogenous RNA to sponge microRNAs (miRNAs). Our objective was to predict the potential miRNA binding sites of circCARD6 using the StarBase database and identify miRNAs that may interact with it. Subsequently, biotin-labeled miRNAs were designed and synthesized in vitro. A circRNA pull-down assay was conducted to confirm the possible existence of the microRNA sponge effect mediated by circCARD6.
Method: I have sequenced circCARD6. Our next step involves identifying the potential miRNA binding sites of circCARD6 within relevant databases. Upon confirmation through database analysis that circCARD6 interacts with two specific miRNAs, namely miRNA-31 and miRNA-29b-3p, these miRNA mimics can be synthesized in vitro to assess their binding affinity to circCARD6. This process necessitates a biotin-miRNA pull-down experiment, wherein biotin-labeled miRNA mimics synthesized in vitro are bound to streptavidin magnetic beads. If circCARD6 serves as a sponge for these miRNAs, it will be co-purified during the pull-down process. To further validate the potential microRNA sponge effect of circCARD6, it is imperative to investigate the mechanism of miRNA function to substantiate its influence on the differentiation and proliferation of Procr+ cells. miRNAs function by binding to mRNA and inhibiting protein production, thereby regulating gene expression [17]. In physiological contexts, miRNAs directly modulate the expression of the majority of mRNAs across diverse biological processes [18]. Additionally, I can use databases to identify the targets of bound miRNAs and their downstream associated proteins. Upon identification of relevant downstream proteins, two parallel in vitro experiments will be conducted using identical co-culture media consisting of Procr+ cells and endothelial cells. In one medium, miRNA will be over-expressed, followed by the evaluation of the colony-forming ability of Procr+ cells in both media. Subsequently, upon over-expression of the downstream protein, circCARD6 will be over-expressed again to assess the colony-forming capacity of Procr+ cells. Expected outcomes include: if miRNA is over-expressed in the co-culture medium of Procr+ cells and endothelial cells, it is anticipated to reduce downstream protein levels and diminish the colony-forming ability of Procr+ cells. Conversely, following over-expression of the downstream protein, if circCARD6 over-expression leads to a recovery in downstream protein content and an enhancement in the colony-forming ability of Procr+ cells, this would indicate that circCARD6 promotes the colony formation and subsequent growth of Procr+ cells into pancreatic tissue.
Expected outcome: If miRNA is over-expressed in the co-culture medium of Procr+ cells and endothelial cells, it is expected to reduce downstream protein levels and diminish the colony-forming ability of Procr+ cells. Conversely, following over-expression of the downstream protein, if circCARD6 over-expression leads to a recovery in downstream protein content and an enhancement in the colony-forming ability of Procr+ cells, this would indicate that circCARD6 promotes the colony formation and subsequent growth of Procr+ cells into pancreatic tissue.
4. Conclusion
In this study, I propose a novel mechanism to elucidate the role of circCARD6 in extracellular vesicles derived from endothelial cells, which promotes the growth of Procr⁺ cells into pancreatic islet organoids. A comprehensive understanding of the specific mechanism by which circCARD6 facilitates the growth of Procr⁺ cells would represent a significant breakthrough in diabetes treatment. This knowledge may facilitate the differentiation of human Procr⁺ cells into organoids, enabling their subsequent differentiation into β, α, δ, and PP cells, thereby restoring normal insulin secretion. However, the factors contributing to the promotion of colony formation in Procr⁺ cells may extend beyond circular RNA, and the influence of circular RNA on the growth of Procr⁺ cells might be negligible. Future exploration of the underlying mechanisms by which endothelial cells promote the growth of Procr⁺ cells into pancreatic islet organoids is a critical step toward advancing diabetes therapy. Collectively, endothelial cell-derived circCARD6 is transferred to Procr⁺ cells via extracellular vesicles and functions as a microRNA sponge, potentially enhancing their differentiation and proliferation. This mechanism underscores circCARD6 as a promising molecular target for promoting islet regeneration. Ultimately, these findings may lay the foundation for innovative therapeutic strategies in diabetes management, leveraging circRNA-mediated intercellular communication to augment islet organoid formation.
References
[1]. Gillespie KM. Type 1 diabetes: pathogenesis and prevention. Canadian Medical Association Journal. 2006 Jun 27; 175(2): 165–70.
[2]. DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 Diabetes Mellitus. Nature Reviews Disease Primers. 2015 Jul 23; 1(1).
[3]. Pan FC, Wright C. Pancreas organogenesis: From bud to plexus to gland. Developmental Dynamics. 2011 Feb 17; 240(3): 530–65.
[4]. Wang D, Wang J, Bai L, Pan H, Feng H, Clevers H, et al. Long-Term Expansion of Pancreatic Islet Organoids from Resident Procr+ Progenitors. Cell. 2020 Mar; 180(6): 1198-1211.e19.
[5]. Wang J, Wang D, Chen X, Yuan S, Bai L, Liu C, et al. Isolation of mouse pancreatic islet Procr+ progenitors and long-term expansion of islet organoids in vitro. Nature Protocols [Internet]. 2022 Apr 8 [cited 2025 Jul 23]; 17(5): 1359–84.
[6]. Lammert E, Cleaver O, Melton D. Induction of Pancreatic Differentiation by Signals from Blood Vessels. Science [Internet]. 2001 Oct 19 [cited 2021 Aug 26]; 294(5542): 564–7.
[7]. Hromada C, Mühleder S, Grillari J, Redl H, Holnthoner W. Endothelial Extracellular Vesicles—Promises and Challenges. Frontiers in Physiology. 2017 May 5; 8.
[8]. Lasda E, Parker R. Circular RNAs Co-Precipitate with Extracellular Vesicles: A Possible Mechanism for circRNA Clearance. Busson P, editor. PLOS ONE. 2016 Feb 5; 11(2): e0148407.
[9]. Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013 Feb 27; 495(7441): 333–8.
[10]. Latreille M, Hausser J, Stützer I, Zhang Q, Hastoy B, Gargani S, et al. MicroRNA-7a regulates pancreatic β cell function. Journal of Clinical Investigation. 2014 May 1; 124(6): 2722–35.
[11]. Xu H, Guo S, Li W, Yu P. The circular RNA Cdr1as, via miR-7 and its targets, regulates insulin transcription and secretion in islet cells. Scientific Reports. 2015 Jul; 5(1).
[12]. Leïla Halidou Diallo, Mariette J, Laugero N, Touriol C, Florent Morfoisse, Prats AC, et al. Specific Circular RNA Signature of Endothelial Cells: Potential Implications in Vascular Pathophysiology. International journal of molecular sciences. 2024 Jan 4; 25(1): 680–0.
[13]. W. A. Bonner, H. R. Hulett, R. G. Sweet, L. A. Herzenberg; Fluorescence Activated Cell Sorting. Rev. Sci. Instrum. 1 March 1972; 43 (3): 404–409.
[14]. Basu S, Campbell HM, Dittel BN, Ray A. Purification of specific cell population by fluorescence activated cell sorting (FACS). J Vis Exp. 2010 Jul 10; (41): 1546.
[15]. Bayani, J. and Squire, J.A. (2004), Fluorescence In Situ Hybridization (FISH). Current Protocols in Cell Biology, 23: 22.4.1-22.4.52.
[16]. Aakash Koppula, Abdelgawad A, Jlenia Guarnerio, Batish M, Parashar V. CircFISH: A Novel Method for the Simultaneous Imaging of Linear and Circular RNAs. Cancers [Internet]. 2022 Jan 15 [cited 2024 May 5]; 14(2): 428–8.
[17]. Krützfeldt J, Poy MN, Stoffel M. Strategies to determine the biological function of microRNAs. Nature Genetics. 2006 May 30; 38(S6): S14–9.
[18]. Lewis BP, Burge CB, Bartel DP. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates that Thousands of Human Genes are MicroRNA Targets. Cell. 2005 Jan; 120(1): 15–20.
Cite this article
Deng,Y. (2025). The Role of Circular RNA Derived from Endothelial Cells in Promoting the Formation of Colonies in Procr+ Islet Cell. Theoretical and Natural Science,139,56-61.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Gillespie KM. Type 1 diabetes: pathogenesis and prevention. Canadian Medical Association Journal. 2006 Jun 27; 175(2): 165–70.
[2]. DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 Diabetes Mellitus. Nature Reviews Disease Primers. 2015 Jul 23; 1(1).
[3]. Pan FC, Wright C. Pancreas organogenesis: From bud to plexus to gland. Developmental Dynamics. 2011 Feb 17; 240(3): 530–65.
[4]. Wang D, Wang J, Bai L, Pan H, Feng H, Clevers H, et al. Long-Term Expansion of Pancreatic Islet Organoids from Resident Procr+ Progenitors. Cell. 2020 Mar; 180(6): 1198-1211.e19.
[5]. Wang J, Wang D, Chen X, Yuan S, Bai L, Liu C, et al. Isolation of mouse pancreatic islet Procr+ progenitors and long-term expansion of islet organoids in vitro. Nature Protocols [Internet]. 2022 Apr 8 [cited 2025 Jul 23]; 17(5): 1359–84.
[6]. Lammert E, Cleaver O, Melton D. Induction of Pancreatic Differentiation by Signals from Blood Vessels. Science [Internet]. 2001 Oct 19 [cited 2021 Aug 26]; 294(5542): 564–7.
[7]. Hromada C, Mühleder S, Grillari J, Redl H, Holnthoner W. Endothelial Extracellular Vesicles—Promises and Challenges. Frontiers in Physiology. 2017 May 5; 8.
[8]. Lasda E, Parker R. Circular RNAs Co-Precipitate with Extracellular Vesicles: A Possible Mechanism for circRNA Clearance. Busson P, editor. PLOS ONE. 2016 Feb 5; 11(2): e0148407.
[9]. Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013 Feb 27; 495(7441): 333–8.
[10]. Latreille M, Hausser J, Stützer I, Zhang Q, Hastoy B, Gargani S, et al. MicroRNA-7a regulates pancreatic β cell function. Journal of Clinical Investigation. 2014 May 1; 124(6): 2722–35.
[11]. Xu H, Guo S, Li W, Yu P. The circular RNA Cdr1as, via miR-7 and its targets, regulates insulin transcription and secretion in islet cells. Scientific Reports. 2015 Jul; 5(1).
[12]. Leïla Halidou Diallo, Mariette J, Laugero N, Touriol C, Florent Morfoisse, Prats AC, et al. Specific Circular RNA Signature of Endothelial Cells: Potential Implications in Vascular Pathophysiology. International journal of molecular sciences. 2024 Jan 4; 25(1): 680–0.
[13]. W. A. Bonner, H. R. Hulett, R. G. Sweet, L. A. Herzenberg; Fluorescence Activated Cell Sorting. Rev. Sci. Instrum. 1 March 1972; 43 (3): 404–409.
[14]. Basu S, Campbell HM, Dittel BN, Ray A. Purification of specific cell population by fluorescence activated cell sorting (FACS). J Vis Exp. 2010 Jul 10; (41): 1546.
[15]. Bayani, J. and Squire, J.A. (2004), Fluorescence In Situ Hybridization (FISH). Current Protocols in Cell Biology, 23: 22.4.1-22.4.52.
[16]. Aakash Koppula, Abdelgawad A, Jlenia Guarnerio, Batish M, Parashar V. CircFISH: A Novel Method for the Simultaneous Imaging of Linear and Circular RNAs. Cancers [Internet]. 2022 Jan 15 [cited 2024 May 5]; 14(2): 428–8.
[17]. Krützfeldt J, Poy MN, Stoffel M. Strategies to determine the biological function of microRNAs. Nature Genetics. 2006 May 30; 38(S6): S14–9.
[18]. Lewis BP, Burge CB, Bartel DP. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates that Thousands of Human Genes are MicroRNA Targets. Cell. 2005 Jan; 120(1): 15–20.









