1. Introduction
Since their introduction, antibiotics have played an irreplaceable role in treating infectious diseases and reducing mortality, becoming a cornerstone of modern medical systems. However, their extensive use in both clinical and daily life has led to the growing problem of resistance caused by irrational use, with some common infections facing treatment failure due to the emergence of resistant bacteria. Communities, as the “last mile” connecting healthcare systems to residents, are not only a primary setting for antibiotic use but also a weak link in resistance prevention and control. In community settings, inadequate public knowledge of antibiotics leads to self-medication; lax prescription management in primary healthcare institutions and regulatory loopholes in retail pharmacy sales further exacerbate the problem, making it difficult to effectively curb antibiotic misuse. Therefore, in-depth research on community-level interventions for the rational use of antibiotics, along with analysis of their implementation logic and actual effectiveness, is of significant value in addressing gaps in grassroots resistance control and improving the public health governance system. This paper explores the issue from multiple dimensions, providing practical references for improving community antibiotic management.
2. Significance of intervention measures
2.1. Enhancing public awareness of rational antibiotic use
Public understanding of antibiotics is directly linked to whether medication behaviors are compliant and thus determines the overall level of standardization in community antibiotic use. As the end-users of antibiotics, residents’ knowledge levels largely dictate the rationality of use [1-2]. In some communities, many residents mistakenly believe “antibiotics are simply anti-inflammatory drugs,” leading to frequent self-purchase and misuse for common ailments such as colds and fevers. Targeted public education initiatives in communities can inform residents about the mechanisms of antibiotics, their appropriate scope of use, and the dangers of misuse. Delivering such key information helps dispel entrenched misconceptions and enables residents to make more rational decisions when seeking medical care or purchasing medicine. This, in turn, reduces unnecessary antibiotic use at the source and lays the cognitive foundation for the scientific use of antibiotics in the community (see Figure 1).
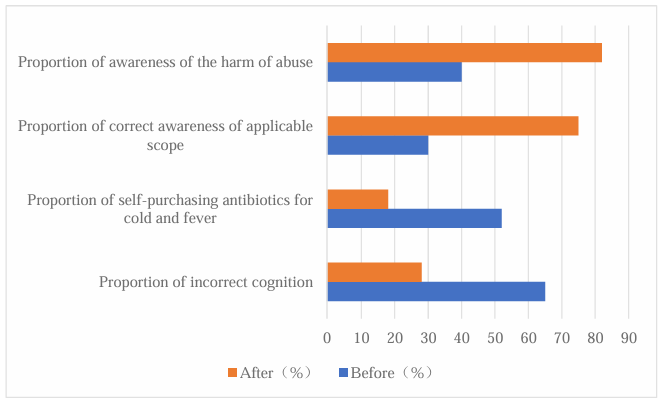
2.2. Standardizing prescription practices of community healthcare personnel
The prescribing behavior of community healthcare personnel is a critical factor in ensuring rational antibiotic use, as their professional competence and adherence to norms directly impact medication safety and effectiveness. In community settings, prescriptions are issued directly by healthcare providers, and their clinical judgment and prescribing habits significantly influence rationality. Some primary healthcare institutions still operate under outdated “medicine-based revenue” models, or healthcare workers fail to update their knowledge according to current antibiotic guidelines, resulting in issues such as excessive dosages, unnecessarily long courses, or overuse of broad-spectrum antibiotics. Intervention measures—such as establishing prescription review systems and conducting clinical medication training—can help healthcare providers accurately understand the indications and contraindications for antibiotic use, ensuring prescriptions align with clinical guidelines [3-4]. Standardized prescribing improves treatment outcomes, reduces unnecessary patient exposure to medication, and minimizes the emergence of resistant bacteria from the very start of medical intervention, thus strengthening community-level resistance control.
2.3. Curbing the development of antibiotic resistance
The spread of antibiotic resistance poses a major global public health threat, and its continued escalation increases the risk of treatment failure for common infections while driving up medical costs. In one community case, prior to targeted interventions in 2022, methicillin-resistant Staphylococcus aureus had a prevalence rate of 40%, and Klebsiella pneumoniae showed a high resistance rate of 35%. By late 2023, following a series of interventions—including public education and prescription standardization—the resistance rate of S. aureus fell to 30%, and K. pneumoniae dropped significantly to 22%. This change clearly demonstrates the positive role of community interventions in curbing resistance (see Table 1). The implementation of resistant bacterial infection monitoring and reporting can provide data support for regional resistance control, facilitating a coherent, end-to-end resistance management system that fundamentally shapes the trajectory of antibiotic resistance.
|
Name |
Before(%) |
After(%) |
|
The drug resistance rate of Staphylococcus aureus |
40 |
30 |
|
The drug resistance rate of Klebsiella pneumoniae |
35 |
22 |
3. Factors influencing the effectiveness of interventions
3.1. Resident-related factors
As the direct recipients of community-based interventions for the rational use of antibiotics, residents’ personal characteristics determine how they receive, understand, and apply intervention messages, thereby influencing the extent to which the intended goals are achieved. Variables such as age, education level, and health beliefs significantly affect the acceptance and effectiveness of interventions. For instance, older adults—given their relatively limited access to information—may have lower adaptability to new forms of health education and thus struggle to fully comprehend intervention content. Residents with lower levels of education may lack the capacity to interpret medical information accurately and may ignore intervention advice due to the influence of traditional medication habits [5-6]. In some cases, entrenched beliefs such as “antibiotics work quickly” persist; even when aware of the harms of misuse, individuals may still opt for inappropriate use under pressure to relieve symptoms, reducing the overall impact of the intervention.
3.2. Community environmental factors
As the primary setting in which residents’ daily healthcare behaviors occur, community environmental characteristics directly shape the conditions for implementing interventions and the pathways through which they operate. Factors such as the allocation of healthcare resources and patterns of neighborhood interaction can indirectly affect intervention outcomes. In resource-poor communities, primary healthcare facilities often have outdated equipment and insufficient staff, reducing the rigor of prescription reviews and narrowing the reach of medication guidance, making it difficult to achieve the desired intervention standards. In resource-rich communities, excessive density of pharmacies can disrupt market balance, leading some stores to bypass prescription requirements to attract customers, thereby undermining regulatory efforts. Additionally, collective perceptions within a community exert strong behavioral influence; if stereotypes about antibiotic use are widely shared among residents, peer imitation can form a negative transmission chain, creating invisible resistance to intervention efforts and diminishing overall effectiveness.
3.3. Intervention-related factors
Intervention measures act as the bridge between objectives and outcomes, and their scientific design and consistent implementation are prerequisites for effectiveness. In some communities, interventions suffer from formalism—for example, relying solely on posters and pamphlets for public education. Such methods lack interactivity and targeting, fail to align with residents’ preferred modes of information intake, and are less likely to stimulate active participation. Short intervention cycles may also hinder habit formation; once interventions end, the absence of sustained external guidance can cause residents to revert to previous patterns. Furthermore, when interventions are not tailored to actual community needs, their applicability diminishes—such as delivering online training courses in an agriculture-focused community, where the approach may be disconnected from residents’ daily contexts, reducing feasibility and limiting integration into daily life.
4. Community-based interventions for the rational use of antibiotics
4.1. Conducting public education campaigns
Some residents, due to a lack of professional knowledge, treat antibiotics as a “cure-all” and use them indiscriminately in non-bacterial infections. Public education activities can specifically address such cognitive gaps and establish a conceptual foundation for rational drug use. Educational efforts should avoid a single, uniform model and instead be tailored to the demographic characteristics of the community. For example, a permanent health education area can be set up in a prominent location at the community health service center, featuring high-quality illustrated displays explaining the mechanisms of antibiotics and using physical specimens to show the morphology of resistant bacteria—visually illustrating the microscopic harms of misuse. For older adults, whose information reception habits differ, experienced healthcare staff can hold regular offline lectures, explaining in clear and simple language the indications and contraindications for antibiotic use, and dispelling common misconceptions such as “injections work faster.” For middle-aged and younger residents, community social media platforms can be used to share weekly short videos and infographics created by professional teams, covering practical topics such as “The difference between antibiotics and anti-inflammatory drugs” and “How to dispose of leftover medications.” Content should avoid overuse of technical jargon, instead using popular health concerns as entry points. Continuous information delivery can gradually correct misconceptions (see Table 2).
|
Formal Name |
Regarding the group |
Implementation Method |
Objective |
|
Permanent Science Popularization Exhibition |
All residents |
The community health center sets up a special area, using graphic display boards and physical specimens to showcase the mechanism of antibiotics and the hazards of drug-resistant bacteria. |
Intuitively convey knowledge, fill the cognitive gap |
|
Offline Lecture |
The elderly group |
Medical staff give lectures regularly, explaining in simple terms the applicable scope, contraindications, and common misunderstandings of antibiotics. |
Adapt to the receiving habits, help them understand and correct their cognition |
|
Online Content Pushing |
The young and middle-aged group |
The community online platform releases professional science popularization short videos and graphics every week, analyzing practical issues. |
Match the information acquisition habits, convey knowledge and correct cognition |
4.2. Strengthening the training of healthcare personnel
Community healthcare personnel are the direct executors of prescription issuance, and their professional competence and attitudes toward medication directly determine the rationality of antibiotic use. However, some primary healthcare staff fail to update their knowledge in line with current guidelines or lack sufficient risk awareness. Accelerating systematic training for this group is a core measure for standardizing prescription behavior, and such training should be embedded in a regular, institutionalized framework [7-8]. Community health service centers should take the lead in formulating annual training plans. Each quarter, infectious disease specialists from higher-level hospitals can be invited to conduct thematic training sessions, explaining the latest clinical guidelines for antibiotic use and analyzing treatment standards for common community conditions such as respiratory tract and urinary tract infections. A prescription review system should be established, with a panel of senior physicians conducting weekly random audits of antibiotic prescriptions to evaluate appropriateness in terms of indications, dosage, and treatment duration. Problematic prescriptions should be traced back to their prescribers, with feedback provided for improvement. Staff should also be encouraged to participate in national continuing education programs, make use of online courses to stay informed about current resistance monitoring data and the pharmacology of new antibiotics, and link training assessment results to professional promotion and performance evaluation. This creates incentives for continuous learning and ensures that professional knowledge evolves in line with clinical practice demands.
4.3. Establishing monitoring and feedback mechanisms
A systematic monitoring and feedback mechanism enables real-time data collection and analysis, providing precise oversight of the entire process of antibiotic use in the community and guiding continuous optimization of interventions. Monitoring systems should cover the full medication process. Community health service centers should develop dedicated information systems requiring healthcare personnel to record, at the time of prescribing antibiotics, details such as the generic name, specifications, dosage, patient demographics, and clinical diagnosis. Dedicated staff can compile and analyze data monthly, generating reports on indicators such as usage frequency of different antibiotic types, average treatment duration, and the proportion of combination therapies. These can be compared with national recommendations to identify anomalies. Cases of resistant bacterial infections should be specially registered, with detailed records of patients’ prior medication histories, exposure circumstances, and treatment outcomes. Epidemiological analysis can be used to trace potential transmission chains. Feedback should be delivered at different levels: healthcare personnel should receive comparative data on their individual prescription quality scores versus departmental averages, while community residents should have access to quarterly updates on local antibiotic misuse rates and warnings about resistant bacterial infection risks. Visualizing these data improves accessibility, helping all stakeholders understand the connection between their behaviors and overall medication safety.
4.4. Strengthening oversight of retail pharmacies
Retail pharmacies are a key point at which antibiotics move from the medical system into the community, and the degree to which their sales practices are regulated directly affects rational antibiotic use [9]. Oversight should adopt a collaborative approach: market supervision departments and community committees should jointly develop regular inspection plans, ensuring quarterly, full-coverage inspections of all pharmacies in the jurisdiction. Checks should focus on sales records and prescription retention, verifying that purchaser information matches prescription details. Technological solutions should be implemented, requiring pharmacies to install intelligent prescription verification systems linked in real time to the community health service center’s electronic prescription platform, automatically blocking purchases without valid prescriptions or with mismatched information. A credit rating system should be established, incorporating compliance records into each pharmacy’s corporate credit file. Pharmacies with no violations for three consecutive years should receive preferential consideration in government procurement and medical insurance designation reviews [10]. In contrast, pharmacies found to have violated sales regulations should be fined in accordance with the law and publicly named via community bulletin boards and government websites, creating a deterrent environment in which “one violation leads to restrictions across the board.” This encourages pharmacies to take their role in safeguarding medication safety seriously.
5. Conclusion
In summary, the implementation of community-level interventions for the rational use of antibiotics is a critical step in addressing the challenge of resistance. Such measures, by enhancing public awareness and standardizing healthcare personnel prescribing practices, play a positive role in curbing misuse and slowing the development of resistance. Their effectiveness is shaped by multiple factors, including residents’ personal characteristics, community environmental conditions, and the scientific soundness of intervention design. Continuous optimization is needed through targeted public education, regular monitoring and feedback, and strict pharmacy regulation. While current practices have provided valuable experience for grassroots antibiotic management, further efforts are required to develop context-specific strategies and strengthen long-term impact monitoring, thereby providing a solid foundation for protecting public health and improving the public health governance system.
References
[1]. World Health Organization. Antimicrobial resistance: key facts. 2021. https: //www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
[2]. Taylor L. COVID-19: antimicrobial misuse in Americas sees drug resistant infections surge, says WHO. BMJ, 2021; 375: n2845.
[3]. Mladenovic-Antic S, Kocic B, Velickovic-Radovanovic R, et al. Correlation between antimicrobial consumption and antimicrobial resistance of Pseudomonas aeruginosa in a hospital setting: a 10-year study. J Clin Pharm Ther, 2016; 41: 532–537.
[4]. Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis, 2020; 71: 2459–2468.
[5]. Lobie TA, Roba AA, Booth JA, et al. Antimicrobial resistance: a challenge awaiting the post-COVID-19 era. Int J Infect Dis, 2021; 111: 322–325.
[6]. Abelenda-Alonso G, Padullés A, Rombauts A, et al. Antibiotic prescription during the COVID-19 pandemic: a biphasic pattern. Infect Control Hosp Epidemiol, 2020; 41: 1371–1372.
[7]. Hendriksen RS, Munk P, Njage P, et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat Commun, 2019; 10: 1124.
[8]. Adebisi YA, Alaran AJ, Okereke M, et al. COVID-19 and antimicrobial resistance: a review. Infect Dis, 2021; 14: 11786337211033870.
[9]. Seethalakshmi PS, Charity OJ, Giakoumis T, et al. Delineating the impact of COVID-19 on antimicrobial resistance: an Indian perspective. Sci Total Environ, 2022; 818: 151702.
[10]. Cassini E, Pezzani MD. Public health burden of antimicrobial resistance in Europe. Lancet Infect Dis, 2019; 19: 4–6.
Cite this article
Tian,J. (2025). Evaluation of the Effectiveness of Community-Level Interventions for the Rational Use of Antibiotics. Theoretical and Natural Science,139,49-55.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. World Health Organization. Antimicrobial resistance: key facts. 2021. https: //www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
[2]. Taylor L. COVID-19: antimicrobial misuse in Americas sees drug resistant infections surge, says WHO. BMJ, 2021; 375: n2845.
[3]. Mladenovic-Antic S, Kocic B, Velickovic-Radovanovic R, et al. Correlation between antimicrobial consumption and antimicrobial resistance of Pseudomonas aeruginosa in a hospital setting: a 10-year study. J Clin Pharm Ther, 2016; 41: 532–537.
[4]. Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis, 2020; 71: 2459–2468.
[5]. Lobie TA, Roba AA, Booth JA, et al. Antimicrobial resistance: a challenge awaiting the post-COVID-19 era. Int J Infect Dis, 2021; 111: 322–325.
[6]. Abelenda-Alonso G, Padullés A, Rombauts A, et al. Antibiotic prescription during the COVID-19 pandemic: a biphasic pattern. Infect Control Hosp Epidemiol, 2020; 41: 1371–1372.
[7]. Hendriksen RS, Munk P, Njage P, et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat Commun, 2019; 10: 1124.
[8]. Adebisi YA, Alaran AJ, Okereke M, et al. COVID-19 and antimicrobial resistance: a review. Infect Dis, 2021; 14: 11786337211033870.
[9]. Seethalakshmi PS, Charity OJ, Giakoumis T, et al. Delineating the impact of COVID-19 on antimicrobial resistance: an Indian perspective. Sci Total Environ, 2022; 818: 151702.
[10]. Cassini E, Pezzani MD. Public health burden of antimicrobial resistance in Europe. Lancet Infect Dis, 2019; 19: 4–6.









