1. Introduction
Tooth enamel is the hardest tissue in the human body, composed of approximately 96–98% inorganic material, mostly carbonated hydroxyapatite (Ca₁₀ (PO4) ₆(OH)2), with the remaining of water and proteins like acerogenins [1]. This highly mineralized structure provides exceptional resistance to mechanical wear, yet it is particularly vulnerable to chemical erosion when exposed to acidic or chemically imbalanced environments, with dissolution initiating at a critical pH of ~5.5 [2]. The general reaction of the dissolution of hydroxyapatite is:
This process weakens the enamel by losing calcium and phosphate ions into the surroundings. Animal bones, especially chicken thigh bones, serve as effective models for enamel demineralization studies due to their chemically analogous hydroxyapatite content, though enamel lacks collagen and has higher mineralization. Bone tissue contains 65–70% hydroxyapatite by weight, making it chemically analogous to enamel in its calcium phosphate composition [3]. Monitoring mass loss in these bone samples under controlled chemical exposure provides data for quantifying calcium loss and erosion. Swimming pool water is chemically treated with chlorine-based disinfectants such as chlorine gas to maintain microbial safety. When chlorine gas is used, it can generate hydrochloric acid (HCl). The general reaction is:
The release of H+ ions lowers the pH, leading to the dissolution of hydroxyapatite:
Sustained exposure to water below pH 5.5 accelerates hydroxyapatite dissolution. Although standard pool protocols aim for pH 7.2–7.8 and free chlorine levels of 1–3 ppm, deficiencies in pH regulation can result in chemically aggressive water that accelerates enamel erosion [4, 5].
This study aims to simulate real-world swimming exposure by immersing chicken thigh bone samples in various aqueous solutions:
Distilled water (control)
Tap water (baseline mineral content)
Chlorinated solutions at 3 ppm (normal pool), 5 ppm (upper regulatory limit), and 10 ppm (shock-level concentration)
Water from Gilmour Academy pool with a pH of 7.5
2. Materials and methods
2.1. Materials
Fresh chicken thigh bones were used as enamel analogs. Additional materials included distilled water, tap water, swimming pool water, and household bleach (Top Job brand, 1.5% sodium hypochlorite). Laboratory equipment consisted of a Gilson Pipetman pipette (10–100 µL), 50 mL plastic or glass containers, a digital scale (sensitivity 0.01 g), aluminum foil or lids, a permanent marker, and a timer.
2.2. Preparation of chlorine solutions
To simulate varying pool conditions, three chlorine concentrations were prepared by diluting 1.5% household bleach (15,000 ppm sodium hypochlorite) with distilled water. All solutions were made fresh prior to use:
• 3 ppm: 10 µL bleach in 50 mL distilled water
• 10 ppm: 33 µL bleach in 50 mL distilled water
The dilution followed the standard equation:
where C1 represents the initial concentration (ppm), V1 represents the volume of stock solution used (µL), C2 represents the final concentration desired (ppm), and V2 represents the final solution volume (µL). Each solution was mixed gently in labeled containers.
2.3. Bone preparation
Chicken thigh bones were cleaned by boiling and gentle scraping to remove residual tissue, followed by air-drying for 12–24 hours at room temperature. Each bone was weighed using a digital scale, and the initial mass was recorded. Bones were then labeled according to their assigned solution group.
2.4. Experimental setup
Each bone was placed in a separate 50 mL container containing one of the following solutions: distilled water, tap water, swimming pool water, 3 ppm chlorine solution, or 10 ppm chlorine solution. Samples were fully submerged, and containers were sealed with lids to reduce chlorine evaporation. Bones were incubated at room temperature (20–25°C) for 48 hours without agitation. After incubation, bones were removed, gently patted dry with a paper towel, and reweighed. Final mass and visible changes in color, texture, or structural integrity were documented.
3. Results
Mass changes in bone samples were recorded over the experimental period, and corresponding pH values of the solutions were measured. Figure 1 shows the percentage of bone mass retained over time across different water conditions, while Figure 2 illustrates the overall percentage change in bone mass.
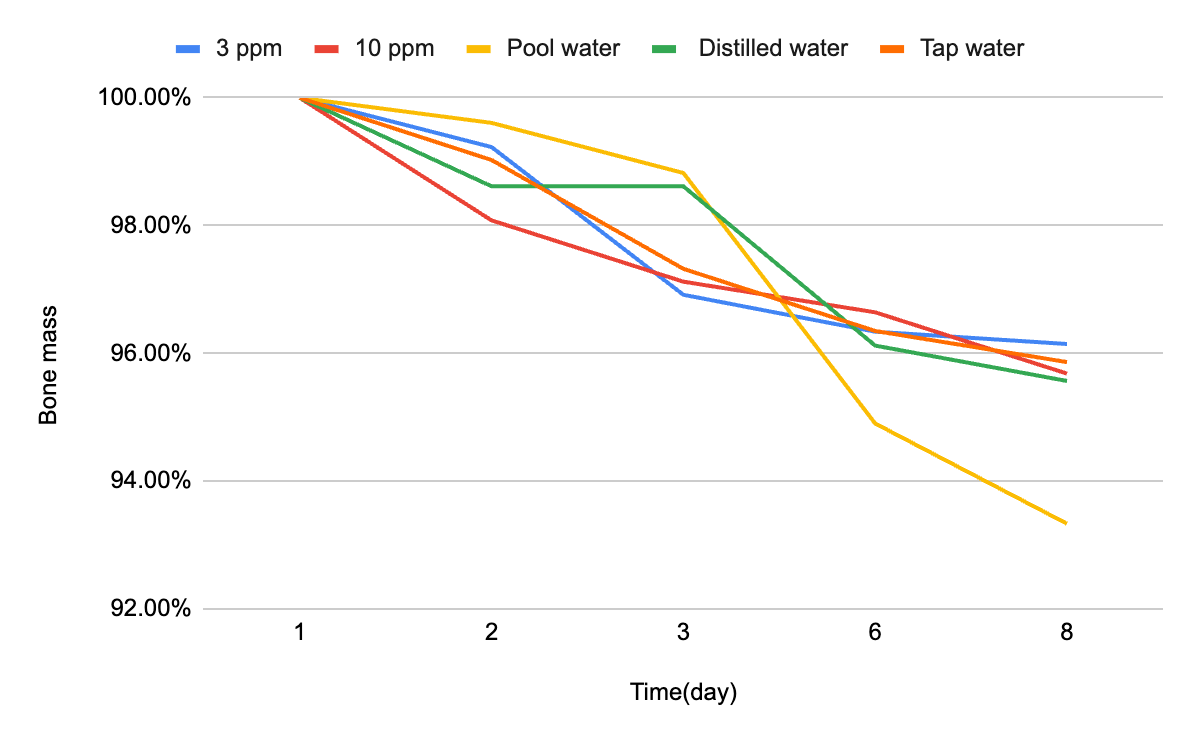
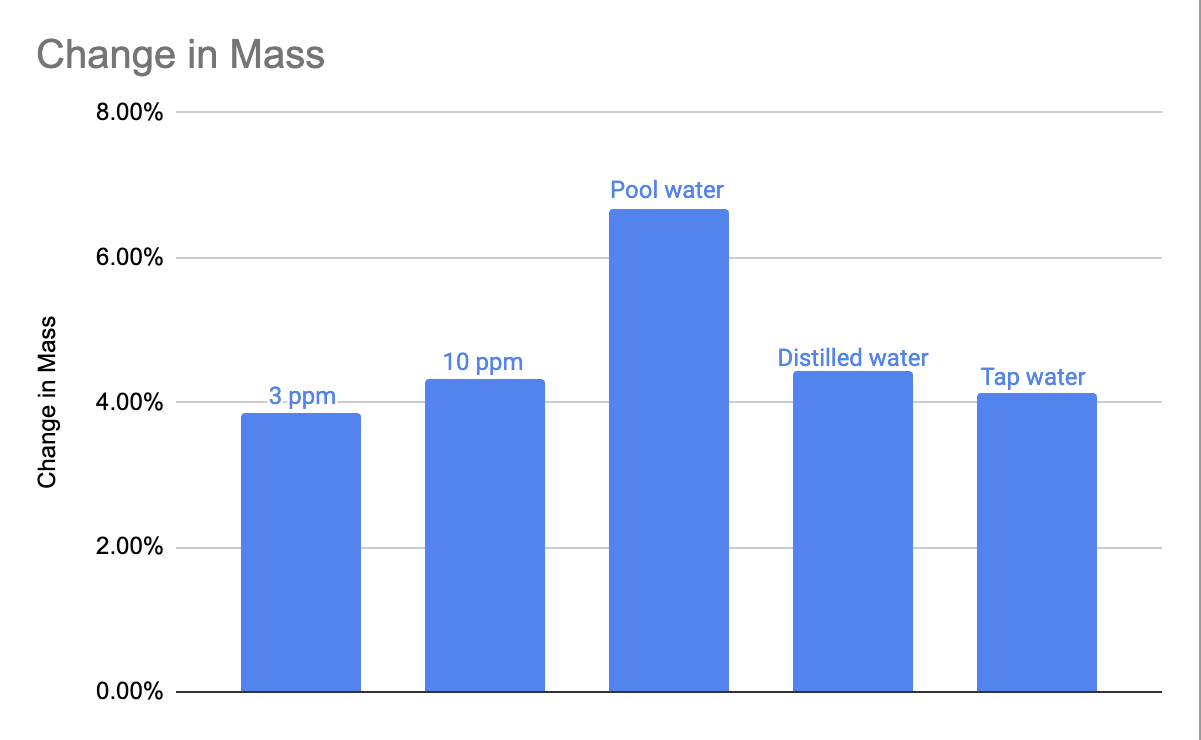
Table 1 summarizes the measured pH values for each solution on Days 1 and 2. Notably, swimming pool water exhibited the lowest pH (6.53 on Day 2), while the 3 ppm chlorine solution maintained the highest pH (7.54 on Day 1).
|
pH |
Distilled water |
Tap water |
Pool water |
3 ppm |
10 ppm |
|
Day 1 |
6.90 |
6.90 |
7.30 |
7.54 |
8.31 |
|
Day 2 |
6.80 |
6.79 |
6.53 |
6.80 |
6.79 |
Table 2 presents the mass changes observed in bone samples. Pool water resulted in the greatest mass loss (6.67%), while the 3 ppm chlorine solution resulted in the least (3.85%).
|
Bone mass (g) |
Day 1 |
Day 2 |
Day 3 |
Day 6 |
Day 8 |
% change in mass |
|
Distilled water |
3.61 |
3.56 |
3.56 |
3.47 |
3.45 |
4.43% |
|
Tap water |
4.11 |
4.07 |
4.00 |
3.96 |
3.94 |
4.13% |
|
Pool water |
2.55 |
2.54 |
2.52 |
2.42 |
2.38 |
6.67% |
|
3 ppm |
5.19 |
5.15 |
5.03 |
5.00 |
4.99 |
3.85% |
|
10 ppm |
4.17 |
4.09 |
4.05 |
4.03 |
3.99 |
4.32% |
The percentage change in bone mass was calculated using Equation (6):
where
4. Discussion
This study examined how different water types influence bone mass loss, serving as a model for enamel erosion. The results showed clear variation: pool water caused the highest loss (6.67%), followed by distilled water (4.43%), 10 ppm chlorine (4.32%), tap water (4.13%), and 3 ppm chlorine (3.85%).
A relationship was observed between pH and degradation. Pool water, with the lowest pH (6.53), produced the greatest loss, while the 3 ppm chlorine solution, with the highest pH (6.80), caused the least. This supports the hypothesis that acidic conditions accelerate hydroxyapatite dissolution, consistent with known mechanisms of enamel erosion.
Distilled water produced more mass loss than either chlorine solution despite a similar pH, likely due to its lack of dissolved ions. In ion-free environments, dissolution continues until the solubility product (Ksp) is reached:
The solubility product is expressed as:
Without common ions present to slow the process, equilibrium shifts toward dissolution until the ion activity product approaches Ksp , explaining the greater loss observed in distilled water.
Chlorine concentration did not correlate directly with erosion. Although the 10 ppm solution caused slightly more loss than 3 ppm, both were less erosive than pool or distilled water. This suggests chlorine acts indirectly by altering pH and overall chemistry, rather than as a direct erosive agent.
Overall, these results indicate that pH stability is a stronger determinant of mineral loss than chlorine concentration. Enamel erosion risk is therefore heightened in poorly maintained pools where pH drops below recommended levels (7.2–7.8), highlighting the importance of proper pool water management for dental health.
5. Conclusion
This study investigated how different water types, varying in chlorine concentration and pH, affect calcium loss in bone samples as a model for enamel erosion. Pool water caused the greatest mass loss (6.67%), likely due to its relatively low pH (6.53) and chemical composition. In contrast, 3 ppm chlorinated water produced the least loss (3.85%), suggesting that pH stability plays a more dominant role in mineral dissolution than chlorine concentration alone.
Distilled water, despite being chlorine-free, also caused significant erosion. This effect is explained by its lack of dissolved ions, which drives dissolution until the solubility product (Ksp) is reached. Thus, both acidic and ion-depleted environments can contribute to enamel weakening.
Overall, these findings highlight that enamel erosion risk is governed primarily by pH and ion balance rather than chlorine level itself. Maintaining proper pool chemistry—especially keeping pH within the recommended 7.2–7.8 range—is therefore essential to protect dental health in individuals with frequent aquatic exposure.
References
[1]. Xu, Jiarong et al. “Advanced materials for enamel remineralization.” Frontiers in bioengineering and biotechnology vol. 10 985881. 13 Sep. 2022, doi: 10.3389/fbioe.2022.985881
[2]. Harper, Robert A et al. “Acid-induced demineralisation of human enamel as a function of time and pH observed using X-ray and polarized light imaging.” Acta biomaterialia vol. 120 (2021): 240-248. doi: 10.1016/j.actbio.2020.04.045
[3]. Habibah TU, Amlani DV, Brizuela M. Hydroxyapatite Dental Material. [Updated 2022 Sep 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https: //www.ncbi.nlm.nih.gov/books/NBK513314/
[4]. Buczkowska-Radlińska, J et al. “Prevalence of dental erosion in adolescent competitive swimmers exposed to gas-chlorinated swimming pool water.” Clinical oral investigations vol. 17, 2 (2013): 579-83. doi: 10.1007/s00784-012-0720-6
[5]. Dawes, Colin, and Carey L Boroditsky. “Rapid and severe tooth erosion from swimming in an improperly chlorinated pool: case report.” Journal (Canadian Dental Association) vol. 74, 4 (2008): 359-61.
Cite this article
Zhang,Y. (2025). The Effect of Pool Water on Enamel Erosion and Dental Health. Theoretical and Natural Science,139,11-16.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Xu, Jiarong et al. “Advanced materials for enamel remineralization.” Frontiers in bioengineering and biotechnology vol. 10 985881. 13 Sep. 2022, doi: 10.3389/fbioe.2022.985881
[2]. Harper, Robert A et al. “Acid-induced demineralisation of human enamel as a function of time and pH observed using X-ray and polarized light imaging.” Acta biomaterialia vol. 120 (2021): 240-248. doi: 10.1016/j.actbio.2020.04.045
[3]. Habibah TU, Amlani DV, Brizuela M. Hydroxyapatite Dental Material. [Updated 2022 Sep 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https: //www.ncbi.nlm.nih.gov/books/NBK513314/
[4]. Buczkowska-Radlińska, J et al. “Prevalence of dental erosion in adolescent competitive swimmers exposed to gas-chlorinated swimming pool water.” Clinical oral investigations vol. 17, 2 (2013): 579-83. doi: 10.1007/s00784-012-0720-6
[5]. Dawes, Colin, and Carey L Boroditsky. “Rapid and severe tooth erosion from swimming in an improperly chlorinated pool: case report.” Journal (Canadian Dental Association) vol. 74, 4 (2008): 359-61.









