1. Introduction
Alzheimer’s disease (AD) is a brain disorder that slowly damages memory and thinking. Changes like amyloid-β (Aβ) plaques and tau tangles start many years before doctors can diagnose them. Therefore, researchers are focusing on identifying early biomarkers that can predict the onset of AD prior to the manifestation of clinical symptoms. One area that is getting a lot of attention is sleep. Sleep is essential for learning, memory, and cleaning the brain. In particular, slow-wave sleep (SWS) can help wash out waste like Aβ. However, prolonged sleep deprivation could exacerbate inflammation and accelerate disease progression [1].
Some studies have found that people with sleep problems are more likely to get dementia. For instance, sleeplessness may nearly double the probability [2]. Sleep apnea is also connected with faster decline and more amyloid [3]. Extensive research, including meta-analyses, indicates that sleep disturbances elevate the risk of Alzheimer's disease by approximately 50% [3]. This shows that sleep is not just something that changes with age but could be part of the disease itself.
Still, normal aging also makes sleep worse. Healthy older adults often sleep less, with less REM and SWS, and their nights are more broken [1]. The real question is when these normal changes become signs of disease. Researchers can try to see which changes are abnormal by comparing normal ageing with early AD. This paper will review (1) sleep structure in normal aging vs. AD, (2) the neurophysiological mechanisms behind these changes, and (3) the predictive value of sleep for AD. Understanding how sleep changes in Alzheimer’s disease compared with normal aging is important since it may provide non-invasive, early-warning markers and open new directions for prevention and treatment.
2. Sleep structure in Alzheimer’s disease
2.1. Sleep in normal aging
Age-related changes in sleep patterns are common. Older individuals typically experience an earlier bedtime and wake time, as well as increased nocturnal awakenings [1]. Their sleep is lighter, too. Polysomnography (PSG) studies have shown more stage N1 light sleep, less SWS and REM, and lower sleep efficiency [1]. EEG studies also report that older adults have weaker delta activity and fewer spindles than young adults. Due to these physiological shifts, a significant portion of the elderly demographic report experiencing non-restorative sleep, irrespective of time spent in bed.
2.2. Abnormal sleep features in early AD
AD patients usually have much bigger sleep problems compared with normal aging. Recent polysomnographic investigations and meta-analyses reveal that individuals exhibit diminished total sleep time (TST), reduced sleep efficiency, and prolonged sleep latency. They wake up more often during the night (WASO); the most clear difference is that they lose a lot of deep sleep [4].
|
PSG Parameter |
AD Patients (mean) |
Controls (mean) |
Effect / Difference |
|
Total Sleep Time (TST) |
~331 min |
~370 min |
↓ AD significantly |
|
Sleep Efficiency (SE) |
~71% |
~81% |
↓ AD significantly |
|
Slow-Wave Sleep (N3/SWS %) |
~4.7% |
~10% |
↓ AD significantly |
|
REM Sleep % |
~14% |
~18% |
↓ AD significantly |
|
Sleep Latency (SL) |
~34 min |
~19 min |
↑ AD significantly |
|
Wake After Sleep Onset (WASO) |
↑ (about +40 min) |
Lower |
↑ AD significantly |
|
REM Latency (REML) |
Longer |
Shorter |
↑ AD significantly |
|
N1 % |
Higher |
Lower |
↑ AD significantly |
|
N2 % |
No significant difference |
— |
— |
|
Number of Awakenings |
Higher |
Lower |
↑ AD significantly |
Table 1 shows that AD patients fall asleep more slowly, have almost no deep N3 sleep, and sleep less efficiently. Although the REM difference looks small, reductions in REM have been linked with future dementia risk [4].
2.3. EEG evidence
EEG gives more detailed evidence. From Figure 1, Alzheimer’s disease (AD) and mild cognitive impairment (MCI) patients show EEG slowing: higher delta/theta power and lower alpha/beta than healthy controls (HC).Sleep spindle activity is also diminished, indicating thalamocortical circuit dysfunction [5]. Coupling between slow waves and spindles is abnormal in early AD, and this predicts memory decline [6].
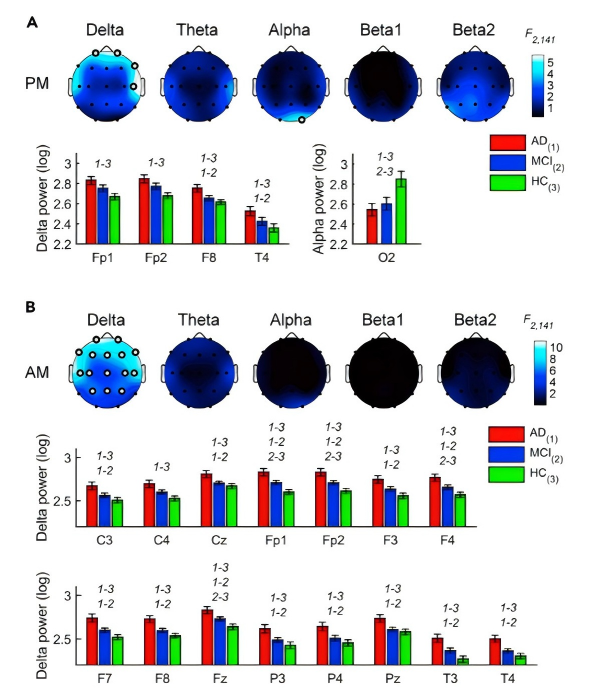
EEG frequency characteristics and spindle density are also suggested as potential electrophysiological biomarkers for Alzheimer's Disease [7]. These results suggest that EEG may be a cheap and non-invasive tool for early diagnosis.
3. Neurophysiological mechanisms underlying sleep changes
3.1. SWS reduction and hippocampal degeneration
Slow-wave sleep (SWS) mainly depends on networks between the hippocampus and cortex. In AD, the hippocampus and the medial prefrontal cortex shrink, breaking the normal slow oscillations. Impaired coupling between slow oscillations and theta bursts correlates with accelerated cognitive decline, suggesting that neuropathology directly modulates sleep architecture [8]. Having less SWS also means the brain clears less waste at night through the glymphatic system, and that can make amyloid build up more quickly.
3.2. REM delay and neurotransmitter systems
Cholinergic neurons control REM sleep in the brainstem and orexin neurons in the hypothalamus. Many cholinergic neurons are lost in AD, making REM take longer to start. At the same time, orexin levels go up, and this makes REM more broken and unstable [7]. Because of that, patients usually have longer REM latency and fewer steady REM periods. So these neurotransmitter changes explain many of the REM problems we see in AD.
3.3. Amyloid and tau effects on sleep centers
Poor sleep promotes amyloid build-up because glymphatic clearance is most active during SWS [9]. Concurrently, the deposition of amyloid plaques and neurofibrillary tangles within sleep-regulating brain regions, such as the hypothalamus and locus coeruleus, exacerbates the disruption of sleep-wake cycles [9].
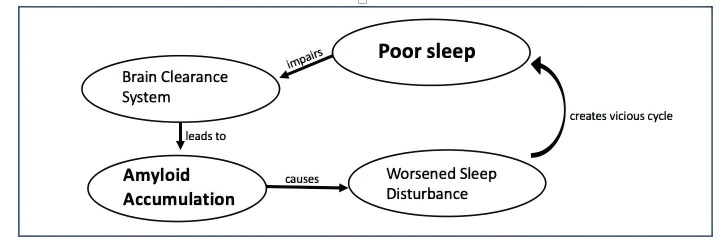
This shows a vicious cycle: pathology worsens sleep, and bad sleep accelerates pathology [6,9].
4. Predictive value of sleep changes in AD
4.1. Multimodal predictive models
Longitudinal research indicates that poor sleep architecture may serve as a predictive biomarker for future dementia development. For instance, reduced REM sleep duration correlates with an elevated dementia risk, with each percentage point decrease in REM sleep potentially increasing the risk by approximately 9% [3]. EEG sleep measures with cerebrospinal fluid (CSF) and magnetic resonance imaging (MRI), and this made their model more accurate, from about 0.84 to 0.90 AUC [8].
Machine learning is also becoming popular in this field. Researchers tested a semi-supervised deep learning method on PSG data, and it could detect AD with over 90% accuracy [2]. Reviews also point out that EEG-based AI models are a growing trend for early detection [10]. These kinds of methods could maybe make it possible to screen large groups of people in the future, even with simple home devices.
4.2. Clinical challenges and ethics
Still, there are many challenges. Sleep problems are widespread in older adults and not always AD [2]. Insomnia, stress, or other diseases may cause similar PSG patterns [2]. This makes specificity low. PSG is expensive and impractical for routine screening, while consumer devices may lack precision.
There are also ethical issues. Communicating an "Alzheimer's Disease risk" to patients solely on the basis of sleep metrics may precipitate undue anxiety, particularly given the current absence of a definitive cure [3,11]. Long-term data collection also raises privacy questions. Therefore, sleep metrics should be used together with other biomarkers.
5. Conclusion
Alzheimer’s disease changes sleep a lot, even in the early stage. Compared with normal aging, patients usually have less slow-wave sleep (SWS) and REM, take longer to fall asleep, and have more broken nights. EEG also shows slower rhythms and fewer spindles. These changes mostly come from brain damage in the hippocampus and cortex, neurotransmitter problems, and amyloid and tau building up in sleep centers. Poor sleep and disease push each other, worsening the decline over time.
More recent studies suggest that sleep itself can be used as a biomarker. Less REM, abnormal coupling between spindles and slow waves, and low efficiency predict cognitive decline. Machine learning studies with PSG and EEG also report strong accuracy. Integrating sleep metrics with cerebrospinal fluid assays and magnetic resonance imaging enhances predictive accuracy, potentially facilitating early identification of individuals at elevated risk.
Undoubtedly, numerous challenges persist. It is difficult to tell which sleep changes are from normal aging and which are from AD. There are also ethical issues and problems with collecting large-scale data. However, sleep is attractive because it is non-invasive and can be improved. If future work shows that better sleep can slow down AD, then sleep will not only be a symptom but maybe also a way to fight the disease.
References
[1]. Pathmanathan, J., Westover, M. B., Sivakumaran, S., Donoghue, J., & Puryear, C. B. (2025). The role of sleep in Alzheimer’s disease: A mini review. Frontiers in Neuroscience, 19, 1428733. https: //doi.org/10.3389/fnins.2025.1428733
[2]. Gallego-Viñaras, L., Mira-Tomás, J. M., Gaeta, A. M., et al. (2024). Alzheimer’s disease detection in PSG signals: Exploring semi-supervised deep learning for sleep EEG. arXiv preprint. arXiv: 2404.03549
[3]. Ungvari, Z., Fekete, M., Lehoczki, A., et al. (2025). Sleep disorders increase the risk of dementia, Alzheimer’s disease, and cognitive decline: A meta-analysis. GeroScience, 47, 4899–4920. https: //doi.org/10.1007/s11357-025-01637-2
[4]. Zhang, Y., Ren, R., Yang, L., et al. (2022). Sleep in Alzheimer’s disease: A systematic review and meta-analysis of polysomnographic findings. Translational Psychiatry, 12(1), 136. https: //doi.org/10.1038/s41398-022-01868-w
[5]. D’Atri, A., Scarpelli, S., Gorgoni, M., et al. (2021). EEG alterations during wake and sleep in mild cognitive impairment and Alzheimer’s disease. iScience, 24(4), 102386. https: //doi.org/10.1016/j.isci.2021.102386
[6]. Liguori, C., Mercuri, N. B., Izzi, F., et al. (2017). Timely coupling of sleep spindles and slow waves linked to early amyloid-β burden in Alzheimer’s disease. Scientific Reports, 7, 9493. https: //doi.org/10.1038/s41598-017-10325-8
[7]. Yu, H., Wang, M., Xu, X., Zhang, R., & Le, W. (2021). Changes in electroencephalography and sleep architecture as potential electrical biomarkers for Alzheimer’s disease. Chinese Medical Journal, 134(6), 662–664. https: //doi.org/10.1097/CM9.0000000000001394
[8]. Tang, Y., Wei, T., Zhao, B., et al. (2025). Coupled sleep rhythm disruption predicts cognitive decline in Alzheimer’s disease. Science Bulletin, 70(12), 1491–1503. https: //doi.org/10.1016/j.scib.2025.03.023
[9]. Astara, K., Tsimpolis, A., Kalafatakis, K., et al. (2024). Sleep disorders and Alzheimer’s disease pathophysiology: The role of the glymphatic system. Mechanisms of Ageing and Development, 217, 111899. https: //doi.org/10.1016/j.mad.2023.111899
[10]. Aviles, M., Sánchez-Reyes, L. M., Álvarez-Alvarado, J. M., & Rodríguez-Reséndiz, J. (2024). Machine and deep learning trends in EEG-based detection and diagnosis of Alzheimer’s disease: A systematic review. Eng, 5(3), 1464–1484. https: //doi.org/10.3390/eng5030078
[11]. Sadeghmousavi, S., Eskian, M., Rahmani, F., & Rezaei, N. (2020). The effect of insomnia on the development of Alzheimer’s disease. Journal of Neuroinflammation, 17(1), 289. https: //doi.org/10.1186/s12974-020-01960-9
Cite this article
Huang,R. (2025). Sleep Structure Alterations in Early Alzheimer’s Disease and Their Predictive Value. Theoretical and Natural Science,141,70-74.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Pathmanathan, J., Westover, M. B., Sivakumaran, S., Donoghue, J., & Puryear, C. B. (2025). The role of sleep in Alzheimer’s disease: A mini review. Frontiers in Neuroscience, 19, 1428733. https: //doi.org/10.3389/fnins.2025.1428733
[2]. Gallego-Viñaras, L., Mira-Tomás, J. M., Gaeta, A. M., et al. (2024). Alzheimer’s disease detection in PSG signals: Exploring semi-supervised deep learning for sleep EEG. arXiv preprint. arXiv: 2404.03549
[3]. Ungvari, Z., Fekete, M., Lehoczki, A., et al. (2025). Sleep disorders increase the risk of dementia, Alzheimer’s disease, and cognitive decline: A meta-analysis. GeroScience, 47, 4899–4920. https: //doi.org/10.1007/s11357-025-01637-2
[4]. Zhang, Y., Ren, R., Yang, L., et al. (2022). Sleep in Alzheimer’s disease: A systematic review and meta-analysis of polysomnographic findings. Translational Psychiatry, 12(1), 136. https: //doi.org/10.1038/s41398-022-01868-w
[5]. D’Atri, A., Scarpelli, S., Gorgoni, M., et al. (2021). EEG alterations during wake and sleep in mild cognitive impairment and Alzheimer’s disease. iScience, 24(4), 102386. https: //doi.org/10.1016/j.isci.2021.102386
[6]. Liguori, C., Mercuri, N. B., Izzi, F., et al. (2017). Timely coupling of sleep spindles and slow waves linked to early amyloid-β burden in Alzheimer’s disease. Scientific Reports, 7, 9493. https: //doi.org/10.1038/s41598-017-10325-8
[7]. Yu, H., Wang, M., Xu, X., Zhang, R., & Le, W. (2021). Changes in electroencephalography and sleep architecture as potential electrical biomarkers for Alzheimer’s disease. Chinese Medical Journal, 134(6), 662–664. https: //doi.org/10.1097/CM9.0000000000001394
[8]. Tang, Y., Wei, T., Zhao, B., et al. (2025). Coupled sleep rhythm disruption predicts cognitive decline in Alzheimer’s disease. Science Bulletin, 70(12), 1491–1503. https: //doi.org/10.1016/j.scib.2025.03.023
[9]. Astara, K., Tsimpolis, A., Kalafatakis, K., et al. (2024). Sleep disorders and Alzheimer’s disease pathophysiology: The role of the glymphatic system. Mechanisms of Ageing and Development, 217, 111899. https: //doi.org/10.1016/j.mad.2023.111899
[10]. Aviles, M., Sánchez-Reyes, L. M., Álvarez-Alvarado, J. M., & Rodríguez-Reséndiz, J. (2024). Machine and deep learning trends in EEG-based detection and diagnosis of Alzheimer’s disease: A systematic review. Eng, 5(3), 1464–1484. https: //doi.org/10.3390/eng5030078
[11]. Sadeghmousavi, S., Eskian, M., Rahmani, F., & Rezaei, N. (2020). The effect of insomnia on the development of Alzheimer’s disease. Journal of Neuroinflammation, 17(1), 289. https: //doi.org/10.1186/s12974-020-01960-9









