1. Introduction
Laos, officially the Lao People’s Democratic Republic, remains one of Southeast Asia’s most rural and under-developed nations in terms of healthcare infrastructure and public health outcomes. Despite national efforts to strengthen primary care, stark disparities persist between urban centers and remote villages.
A survey of ten remote villages in Savannakhet Province, the country’s largest and most populated region, revealed high prevalence of diarrhea, malaria, and respiratory illnesses linked to poor sanitation, inadequate handwashing, open defecation, unsafe drinking water, indoor air pollution from biomass cooking, and malnutrition [1]. Many of these causes could be addressed through low-cost, community-led interventions, yet knowledge gaps and weak intersectoral coordination remain [2]. Village health volunteers play a crucial role in bridging the gap between formal healthcare systems and rural communities, acting as cultural and linguistic mediators. However, challenges remain in sustaining these roles and fully integrating them into the national health system [2].
At the facility level, conditions are equally concerning. A 2021 WHO assessment reported that only 16% of health centers had basic hygiene services and 19% had minimal waste management; just 2% met sanitation standards. Nearly all had been damaged by extreme weather in the past two decades, yet only 1% had implemented resilience measures [3]. Systemic constraints further limit equity of care. Public hospitals are chronically underfunded, understaffed, and overcrowded, forcing patients to pay out-of-pocket costs often exceeding their annual insurance contributions. Non-communicable diseases (NCDs) now account for roughly 60% of adult deaths, reflecting rapid epidemiological transition [4].
These pressures are compounded by climate-related threats—droughts, floods, and heatwaves—amplifying waterborne and vector-borne diseases [5]. My fieldwork documents these realities, from household hygiene practices to provincial hospital limitations, illustrating the gap between local health behaviors and systemic requirements. Addressing this gap demands coordinated, multi-level action: strengthening primary care, improving resilience, expanding financing and governance, and empowering communities through health literacy and resources.
2. Methods
2.1. Study design and setting
A mixed-methods cross-sectional study was conducted in December 2024 in ten remote villages in Savannakhet Province, Laos—the country’s largest and most populated province. The region faces wide healthcare disparities, under-resourced primary facilities, and recurrent mosquito- and waterborne disease outbreaks. Data collection targeted both community members and frontline healthcare providers.
2.2. Community survey
Structured face-to-face surveys assessed community awareness of infectious diseases, health-seeking behaviors, preventive practices, and hygiene conditions. Participants were recruited from primary health centers and surrounding villages through purposive sampling to capture diverse household situations. Questions addressed knowledge of disease names and symptoms, reasons for seeking care, mosquito prevention methods, vaccination status, drinking water use and boiling practices, and sources of health education. Responses were recorded by trained interviewers fluent in Lao and local dialects, digitized into Excel, and analyzed descriptively in Python.
2.3. Healthcare provider survey
A parallel survey was administered to rural healthcare workers, focusing on commonly treated infectious diseases, diagnostic and treatment practices, infection control methods, and seasonal case trends. Providers were also asked about systemic challenges, governmental support, and community outreach activities, and offered qualitative input on barriers and resource needs. Data were anonymized and aggregated prior to analysis.
2.4. Data analysis
Quantitative data were analyzed in Python (pandas, matplotlib, seaborn). Frequencies and distributions were summarized in bar charts, pie charts, and composite figures, while provider responses on barriers and support needs were coded and quantified through keyword frequency analysis. Figures were standardized for clarity and comparability.
3. Results
3.1. Citizens’ knowledge and health-seeking behavior
Survey data from rural communities revealed substantial limitations in disease literacy and preventive behaviors. As shown in Figure 1A, persistent fever was the overwhelming driver of healthcare-seeking, followed by more general complaints such as feeling unwell, fatigue, and pain. Only a small proportion cited diarrhea or bleeding as a primary reason to visit a clinic. When asked if they knew the name of their illness, most respondents admitted they did not know or could only describe symptoms; relatively few correctly named diseases such as dengue fever or malaria (Figure 1B).
Preventive practices were inconsistently adopted. Over half of respondents reported using mosquito nets and ~43% reported mosquito repellents, though very few mentioned medical-grade nets (Figure 1C). Vaccination coverage was strikingly low, with only 3.2% of participants reporting having received vaccinations (Figure 1D). These findings underscore a persistent gap between community perceptions of illness and effective prevention strategies.
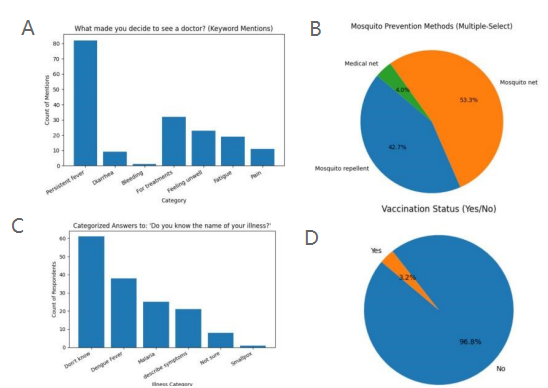
(A) Reasons for seeking healthcare, dominated by persistent fever. (B) Ability to name illnesses, with most reporting only symptoms. (C) Preventive practices, mainly mosquito nets and repellents. (D) Vaccination coverage, reported by only 3.2% of respondents.
3.2. Clinical perspectives on disease burden
Frontline providers emphasized mosquito-borne illnesses as the dominant clinical challenge. According to provider reports, dengue fever and malaria were the most common epidemic diseases, far outnumbering nonspecific fever cases (Figure 2A). Nearly all healthcare workers linked case surges to the rainy season, with minimal reports of cases being unaffected by seasonal patterns (Figure 2B).
This strong seasonal signal suggests that clinics face predictable but overwhelming patient loads during certain months, exacerbating the limitations of rural health facilities.
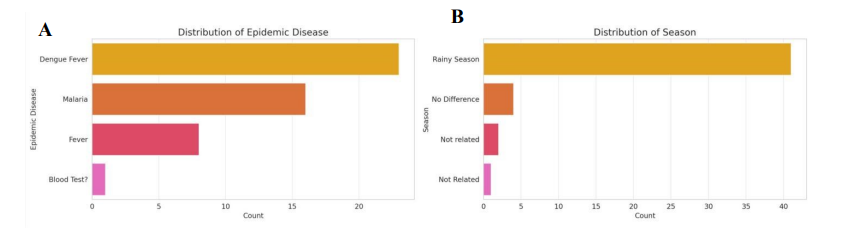
(A) Most frequently reported epidemic diseases, led by dengue and malaria. (B) Seasonal variation in cases, with peaks during the rainy season.
3.3. Diagnosis, treatment, and infection control practices
Clinical management of infectious diseases was heavily constrained by limited resources. Providers reported relying primarily on patient symptoms and physical examinations to guide diagnosis, with laboratory tests or medical history only occasionally available (Figure 3C).
Treatment recommendations were dominated by hydration, symptomatic management, and antipyretics, with antibiotics prescribed far less frequently (Figure 3A). This reflects both cautious prescribing practices and limited drug availability. Beyond treatment, doctors also engaged in public health messaging, most commonly delivering general well-wishes, mosquito control advice, and hygiene reminders, while structured health education campaigns were less consistently emphasized (Figure 3B).
Infection control practices further revealed systemic constraints. Heat treatment and alcohol disinfection were the most widely used sterilization methods, with fewer facilities reporting access to UV sterilization and almost none using advanced equipment (Figure 3D). These findings indicate that infection control remains dependent on basic, low-cost methods, leaving both patients and staff vulnerable during outbreak seasons.
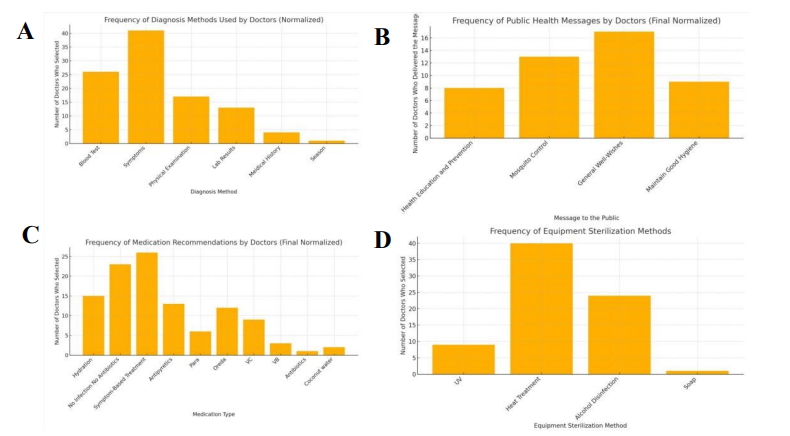
(A) Common treatment recommendations, mainly hydration and antipyretics. (B) Public health messages, including mosquito control and hygiene. (C) Diagnostic approaches, largely symptom-based with limited testing. (D) Sterilization methods, dominated by heat and alcohol disinfection.
3.4. Structural barriers, systemic support, and environmental risks
Doctors cited numerous systemic challenges that constrained their ability to provide care. Lack of medical equipment emerged as the most frequent and pressing concern, followed by shortages of trained experts, limited clinical experience, and low levels of disease awareness among the public (Figure 4A).
Community health education was reported as highly centralized. Hospitals were overwhelmingly identified as the main source of health messaging, with limited input from community, family, or government sources (Figure 4B). Providers also expressed a strong desire for expanded training opportunities; most requested additional medical training as the most urgent form of government support, while only a minority mentioned broader systemic reforms or infection-source elimination (Figure 4C).
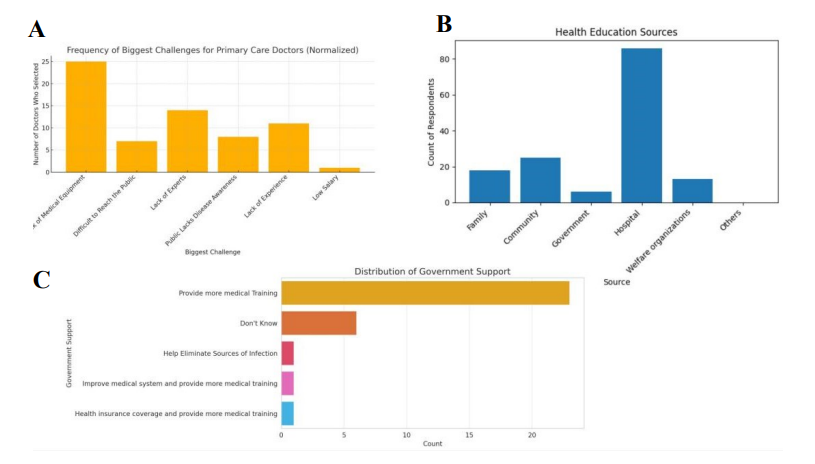
(A) Key provider-reported challenges, led by equipment shortages. (B) Main sources of health education, dominated by hospitals. (C) Perceived government support, with most requesting additional training.
3.5. Environmental and structural risk factors
Field observations reinforced the quantitative findings and highlighted household-level risks. Drinking water was often drawn from sources near contaminated bodies, without barriers or treatment (Figure 5B). Residents also kept large open containers of stagnant water immediately outside their homes, reportedly for cooling, though these served as mosquito breeding sites (Figure 5A).
In addition, livestock pens with pigs and poultry were frequently located next to dwellings, with no separation from human living spaces. Combined with stagnant water, this created ideal conditions for mosquito proliferation and increased vector-borne disease risk (Figure 5C). No coordinated community-level vector control measures were observed, underscoring how environmental practices directly amplify rural health vulnerabilities.

(A) Stagnant household water containers placed outside homes. (B) Open drainage with unclear separation of clean and contaminated water. (C) Livestock kept adjacent to dwellings, increasing exposure to vectors.
4. Discussion
This study shows how community knowledge gaps, limited clinical resources, and environmental exposures sustain health vulnerabilities in rural Laos. Care-seeking was usually triggered by persistent fever, with few respondents able to name specific diseases (Figure 1A–B), consistent with findings from Savannakhet that households often seek care only after prolonged fever with little diagnostic awareness [6]. Preventive practices such as mosquito net use were reported, yet vaccination coverage remained extremely low (Figure 1C–D).
Clinicians identified dengue and malaria as the dominant infectious diseases, with sharp rainy-season surges (Figure 2). Diagnosis relied largely on symptoms and treatment on hydration and antipyretics, reflecting minimal access to laboratory testing or disease-specific therapies (Figure 3A–C). Similar diagnostic challenges have been reported in Laos, where non-malarial fevers such as rickettsial infections are frequently overlooked [7]. Infection control depended on basic methods such as heat and alcohol sterilization (Figure 3D).
Systemic barriers, including shortages of equipment, training, and staff, further constrained care delivery (Figure 4A–C). Field observations underscored how household practices amplified risks: stagnant water, untreated sources, and livestock proximity created favorable conditions for diarrheal and vector-borne transmission (Figure 5A–C). Such patterns mirror studies showing household water storage drives Aedes aegypti breeding in Lao and Thai villages [8].
Overall, these findings emphasize the need for targeted health education, expanded diagnostic and training resources, and policies linking clinical interventions with environmental management to reduce preventable disease burdens.
5. Conclusion
Rural healthcare in Laos is challenged by low community health literacy, resource-constrained clinical practice, and high environmental risk. Citizens recognize symptoms but rarely diseases, clinicians treat under conditions of scarcity, and systemic shortages compound the burden of preventable illness.
Improving outcomes will require multi-level action: sustained community health education, expanded diagnostic and training resources for clinics, and coordinated policies that link health interventions with environmental and agricultural practices. Bridging these gaps is essential to strengthening equity and resilience in Laos’ healthcare system.
References
[1]. Geng, J., Yuasa, M., Kobayashi, J., Xeuatvongsa, A., & Kuroiwa, C. (2015). Preparing for the introduction of hospital autonomy in Laos: An assessment of current situation and suggestions for policy-making. The International Journal of Health Planning and Management, 31(2), 148–166. https: //doi.org/10.1002/hpm.2283
[2]. Nonaka, D., Sato, Y., Nakamura, Y., Vongphrachanh, P., & Kobayashi, J. (2022). Primary health care situations in remote rural villages of the Savannakhet Province, Lao People’s Democratic Republic. Tropical Medicine and Health, 50(1), 1–10. https: //doi.org/10.1186/s41182-022-00482-9
[3]. World Health Organization. (2024, February 8). Survey reveals gaps in water, sanitation and climate-resilience in health care facilities in Lao PDR. https: //www.who.int/laos/news/detail/08-02-2024-survey-reveals-gaps-in-water--sanitation-and-climate-resilience-in-health-care-facilities-in-lao-pdr
[4]. World Health Organization. (n.d.). Improving health systems to ensure health for all in Lao People’s Democratic Republic. Retrieved July 19, 2025, from https: //www.who.int/laos/our-work/improving-health-systems-to-ensure-health-for-all
[5]. World Health Organization. (2021, July 20). Building climate-resilient health systems in Lao People’s Democratic Republic. https: //www.who.int/news-room/feature-stories/detail/building-climate-resilient-health-systems-in-lao
[6]. Adhikari, B., Phommasone, K., Pongvongsa, T., Soundala, X., Koummarasy, P., Henriques, G., … Newton, P. N. (2019). Treatment-seeking behaviour for febrile illnesses and its implications for malaria control and elimination in Savannakhet Province, Lao PDR: A mixed-method study. BMC Health Services Research, 19(1), 252. https: //doi.org/10.1186/s12913-019-4070-9
[7]. Mayxay, M., Castonguay-Vanier, J., Chansamouth, V., Dubot-Pérès, A., Paris, D. H., Phetsouvanh, R., … Newton, P. N. (2013). Causes of non-malarial fever in Laos: a prospective study. The Lancet Global Health, 1(1), e46–e54. https: //doi.org/10.1016/S2214-109X(13)70008-1
[8]. Vannavong, N., Seidu, R., Stenström, T.-A., Dada, N., & Overgaard, H. J. (2017). Effects of socio-demographic characteristics and household water management on Aedes aegypti production in suburban and rural villages in Laos and Thailand. Parasites & Vectors, 10(1), 170. https: //doi.org/10.1186/s13071-017-2107-7
Cite this article
Wang,A. (2025). Local Realities and Public Health Gaps: Insights from Community and Clinical Perspectives in Laos. Theoretical and Natural Science,144,13-19.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Geng, J., Yuasa, M., Kobayashi, J., Xeuatvongsa, A., & Kuroiwa, C. (2015). Preparing for the introduction of hospital autonomy in Laos: An assessment of current situation and suggestions for policy-making. The International Journal of Health Planning and Management, 31(2), 148–166. https: //doi.org/10.1002/hpm.2283
[2]. Nonaka, D., Sato, Y., Nakamura, Y., Vongphrachanh, P., & Kobayashi, J. (2022). Primary health care situations in remote rural villages of the Savannakhet Province, Lao People’s Democratic Republic. Tropical Medicine and Health, 50(1), 1–10. https: //doi.org/10.1186/s41182-022-00482-9
[3]. World Health Organization. (2024, February 8). Survey reveals gaps in water, sanitation and climate-resilience in health care facilities in Lao PDR. https: //www.who.int/laos/news/detail/08-02-2024-survey-reveals-gaps-in-water--sanitation-and-climate-resilience-in-health-care-facilities-in-lao-pdr
[4]. World Health Organization. (n.d.). Improving health systems to ensure health for all in Lao People’s Democratic Republic. Retrieved July 19, 2025, from https: //www.who.int/laos/our-work/improving-health-systems-to-ensure-health-for-all
[5]. World Health Organization. (2021, July 20). Building climate-resilient health systems in Lao People’s Democratic Republic. https: //www.who.int/news-room/feature-stories/detail/building-climate-resilient-health-systems-in-lao
[6]. Adhikari, B., Phommasone, K., Pongvongsa, T., Soundala, X., Koummarasy, P., Henriques, G., … Newton, P. N. (2019). Treatment-seeking behaviour for febrile illnesses and its implications for malaria control and elimination in Savannakhet Province, Lao PDR: A mixed-method study. BMC Health Services Research, 19(1), 252. https: //doi.org/10.1186/s12913-019-4070-9
[7]. Mayxay, M., Castonguay-Vanier, J., Chansamouth, V., Dubot-Pérès, A., Paris, D. H., Phetsouvanh, R., … Newton, P. N. (2013). Causes of non-malarial fever in Laos: a prospective study. The Lancet Global Health, 1(1), e46–e54. https: //doi.org/10.1016/S2214-109X(13)70008-1
[8]. Vannavong, N., Seidu, R., Stenström, T.-A., Dada, N., & Overgaard, H. J. (2017). Effects of socio-demographic characteristics and household water management on Aedes aegypti production in suburban and rural villages in Laos and Thailand. Parasites & Vectors, 10(1), 170. https: //doi.org/10.1186/s13071-017-2107-7









