1. Introduction
Staphylococcus aureus has proven to be an adaptable and versatile opportunist with considerable documented effects on human and animal life for many years. This bacterium has normal biotic habitats on the skin, in the nasal passages, and on mucous membranes in different host organisms, including humans and domestic animals such as dogs, cats, pigs, and horses [1]. While in humans, S. aureus has been recognized to be one of the most frequently isolated microbes responsible for numerous infectious diseases ranging in severity from mild to severe Diseases, including life-threatening ailments such as pulmonary infection, endocarditis, and bacteremia in humans [2,3]. In companion animals, S. aureus is predominantly of human origin, with dogs and cats typically exhibiting transient colonization rather than persistent carriage [4].
Among these pathogens, the emergence and prevalence of methicillin-resistant Staphylococcus aureus (MRSA) pose a challenging problem in medicine. MRSA strains are characterized by resistance to β-lactam antibiotics (e.g., methicillin, oxacillin, or other penicillin-derived drugs) by acquiring the mecA gene (or less commonly mecC), which encodes an alternative penicillin-binding protein (PBP2a) with low affinity for these drugs [5]. The worldwide spread of MRSA is linked to morbidity, mortality, and economic burden in healthcare facilities. In healthcare settings, MRSA is a persistent pathogen that causes outbreaks and is a difficult-to-treat infection, particularly in susceptible patients with weakened immune function or indwelling medical devices. In companion animals, MRSA infections most commonly manifest as skin and soft tissue infections; however, in horses, more severe conditions such as bacteremia, suppurative arthritis, and osteomyelitis have been reported [6].
In recent years, the rising number of companion animals worldwide has increased concern for the epidemiology of MRSA, especially because of its zoonotic potential. The long-term cohabitation of humans and companion animals and close daily contact provide direct and favorable conditions for cross-infection of MRSA between humans and animals [6]. More particularly, it should be noted that the MRSA typed in dogs and cats is most frequently related to human clonal complexes, such as CC398 [4]. Such strains, with strong environmental adaptability and human-to-human transmission capacity, further increase the risk of zoonosis and pose significant challenges to MRSA epidemic control.
Despite the work done in identifying the prevalence of MRSA in companion animals, there has been considerable variation in the data, pointing to discrepancies in methodology and geographic variations. For instance, a study in London identified MRSA in 26 out of 1,692 samples from dogs, cats, and horses, yielding a prevalence of 1.53% [7]. In contrast, a large-scale study in veterinary clinics across Germany reported much higher rates—20.4% in dogs and 15.6% in cats [8]. These variations can be explained by variations in study methods and geographic variations in antibiotic consumption [9,10]. Moreover, recent evidence indicates that the rising prevalence of methicillin-resistant S. pseudintermedius (MRSP) in companion animals (particularly in North America) may lead to diagnostic confounding and underestimation of true MRSA prevalence [11].
Although there has been the establishment of efficient surveillance programs in various areas, such as various European countries, there is disparate and, in many cases, incompatible data in Asia and North America [12,13]. Furthermore, molecular epidemiological studies reveal divergent dominant clones across regions—for example, CC398 in Europe and Thailand versus ST1 and ST5 in Malaysia—suggesting complex and geographically distinct transmission dynamics that are not yet fully understood [14,15].
The complexity of MRSA transmission among humans, companion animals, and the environment is illustrated in Figure 1, which depicts the continuous bidirectional transmission cycle of MRSA within the human–pet–environment interface [16,17]. This cycle is further characterized by major factors —veterinary clinics, household contact, and wound sites—as summarized in Figure 2.
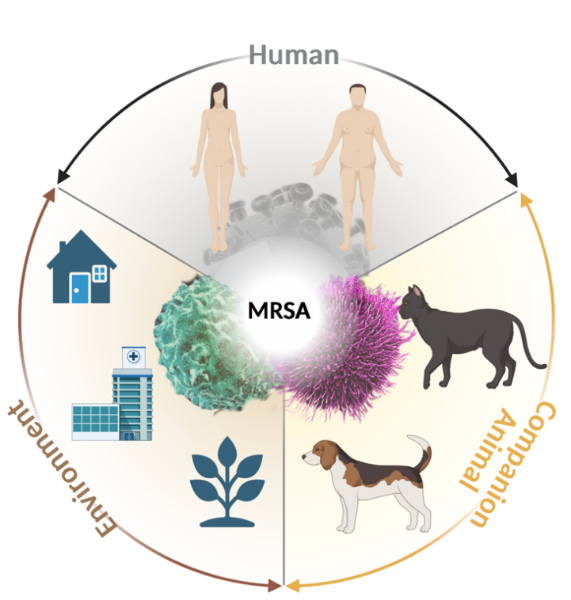
MRSA persists in bidirectional transmission between humans, companion animals, and the environment. Humans may transmit MRSA to pets through close contact (e.g., petting or sharing beds), while infected or colonized pets shed the bacteria into the environment (e.g., household bedding and toys, veterinary clinic cages and equipment, and community settings like parks). Environmental contamination serves as a reservoir for the bacteria, enabling persistent transmission back to both humans and animals, thereby perpetuating a vicious cycle. This underscores the imperative for effective control through a One Health approach.

There are three significant risk factors that play an essential role in MRSA transmission and infection in companion animals: (1) Veterinary clinics: serve as hotspots for MRSA transmission due to the concentration of susceptible animals, surgical procedures, and possible contamination of instruments and surfaces. (2) Household contact: close physical interaction between pets and owners (e.g., shared living spaces, grooming) facilitates efficient cross-species transmission. (3) Wounds: skin abrasions, surgical incisions, or other wounds provide entry points for MRSA colonization and infection, further promoting dissemination within households and clinical settings.
Therefore, the purpose of this review is to: (1) synthesize the global geographical distribution of MRSA in companion animals, critically analyzing the factors driving disparities; (2) elucidate the molecular epidemiology and predominant clones; (3) detail the antimicrobial resistance mechanisms and patterns; and (4) examine the clinical and public health implications in terms of One Health.
2. Geographical distribution of MRSA in companion animals: a contemporary review (2021–2025 update)
Globally, the epidemiology of MRSA in companion animals is complex and heterogeneous. Current evidence indicates that companion animals act as reservoirs and victims of MRSA transmission, and in many cases, their epidemiology is related to that circulating in the community [18]. Within the current estimate for the period 2021–2025, it has been revealed that MRSA is very prevalent, but in-depth studies reveal that the current cases of diseases are not only indicative of real prevalence but are firmly influenced by regional variations in surveillance and different standards in diagnostic and therapeutic methods and socio-economic status. It is noteworthy that data from Africa, South America, and Oceania are extremely scarce—a critical finding that highlights significant blind spots in global surveillance and points to essential directions for future research. This section critically addresses these disparities and the significant knowledge gaps that remain. The compiled data on current studies in different geographical areas on MRSA can be seen in Table 1, where it has been demonstrated that there is major variation in disease prevalence.in MRSA prevalence.
2.1. In North America: widespread detection and diagnostic bias
Data from North America, specifically the United States point to core challenges in MRSA epidemiology [10]. A large sample (n=5,618 isolates) from dogs and cats across the United States demonstrated widespread MRSA detection, primarily from skin and soft tissue infections (57.1% of samples), as detailed in Table 1. This observation is consistent with the clinical profile of MRSA as an opportunistic pathogen.
On the other hand, the relatively low prevalence rate of 5.8% (202/3,482) demonstrated in the veterinary diagnostic laboratory in the US Midwest by Adiguzel et al., with 3,482 cases, emphasizes an essential change in diagnostic methods (Table 1). Staphylococcus pseudintermedius appears to be evolving into a major veterinary pathogen. The high prevalence of MRSP (68.3%) in canine cases suggests it may be a more common pathogen than MRSA in the American Midwest, potentially leading to an underestimation of MRSA's true prevalence due to diagnostic focus or misclassification. The geographical variations summarized above appear to occur because of differences in veterinary referral rates and submission to veterinary diagnostic laboratories in the US [11].
2.2. In Europe: robust surveillance and zoonotic links
The epidemiological picture in Europe is clearer, though no less complex. A large-scale German study [8], encompassing 5,430 samples from dogs and cats, reported an overall MRSA prevalence of 17.8% (968/5,430), which is substantially higher than typical figures from North America (Table 1). The study further revealed a higher prevalence in dogs (20.4%) compared to cats (15.6%), with over 30% of canine infections originating from wounds. This higher prevalence in dogs may be linked to their greater outdoor exposure and the fact that dogs are more popular pets in Europe—approximately 25% of households own at least one dog [19]. Temporal trends from the same German study (2019–2021), shown in Table 2, indicate fluctuating but persistently significant MRSA prevalence, ranging from 16.0% to 19.3%. This study challenged the hypothesis that dogs, due to close daily contact with humans, are more likely to acquire MRSA than other companion animals and more likely to transmit MRSA of human origin. Dogs undergo surgical procedures more frequently than cats, and infections originating from canine wounds account for a third of all infections. Variations in the microbial environment between species also exist: canine skin is more permissive to MRSA colonization [8].
Perhaps the most critical result from European data is the close association between MRSA strains carried by pets and local human healthcare-associated MRSA clonal lineages [9]. The prevalence of lineages such as CC398 in companion animals is not a random occurrence but a direct result of zoonotic transfer. Therefore, the prevalence of MRSA in European companion animals is not an isolated issue but reflects the circulation of MRSA in human populations. Consequently, companion animal surveillance could serve as a sensitive early warning system for human MRSA circulation. The downward trend observed in France (2022–2023), with a prevalence of 13.8% (66/479) in a sample of 479 animals (Table 1), may reflect the success of human antimicrobial stewardship programs, which also benefit the pet population [9].
2.3. In Asia: an emerging picture with significant knowledge gaps
While in comparison the situation in Western countries is relatively well understood, there are only initial data on the MRSA epidemiology in companion animals on the continent of Asia, and these data are not well characterized. The sparse and scattered data available suggest that Asia might be a potential hotspot due to distinct socioeconomic conditions. Studies from Malaysia and Indonesia report varying prevalence rates, such as 6.7% in dogs in Malaysia and 2.6%–4.87% in cats in Indonesia, often based on smaller sample sizes (e.g., n=70, n=150) (Table 1). A concerning study in Malaysia reported high MRSA infection rates in dogs, cats, and their owners, demonstrating active zoonotic transmission within households in an "alarming manner" [20]. Similarly, additional Indonesian studies confirm that MRSA remains a significant concern for both companion animal and human health. MRSA has also been detected in cats in Surabaya (East Java Province) and West Tegal (West Java Province) on multiple occasions [21,22]. The occurrence of these findings in close geographic and temporal proximity in East Java over subsequent years strongly indicates important public health risks in this region, rather than sporadic cases. A systematic review of livestock-associated MRSA in China and other South Asian countries—including Hong Kong, Sri Lanka, and Bangladesh—while not focused on companion animals, provides valuable insights regarding these reservoir hosts [23]. Companion animals likely play a role in outbreaks affecting livestock, humans, and communities due to their close interaction with humans and frequent environmental exposure. This contrasts sharply with the scarcity of data from Africa, South America, and Oceania. Asia represents a significant knowledge gap in the global epidemiology of MRSA. Filling this gap is not purely an academic endeavor but an urgent global public health priority.
2.4. A critical blind spot: the scarcity of data from Africa, South America, and Oceania
Although it has been useful in compiling information from areas where studies are in progress, it has, on the one hand, brought out the chasm in knowledge of MRSA in companion animals on the other side of the world: namely, the absence of any data coming from extensive areas, especially in Africa, South America, and Oceania specifically, where studies or surveillance are not being conducted. This lack of data does not imply an absence of MRSA but represents a critical blind spot in global health security. Factors such as limited funding for veterinary antimicrobial resistance (AMR) surveillance, competing public health priorities, and a lack of diagnostic infrastructure likely contribute to this disparity. However, the growing pet ownership and close human-animal bonds in these regions create conditions ripe for zoonotic transmission. The failure to characterize the prevalence, dominant clones, and resistance patterns of MRSA in companion animals across these continents hinders the development of effective local control measures and distorts the true global picture of AMR spread. The compilation of data on MRSA in companion animals in these areas would not only serve an academic purpose but would be essential in fighting MRSA on the global level in an integrated manner in accordance with the One Health policy plan.

Adapted from Li et al. [24].
A world map visualizing the pooled prevalence estimates of MRSA colonization/infection in dogs, cats, and horses across different regions based on the data synthesized in this review. The shading/color gradient and data points reflect the varying prevalence rates reported in North America, Europe, and Asia, highlighting significant geographical disparities. Regions with insufficient data (e.g., Africa, South America) are indicated.
|
Region |
Country |
Sample Source |
Method |
Host |
Sample Categories |
Sample Size |
MRSA Prevalence /MRSA Separation Rate (%) |
Predominant Strain/Notes |
Reference |
|
North America |
USA (50 States and Columbia) |
Clinical and Laboratory |
MALDI-TOF |
Dogs, Cats |
Skin and Soft tissue: 3,207(57.1%); Urinary System: 449(8.0%); Ear:443(7.9%) |
Total: 5,618; Dogs: 3,835(68.3%); Cats: 1,783(31.7%) |
Data missing in original |
Data missing in original |
[10] |
|
North America |
USA (Midwest and Northeast) |
Iowa State University Veterinary Diagnostic Laboratory |
MALDI-TOF |
Companion Animal (mostly dog and cat) |
- |
3,482 |
5.8% (202/3,482) |
High MRSP prevalence (68.3%) noted |
[11] |
|
Europe |
Germany |
Country's Veterinary Clinics |
MALDI-TOF |
Dogs, Cats |
Dogs wound: >30%; Dogs other source: Around 10%; Cats: No significant differences between sample types. |
5,430 |
17.8% (968/5,430) |
Prevalence higher in dogs (20.4%) than cats (15.6%) |
[8] |
|
Europe |
Finland |
Clinical Microbiology Laboratory |
- |
Dogs, Cats |
- |
94 |
7%(6/94) |
- |
[25] |
|
Europe |
France |
Monitoring system |
- |
Dogs, Cats, Horses |
- |
479 |
13.8% (66/479) |
Decreasing trend (2022-2023) |
[9] |
|
Europe |
Greece |
- |
PCR |
Dogs, Cats |
53 |
26.7% (16/53) |
- |
[26] |
|
|
Asia |
Malaysia |
- |
PCR |
Dogs, Cats |
Mouth and nose |
70 |
Dogs: 6.7%(2/30) Cats: 0%(0/30) |
- |
[20] |
|
Asia |
Indonesia |
Clinical |
ORSAB |
Dogs, Cats |
- |
150 |
2.6%(4/150) |
Detected only in the southern region |
[21] |
|
Asia |
Indonesia |
Clinical |
ORSAB |
Dogs, Cats |
- |
82 |
4.87%(4/82) |
- |
[22] |
Note: Variations in prevalence are heavily influenced by sample source (community vs. clinical) and detection methods.
|
Region |
Year |
MRSA Prevalence (%) |
MRSA Separation Rate (%) |
Reference |
|
North America |
2019 |
- |
20.6%(1,160/5,643) |
[10] |
|
2020 |
19.6%(1,236/6,296) |
|||
|
2021 |
20.5%(1,568/7,637) |
|||
|
Europe |
2019 |
19.3% |
- |
[8] |
|
2020 |
16.0% |
|||
|
2021 |
17.8% |
3. MRSA types and epidemiological characteristics
Molecular typing reveals that the global distribution of MRSA clones in companion animals is geographically structured, yet frequently overlaps with human-associated lineages—underscoring the significance of zoonotic transmission.
3.1. Major epidemic clones and molecular typing
Molecular epidemiological surveys demonstrate distinct regional patterns. For instance, a 5-year molecular epidemiological survey in France revealed that CC398 was the dominant lineage among MRSA isolates from equine, feline, and canine hosts, with spa types t011 and t034 showing the highest detection rates [27]. Consistently, studies in Thailand also showed that CC398 is the dominant MRSA lineage in dogs and cats, with companion animals mostly colonized by spa type t034 [15]. The molecular epidemiology of MRSA is strongly geographically structured. Studies in Malaysia found different patterns, with ST1 and ST5 being the most common sequence types (STs) among isolates from pets and owners [14,20] field gel electrophoresis further demonstrated genetic relatedness between human and animal isolates in Malaysian households, strongly supporting that MRSA can be transmitted bidirectionally between companion animals and their owners [14]. In Japan, a significant clonal MRSA outbreak was reported in an equine hospital, involving spa type t1784 and ST1—this finding demonstrates the potential of certain clonal lineages to drive institutional spread in veterinary healthcare settings [28].
3.2. Key epidemiological characteristics of MRSA
The epidemiology of MRSA in companion animals is defined by dynamic bidirectional interactions between humans, animals, and the environment, forming complex transmission networks that transcend traditional infection control boundaries.
The primary transmission pathway occurs through direct animal-human contact via close physical interaction. Studies in Egypt have shown that cats can act as reservoir hosts for MRSA strains, with carriage isolates exhibiting a high degree of genetic similarity to human strains—specifically, 25% of cats and 20% of their human contacts carried genetically related MRSA strains [29]. Such close human-animal interaction facilitates bacterial exchange, thereby promoting cross-species transmission.
Molecular studies in Malaysia have provided robust evidence for bidirectional transmission, identifying identical MRSA strains in pets and their owners—particularly within households where close contact is common [14,20]. This creates a “vicious cycle” of reinfection within households, posing particular risks to immunocompromised individuals.
The hospital environment in veterinary settings is recognized as a critical reservoir and transmission site for MRSA. An MRSA outbreak in a Japanese equine hospital emphasized that specific clonal lineages (e.g., ST1, spa type t1784) can persist in the hospital environment and cause hospital-acquired infections in horses [28]. These hospital-acquired strains can persist in the environment, contributing to indirect transmission. Consequently, robust infection control measures are essential to prevent outbreaks in multi-pet households and veterinary clinics.
The role of companion animals as MRSA reservoirs capable of transmitting infection to humans is increasingly recognized, and pets can serve as sources of community-acquired MRSA infections in humans [13]. A One Health approach is therefore necessary to understand MRSA transmission dynamics [24].
The consistent identification of genetically related MRSA strains in pets and their owners—from France to Malaysia—provides irrefutable evidence that households serve as key units for MRSA transmission and persistence.
4. MRSA resistance mechanisms
The resilience of MRSA stems from a complex, multi-layered network of resistance mechanisms. Understanding these mechanisms is critical for elucidating treatment failures in companion animals and for developing novel therapeutic strategies.
The primary resistance mechanism of MRSA is acquired through the SCCmec gene cluster, which harbors the mecA or mecC gene. These genes encode penicillin-binding protein 2a (PBP2a)—a protein with low affinity for β-lactams—thereby conferring resistance to all β-lactam antibiotics. Intriguingly, SCCmec types IV and V are not only recognized as reservoirs for community-associated MRSA but are also prevalent in companion animal populations globally. Notably, the CC398 lineage is frequently isolated from companion animals across continents, from Europe to Thailand. This lineage can balance resistance and adaptability through specific SCCmec types (particularly Type V), which in turn facilitates widespread zoonotic and reverse zoonotic transmission [15,30].
As summarized in Table 3, MRSA isolates from companion animals exhibit high rates of multidrug resistance (MDR). Comparative analysis of different detection methods reveals that companion animal MRSA strains display complex MDR patterns and similarities across multiple antibiotic resistance indices, indicating the presence of potential non-mec-mediated resistance [31]. Variations in detection technologies significantly impact estimates of the true resistance burden and the consistency of cross-study comparisons.
|
Antibiotic Class |
Antibiotic |
Primary Resistance Mechanism(s) |
Genetic Determinant(s) |
Notes |
Reference |
|
β-lactam |
Oxacillin, Cefoxitin |
Acquired PBP2a (low affinity for β-lactams) |
mecA, mecC |
Definitive resistance to MRSA. Note the need to distinguish misleading results from MRSP. |
[11,14,23,25,30] |
|
Macrolides |
Erythromycin, Azithromycin |
Ribosomal target site modification (MLSB resistance) |
erm(A), erm(B), erm(C), msr(A) |
The rate of drug resistance is exceptionally high in Asian isolates (for example, as high as 85% has been reported in. |
[14,23] |
|
Lincosamides |
Clindamycin |
Ribosomal target site modification (MLSB resistance) |
erm genes (conferring inducible resistance), lnu(A) |
Note: Inducible clindamycin resistance (iMLSB) requires D-test detection. |
[25] |
|
Tetracyclines |
Tetracycline, Doxycycline |
Ribosomal protection protein / Active export pump |
tet(K), tet(M), tet(L) |
tet(K) is frequently associated with plasmids and is commonly found in companion animal isolates. |
[23,25] |
|
Aminoglycosides |
Gentamicin, Kanamycin |
Aminoglycoside-modifying enzyme |
aac(6')-Ie-aph(2'')-Ia (bifunctional ), aph (3')-IIIa, ant (4')-Ia |
The aac(6')-Ie-aph(2'')-Ia gene simultaneously mediates resistance to gentamicin and kanamycin. |
[23] |
|
Fluoroquinolones |
Enrofloxacin, Ciprofloxacin |
DNA gyrase (GYR) and topoisomerase IV (PARC) mutations |
gyrA mutations, parC mutations |
Antimicrobial resistance is associated with the historical use of fluoroquinolones in veterinary and human medicine. |
[23,30] |
|
Folate pathway inhibitors |
Trimethoprim-Sulfamethoxazole |
Dihydrofolate reductase variants / Dihydrofolate synthase variants |
dfrA, dfrG, dfrK |
[25] |
Note: MLSB, Macrolide-Lincosamide-Streptogramin B; MRSP, Methicillin-resistant Staphylococcus pseudintermedius; aac, Aminoglycoside Acetyltransferase; aph, Aminoglycoside Phosphotransferase; ant, Aminoglycoside Transferase; dfr, Dihydrofolate Reductase.
Studies employing culture-based antimicrobial susceptibility testing (e.g., following CLSI or EUCAST guidelines) and PCR-based methods for resistance gene detection have generated extensive phenotypic and genotypic data [14,23]. However, these studies primarily rely on convenience sampling (e.g., specimens submitted to a referral hospital or clinical cases from a specific region). This potential selection bias may lead to overestimation of reported resistance rates relative to the true community-level prevalence—for example, macrolide resistance rates of up to 85% have been reported in Asia. Although national passive surveillance systems [15] provide relatively systematic data coverage, they may underestimate resistance because they depend on clinical samples submitted to diagnostic laboratories rather than data collected through active epidemiological investigations. Notably, in Thai studies, whole-genome sequencing (WGS) of paired human-animal isolates, combined with comparative genomics, has directly revealed the transmission of resistant clones and shared resistance genes [23]. Comparative genomics is significantly more effective than traditional phenotypic typing techniques at delineating transmission chains.
Despite methodological variations, a consistent pattern emerges: MRSA in companion animals frequently exhibits MDR, mediated by multiple genes clustered on mobile genetic elements (e.g., plasmids and transposons). Genes such as erm(C), aac(6')-Ie-aph(2'')-Ia, and tet(K) frequently co-occur in isolates from Asia (Thailand and Malaysia), indicating strong co-selection pressure [14,23]. The rise in fluoroquinolone resistance likely reflects the historical and current use of these agents in both veterinary and human healthcare [30]. Critically, comparative genomics has now confirmed the bidirectional transmission of resistant clones between pets and owners [23]—this finding establishes the household as a key ecological niche for resistance evolution, rather than merely a transmission site.
A key insight from this synthesis is the high prevalence of co-resistance—particularly in Asia—where genes conferring resistance to macrolides (erm), aminoglycosides (aac-aph), and tetracyclines (tet) are frequently co-localized on mobile genetic elements, enabling their simultaneous spread.
5. Conclusion
This review confirms that MRSA prevalence in companion animals is a global concern, with major clones displaying zoonotic potential and complex multidrug resistance patterns. MRSA is a highly resistant pathogen primarily transmitted through close contact between humans and animals. As global pet ownership increases and MRSA rates remain high in companion animals (particularly dogs and cats), cross-species MRSA transmission events have become frequent.
Epidemiological data from North America, Europe, and Asia reveal variable prevalence and resistance patterns, underscoring the importance of region-specific surveillance and targeted control measures. Critically, the epidemiology of MRSA in companion animals is not an isolated veterinary concern but a direct reflection of community-associated MRSA dynamics in human populations—one that is significantly shaped by diagnostic practices and regional healthcare policies.
6. Prospects
Controlling MRSA in companion animals requires enhanced international surveillance, optimized diagnostic and treatment protocols, and a robust One Health approach that jointly considers human, animal, and environmental health. A deeper understanding of MRSA’s genetic diversity and resistance mechanisms is also necessary, as this knowledge can inform the development of new antimicrobial agents and strategies.
As the recognition of companion animals as MRSA reservoirs grows, international collaboration will be essential to address this public health challenge. Additionally, given the dynamic nature of animal-to-human transmission, robust infection control in both veterinary and human healthcare settings will be critical for reducing MRSA prevalence and preventing future outbreaks.
1.Surveillance: Implement mandatory molecular typing (e.g., whole-genome sequencing [WGS]) of MRSA isolates from companion animals in national AMR surveillance programs to track zoonotic clones.
2.Diagnosis: Develop and validate rapid diagnostic tools for differentiating MRSA from MRSP in clinical settings.
3.Stewardship: Establish international guidelines for antimicrobial use in companion animals, mirroring the stewardship efforts implemented in human medicine.
References
[1]. González-García, S., Hamdan-Partida, A., Bustos-Hamdan, A., Bustos-Martínez, J., González-García, S., Hamdan-Partida, A., Bustos-Hamdan, A., & Bustos-Martínez, J. (2021). Factors of Nasopharynx that Favor the Colonization and Persistence of Staphylococcus aureus. In Z. Z. Xiaoying Zhou (Ed.), Pharynx - Diagnosis and Treatment (Vol. 11). IntechOpen. https: //doi.org/10.5772/INTECHOPEN.95843
[2]. Grema, H. A. (2015). Methicillin Resistant Staphylococcus aureus (MRSA): A Review. Advances in Animal and Veterinary Sciences, 3(2), 79–98. https: //doi.org/10.14737/journal.aavs/2015/3.2.79.98
[3]. Klevens, R. M., Morrison, M. A., Nadle, J., Petit, S., Gershman, K., Ray, S., Harrison, L. H., Lynfield, R., Dumyati, G., Townes, J. M., Craig, A. S., Zell, E. R., Fosheim, G. E., McDougal, L. K., Carey, R. B., & Fridkin, S. K. (2007). Invasive Methicillin-Resistant Staphylococcus aureus Infections in the United States. JAMA, 298(15), 1763–1771. https: //doi.org/10.1001/JAMA.298.15.1763
[4]. Haag, A. F., Fitzgerald, J. R., & Penadés, J. R. (2019). Staphylococcus aureus in Animals . Microbiology Spectrum, 7(3). https: //doi.org/10.1128/microbiolspec.gpp3-0060-2019
[5]. Mustapha, M., Maryam Bukar-Kolo, Y., Geidam, Y. A., & Gulani, I. A. (2014). Review on Methicillin-resistant Staphylococcus aureus (MRSA) in Dogs and Cats. International Journal of Animal and Veterinary Advances, 6(2), 61–73.
[6]. Favier, P., Raffo, C., Torres, D., Gismondi, M., Piñeiro, F., Blugerman, G., Erbin, M., Pérez, J., Sued, O., & Rolón, M. J. (2025). Living with dogs and cats: Is it a risk factor for community acquired methicillin-resistant Staphylococcus aureus skin and soft tissue infections in humans? Revista Espanola de Quimioterapia, 38(3), 187–196. https: //doi.org/10.37201/req/114.2024
[7]. Loeffler, A., Pfeiffer, D. U., Lindsay, J. A., Soares, R. J., Ga L H, M. A., & Es, A. ˜. (2010). Prevalence of and risk factors for MRSA carriage in companion animals: a survey of dogs, cats and horses. Cambridge.OrgA Loeffler, DU Pfeiffer, JA Lindsay, RJS Magalhães, DH LloydEpidemiology & Infection, 2011•cambridge.Org. https: //doi.org/10.1017/S095026881000227X
[8]. Feuer, L., Frenzer, S. K., Merle, R., Leistner, R., Bäumer, W., Bethe, A., Lübke-Becker, A., Klein, B., & Bartel, A. (2024). Prevalence of MRSA in canine and feline clinical samples from one-third of veterinary practices in Germany from 2019–2021. Academic.Oup.ComL Feuer, SK Frenzer, R Merle, R Leistner, W Bäumer, A Bethe, A Lübke-Becker, B KleinJournal of Antimicrobial Chemotherapy, 2024•academic.Oup.Com, 00. https: //doi.org/10.1093/jac/dkae225
[9]. Amat, J.-P., Cazeau, G., Collineau, L., Haenni, M., Jarrige, N., Jouy, E., Lupo, A., Madec, J.-Y., Chatre, P., Chauvin, C., Du Fraysseix, L., Drapeau, A., Francois, P., Le Devendec, L., Metayer, V., Murri, S., Philippon, C., Saras, E., & Vinard, J.-L. (2024.). November 2024 Authors (alphabetical order). www.resapath.anses.fr
[10]. Sobkowich, K. E., Hui, A. Y., Poljak, Z., Szlosek, D., Plum, A., & Scott Weese, J. (2025). Nationwide analysis of methicillin-resistant staphylococci in cats and dogs: resistance patterns and geographic distribution. American Journal of Veterinary Research, 86(3). https: //doi.org/10.2460/ajvr.24.09.0253
[11]. Adiguzel, M. C., Schaefer, K., Rodriguez, T., Ortiz, J., & Sahin, O. (2022). Prevalence, Mechanism, Genetic Diversity, and Cross-Resistance Patterns of Methicillin-Resistant Staphylococcus Isolated from Companion Animal Clinical Samples Submitted to a Veterinary Diagnostic Laboratory in the Midwestern United States. Antibiotics, 11(5). https: //doi.org/10.3390/antibiotics11050609
[12]. Barua, N., Rahman, N., Tin, M. C. F., Yang, L., Alim, A., Akther, F., Handapangoda, N., Manathunga, T. A., Jinadasa, R. N., Liyanapathirana, V., Luo, M., & Ip, M. (2025). Prevalence of MRSA in Livestock, Including Cattle, Farm Animals, and Poultry, in Mainland China, Hong Kong Special Administrative Region, Sri Lanka, and Bangladesh: A Systematic Review and Meta-Analysis. In Microorganisms (Vol. 13, Issue 4). Multidisciplinary Digital Publishing Institute (MDPI). https: //doi.org/10.3390/microorganisms13040704
[13]. Khairullah, A. R., Sudjarwo, S. A., Effendi, M. H., Ramandinianto, S. C., Gelolodo, M. A., Widodo, A., Riwu, K. H. P., & Kurniawati, D. A. (2023). Pet animals as reservoirs for spreading methicillin-resistant Staphylococcus aureus to human health. In Journal of Advanced Veterinary and Animal Research (Vol. 10, Issue 1, pp. 1–13). Network for the Veterinarians of Bangladesh. https: //doi.org/10.5455/javar.2023.j641
[14]. Chai, M. H., Sukiman, M. Z., Liew, Y. W., Shapawi, M. S., Roslan, F. S., Hashim, S. N., Mohamad, N. M., Ariffin, S. M. Z., & Ghazali, M. F. (2021). Detection, molecular characterization, and antibiogram of multi-drug resistant and methicillin-resistant Staphylococcus aureus (MRSA) isolated from pets and pet owners in Malaysia. Iranian Journal of Veterinary Research, 22(4), 277–287. https: //doi.org/10.22099/ijvr.2021.39586.5752
[15]. Chueahiran, S., Yindee, J., Boonkham, P., Suanpairintr, N., & Chanchaithong, P. (2021). Methicillin-resistant staphylococcus aureus clonal complex 398 as a major mrsa lineage in dogs and cats in Thailand. Antibiotics, 10(3), 1–12. https: //doi.org/10.3390/antibiotics10030243
[16]. Garcês, A., Silva, A., Lopes, R., Sampaio, F., Duque, D., & Brilhante-Simões, P. (2022). Methicillin-Resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus pseudintermedius (MRSP) in Skin Infections from Company Animals in Portugal (2013–2021). 24. https: //doi.org/10.3390/ECA2022-12689
[17]. Shaban Ali et al. (2023). Methicillin-Resistant Staphylococcus aureus (MRSA) and its Intersection with Animals. In Emerging Infectious Diseases (Vol. 17, Issue 2). Centers for Disease Control and Prevention (CDC). https: //doi.org/10.3201/EID1702.101070
[18]. Kasela, M., Ossowski, M., Dzikoń, E., Ignatiuk, K., Antibiotics, Ł. W.-, & 2023, undefined. (2023). The Epidemiology of Animal-Associated Methicillin-Resistant Staphylococcus aureus. Mdpi.Com. Retrieved September 4, 2025, from https: //www.mdpi.com/2079-6382/12/6/1079
[19]. European pet population and market data 2023. (2025). https: //europeanpetfood.org/about/statistics/
[20]. Afshar, M. F., Zakaria, Z., Cheng, C. H., & Ahmad, N. I. (2023). Prevalence and multidrug-resistant profile of methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus pseudintermedius in dogs, cats, and pet owners in Malaysia. Veterinary World, 16(3), 536–545. https: //doi.org/10.14202/vetworld.2023.536-545
[21]. Afnani, D. A., Fatih, N., Effendi, M. H., Tyasningsih, W., Khairullah, A. R., Kurniawan, S. C., Silaen, O. S. M., Ramandinianto, S. C., Widodo, A., & Riwu, K. H. P. (2022). Profile of Multidrug Resistance and Methicillin-Resistant Staphylococcus aureus (MRSA) isolated from cats in Surabaya, Indonesia. Biodiversitas, 23(11), 5703–5709. https: //doi.org/10.13057/biodiv/d231121
[22]. Waruwu, Y. K. K., Khairullah, A. R., Effendi, M. H., Lukiswanto, B. S., Afnani, D. A., Kurniawan, S. C., Silaen, O. S. M., Riwu, K. H. P., Widodo, A., & Ramandinianto, S. C. (2023). Detection of methicillin-resistant Staphylococcus aureus and multidrug resistance isolated from cats in animal clinic at Sidoarjo District, East Java, Indonesia. Biodiversitas, 24(1), 106–111. https: //doi.org/10.13057/biodiv/d240114
[23]. Buranasinsup, S., Wiratsudakul, A., Suwanpakdee, S., Jiemtaweeboon, S., Maklon, K., Sakcamduang, W., & Chantong, B. (2025). A Comparative Analysis of Antimicrobial Resistance Patterns and Genes in Staphylococcus aureus From Humans and Animals in Veterinary Clinics Across Thailand. Transboundary and Emerging Diseases, 2025(1). https: //doi.org/10.1155/tbed/5541655
[24]. Li, J., Cheng, F., Wei, X., Bai, Y., Wang, Q., Li, B., Zhou, Y., Zhai, B., Zhou, X., Wang, W., & Zhang, J. (2025). Methicillin-Resistant Staphylococcus aureus (MRSA): Resistance, Prevalence, and Coping Strategies. Antibiotics, 14(8), 771. https: //doi.org/10.3390/antibiotics14080771
[25]. turvallisuus ja kehittämiskeskus Fimea, L., & yliopisto, H. (2022). Finnish Veterinary Antimicrobial Resistance Monitoring and Consumption of Antimicrobial Agents.
[26]. Drougka, E., Foka, A., Koutinas, C. K., Jelastopulu, E., Giormezis, N., Farmaki, O., Sarrou, S., Anastassiou, E. D., Petinaki, E., & Spiliopoulou, I. (2016). Interspecies spread of Staphylococcus aureus clones among companion animals and human close contacts in a veterinary teaching hospital. A cross-sectional study in Greece. Preventive Veterinary Medicine, 126, 190–198. https: //doi.org/10.1016/J.PREVETMED.2016.02.004
[27]. Haenni, M., Châtre, P., Dupieux-Chabert, C., Métayer, V., Bes, M., Madec, J. Y., & Laurent, F. (2017). Molecular epidemiology of methicillin-resistant staphylococcus aureus in horses, cats, and dogs over a 5-year period in France. Frontiers in Microbiology, 8(DEC). https: //doi.org/10.3389/fmicb.2017.02493
[28]. Uchida-Fujii, E., Niwa, H., Kanai, K., Kinoshita, Y., Kuroda, T., Nukada, T., & Ueno, T. (2022). Outbreak of methicillin-resistant Staphylococcus aureus sequence type 1, spa type t1784, in an equine hospital in Japan. Veterinary and Animal Science, 17, 100259. https: //doi.org/10.1016/J.VAS.2022.100259
[29]. Zafir Z, Alsadi I, Gomaa A, et al.Occurrence of Methicillin Resistant Staphylococcus Aureus in Cats and Man [J].Alexandria Journal of Veterinary Sciences, 2022.DOI: 10.5455/ajvs.111225.
[30]. Olanipekun, T. A., Demilade Ojeniyi, F., Itunuoluwa Celestina, O., Deborah Ayanyinka, A., Olaide Habeeb, A., Rita Ayanbolade, O., Ojurongbe, O., Oladele Opaleye, O., & Adekunle Olowe, O. (2025). Emerging Pathogenic Methicillin-Resistant Staphylococcus aureus (MRSA) Strains in Veterinary Medicine (Narrative Review). Advanced Analytical Pathology, 1, 39–51. https: //doi.org/10.17582/journal.aap/2025/1.39.51
[31]. Abdulkadir, A., Kabir, J., Bello, M., & Olayinka, B. (2025). Genetic resistance factors and antimicrobialresistance phenotypes in methicillin- resistant Staphylococcus aureus isolates of animals and humans. One Health Bulletin. https: //doi.org/10.4103/OHBL.OHBL_55_24
Cite this article
Chan,Y. (2025). Global Prevalence, Drivers, and One Health Implications of Methicillin-Resistant Staphylococcus Aureus (MRSA) in Companion Animals: A Systematic Review. Theoretical and Natural Science,149,35-47.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICMMGH 2026 Symposium: Environmental Engineering and Climate Change
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. González-García, S., Hamdan-Partida, A., Bustos-Hamdan, A., Bustos-Martínez, J., González-García, S., Hamdan-Partida, A., Bustos-Hamdan, A., & Bustos-Martínez, J. (2021). Factors of Nasopharynx that Favor the Colonization and Persistence of Staphylococcus aureus. In Z. Z. Xiaoying Zhou (Ed.), Pharynx - Diagnosis and Treatment (Vol. 11). IntechOpen. https: //doi.org/10.5772/INTECHOPEN.95843
[2]. Grema, H. A. (2015). Methicillin Resistant Staphylococcus aureus (MRSA): A Review. Advances in Animal and Veterinary Sciences, 3(2), 79–98. https: //doi.org/10.14737/journal.aavs/2015/3.2.79.98
[3]. Klevens, R. M., Morrison, M. A., Nadle, J., Petit, S., Gershman, K., Ray, S., Harrison, L. H., Lynfield, R., Dumyati, G., Townes, J. M., Craig, A. S., Zell, E. R., Fosheim, G. E., McDougal, L. K., Carey, R. B., & Fridkin, S. K. (2007). Invasive Methicillin-Resistant Staphylococcus aureus Infections in the United States. JAMA, 298(15), 1763–1771. https: //doi.org/10.1001/JAMA.298.15.1763
[4]. Haag, A. F., Fitzgerald, J. R., & Penadés, J. R. (2019). Staphylococcus aureus in Animals . Microbiology Spectrum, 7(3). https: //doi.org/10.1128/microbiolspec.gpp3-0060-2019
[5]. Mustapha, M., Maryam Bukar-Kolo, Y., Geidam, Y. A., & Gulani, I. A. (2014). Review on Methicillin-resistant Staphylococcus aureus (MRSA) in Dogs and Cats. International Journal of Animal and Veterinary Advances, 6(2), 61–73.
[6]. Favier, P., Raffo, C., Torres, D., Gismondi, M., Piñeiro, F., Blugerman, G., Erbin, M., Pérez, J., Sued, O., & Rolón, M. J. (2025). Living with dogs and cats: Is it a risk factor for community acquired methicillin-resistant Staphylococcus aureus skin and soft tissue infections in humans? Revista Espanola de Quimioterapia, 38(3), 187–196. https: //doi.org/10.37201/req/114.2024
[7]. Loeffler, A., Pfeiffer, D. U., Lindsay, J. A., Soares, R. J., Ga L H, M. A., & Es, A. ˜. (2010). Prevalence of and risk factors for MRSA carriage in companion animals: a survey of dogs, cats and horses. Cambridge.OrgA Loeffler, DU Pfeiffer, JA Lindsay, RJS Magalhães, DH LloydEpidemiology & Infection, 2011•cambridge.Org. https: //doi.org/10.1017/S095026881000227X
[8]. Feuer, L., Frenzer, S. K., Merle, R., Leistner, R., Bäumer, W., Bethe, A., Lübke-Becker, A., Klein, B., & Bartel, A. (2024). Prevalence of MRSA in canine and feline clinical samples from one-third of veterinary practices in Germany from 2019–2021. Academic.Oup.ComL Feuer, SK Frenzer, R Merle, R Leistner, W Bäumer, A Bethe, A Lübke-Becker, B KleinJournal of Antimicrobial Chemotherapy, 2024•academic.Oup.Com, 00. https: //doi.org/10.1093/jac/dkae225
[9]. Amat, J.-P., Cazeau, G., Collineau, L., Haenni, M., Jarrige, N., Jouy, E., Lupo, A., Madec, J.-Y., Chatre, P., Chauvin, C., Du Fraysseix, L., Drapeau, A., Francois, P., Le Devendec, L., Metayer, V., Murri, S., Philippon, C., Saras, E., & Vinard, J.-L. (2024.). November 2024 Authors (alphabetical order). www.resapath.anses.fr
[10]. Sobkowich, K. E., Hui, A. Y., Poljak, Z., Szlosek, D., Plum, A., & Scott Weese, J. (2025). Nationwide analysis of methicillin-resistant staphylococci in cats and dogs: resistance patterns and geographic distribution. American Journal of Veterinary Research, 86(3). https: //doi.org/10.2460/ajvr.24.09.0253
[11]. Adiguzel, M. C., Schaefer, K., Rodriguez, T., Ortiz, J., & Sahin, O. (2022). Prevalence, Mechanism, Genetic Diversity, and Cross-Resistance Patterns of Methicillin-Resistant Staphylococcus Isolated from Companion Animal Clinical Samples Submitted to a Veterinary Diagnostic Laboratory in the Midwestern United States. Antibiotics, 11(5). https: //doi.org/10.3390/antibiotics11050609
[12]. Barua, N., Rahman, N., Tin, M. C. F., Yang, L., Alim, A., Akther, F., Handapangoda, N., Manathunga, T. A., Jinadasa, R. N., Liyanapathirana, V., Luo, M., & Ip, M. (2025). Prevalence of MRSA in Livestock, Including Cattle, Farm Animals, and Poultry, in Mainland China, Hong Kong Special Administrative Region, Sri Lanka, and Bangladesh: A Systematic Review and Meta-Analysis. In Microorganisms (Vol. 13, Issue 4). Multidisciplinary Digital Publishing Institute (MDPI). https: //doi.org/10.3390/microorganisms13040704
[13]. Khairullah, A. R., Sudjarwo, S. A., Effendi, M. H., Ramandinianto, S. C., Gelolodo, M. A., Widodo, A., Riwu, K. H. P., & Kurniawati, D. A. (2023). Pet animals as reservoirs for spreading methicillin-resistant Staphylococcus aureus to human health. In Journal of Advanced Veterinary and Animal Research (Vol. 10, Issue 1, pp. 1–13). Network for the Veterinarians of Bangladesh. https: //doi.org/10.5455/javar.2023.j641
[14]. Chai, M. H., Sukiman, M. Z., Liew, Y. W., Shapawi, M. S., Roslan, F. S., Hashim, S. N., Mohamad, N. M., Ariffin, S. M. Z., & Ghazali, M. F. (2021). Detection, molecular characterization, and antibiogram of multi-drug resistant and methicillin-resistant Staphylococcus aureus (MRSA) isolated from pets and pet owners in Malaysia. Iranian Journal of Veterinary Research, 22(4), 277–287. https: //doi.org/10.22099/ijvr.2021.39586.5752
[15]. Chueahiran, S., Yindee, J., Boonkham, P., Suanpairintr, N., & Chanchaithong, P. (2021). Methicillin-resistant staphylococcus aureus clonal complex 398 as a major mrsa lineage in dogs and cats in Thailand. Antibiotics, 10(3), 1–12. https: //doi.org/10.3390/antibiotics10030243
[16]. Garcês, A., Silva, A., Lopes, R., Sampaio, F., Duque, D., & Brilhante-Simões, P. (2022). Methicillin-Resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus pseudintermedius (MRSP) in Skin Infections from Company Animals in Portugal (2013–2021). 24. https: //doi.org/10.3390/ECA2022-12689
[17]. Shaban Ali et al. (2023). Methicillin-Resistant Staphylococcus aureus (MRSA) and its Intersection with Animals. In Emerging Infectious Diseases (Vol. 17, Issue 2). Centers for Disease Control and Prevention (CDC). https: //doi.org/10.3201/EID1702.101070
[18]. Kasela, M., Ossowski, M., Dzikoń, E., Ignatiuk, K., Antibiotics, Ł. W.-, & 2023, undefined. (2023). The Epidemiology of Animal-Associated Methicillin-Resistant Staphylococcus aureus. Mdpi.Com. Retrieved September 4, 2025, from https: //www.mdpi.com/2079-6382/12/6/1079
[19]. European pet population and market data 2023. (2025). https: //europeanpetfood.org/about/statistics/
[20]. Afshar, M. F., Zakaria, Z., Cheng, C. H., & Ahmad, N. I. (2023). Prevalence and multidrug-resistant profile of methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus pseudintermedius in dogs, cats, and pet owners in Malaysia. Veterinary World, 16(3), 536–545. https: //doi.org/10.14202/vetworld.2023.536-545
[21]. Afnani, D. A., Fatih, N., Effendi, M. H., Tyasningsih, W., Khairullah, A. R., Kurniawan, S. C., Silaen, O. S. M., Ramandinianto, S. C., Widodo, A., & Riwu, K. H. P. (2022). Profile of Multidrug Resistance and Methicillin-Resistant Staphylococcus aureus (MRSA) isolated from cats in Surabaya, Indonesia. Biodiversitas, 23(11), 5703–5709. https: //doi.org/10.13057/biodiv/d231121
[22]. Waruwu, Y. K. K., Khairullah, A. R., Effendi, M. H., Lukiswanto, B. S., Afnani, D. A., Kurniawan, S. C., Silaen, O. S. M., Riwu, K. H. P., Widodo, A., & Ramandinianto, S. C. (2023). Detection of methicillin-resistant Staphylococcus aureus and multidrug resistance isolated from cats in animal clinic at Sidoarjo District, East Java, Indonesia. Biodiversitas, 24(1), 106–111. https: //doi.org/10.13057/biodiv/d240114
[23]. Buranasinsup, S., Wiratsudakul, A., Suwanpakdee, S., Jiemtaweeboon, S., Maklon, K., Sakcamduang, W., & Chantong, B. (2025). A Comparative Analysis of Antimicrobial Resistance Patterns and Genes in Staphylococcus aureus From Humans and Animals in Veterinary Clinics Across Thailand. Transboundary and Emerging Diseases, 2025(1). https: //doi.org/10.1155/tbed/5541655
[24]. Li, J., Cheng, F., Wei, X., Bai, Y., Wang, Q., Li, B., Zhou, Y., Zhai, B., Zhou, X., Wang, W., & Zhang, J. (2025). Methicillin-Resistant Staphylococcus aureus (MRSA): Resistance, Prevalence, and Coping Strategies. Antibiotics, 14(8), 771. https: //doi.org/10.3390/antibiotics14080771
[25]. turvallisuus ja kehittämiskeskus Fimea, L., & yliopisto, H. (2022). Finnish Veterinary Antimicrobial Resistance Monitoring and Consumption of Antimicrobial Agents.
[26]. Drougka, E., Foka, A., Koutinas, C. K., Jelastopulu, E., Giormezis, N., Farmaki, O., Sarrou, S., Anastassiou, E. D., Petinaki, E., & Spiliopoulou, I. (2016). Interspecies spread of Staphylococcus aureus clones among companion animals and human close contacts in a veterinary teaching hospital. A cross-sectional study in Greece. Preventive Veterinary Medicine, 126, 190–198. https: //doi.org/10.1016/J.PREVETMED.2016.02.004
[27]. Haenni, M., Châtre, P., Dupieux-Chabert, C., Métayer, V., Bes, M., Madec, J. Y., & Laurent, F. (2017). Molecular epidemiology of methicillin-resistant staphylococcus aureus in horses, cats, and dogs over a 5-year period in France. Frontiers in Microbiology, 8(DEC). https: //doi.org/10.3389/fmicb.2017.02493
[28]. Uchida-Fujii, E., Niwa, H., Kanai, K., Kinoshita, Y., Kuroda, T., Nukada, T., & Ueno, T. (2022). Outbreak of methicillin-resistant Staphylococcus aureus sequence type 1, spa type t1784, in an equine hospital in Japan. Veterinary and Animal Science, 17, 100259. https: //doi.org/10.1016/J.VAS.2022.100259
[29]. Zafir Z, Alsadi I, Gomaa A, et al.Occurrence of Methicillin Resistant Staphylococcus Aureus in Cats and Man [J].Alexandria Journal of Veterinary Sciences, 2022.DOI: 10.5455/ajvs.111225.
[30]. Olanipekun, T. A., Demilade Ojeniyi, F., Itunuoluwa Celestina, O., Deborah Ayanyinka, A., Olaide Habeeb, A., Rita Ayanbolade, O., Ojurongbe, O., Oladele Opaleye, O., & Adekunle Olowe, O. (2025). Emerging Pathogenic Methicillin-Resistant Staphylococcus aureus (MRSA) Strains in Veterinary Medicine (Narrative Review). Advanced Analytical Pathology, 1, 39–51. https: //doi.org/10.17582/journal.aap/2025/1.39.51
[31]. Abdulkadir, A., Kabir, J., Bello, M., & Olayinka, B. (2025). Genetic resistance factors and antimicrobialresistance phenotypes in methicillin- resistant Staphylococcus aureus isolates of animals and humans. One Health Bulletin. https: //doi.org/10.4103/OHBL.OHBL_55_24









