1. Introduction
The discovery of the molecular structure of benzene in middle school textbooks has been described by the anecdote of Kekule’s “wonderful” dream, and some chemistry history textbooks refer to the “structure theory of benzene” as an example of chemical intuition [1]. High school textbooks are limited in length, present students with little knowledge, and attribute great scientific discoveries to an accident, which always feel unconvincing. As we all know, the revelation of scientific laws is a slow accumulation process, a process in which quantitative changes cause qualitative changes. Kekule’s revelation of the structure of benzene should also be a process of quantitative change causing qualitative change. What is the process of discovery of Kekule’s “dream”? What about the large π bond in the benzene ring in modern valence bond theory?
2. Background
2.1. Discovery of benzene
In the early 19th century, the United Kingdom and other European countries were widely used in urban lighting gas. Gas production left a kind of oily, smelly, viscous liquid for a long time no one. As shown in Figure 1, English scientist Faraday was the first to become interested in this oily liquid. After five years of research, in 1825, he isolated a new hydrocarbon from the liquid. Faraday called it a “heavy carbon compound of hydrogen” and determined its experimental formula CH by analysis. In 1834, German chemist Michilrich named it benzene, and then French chemist Gerard determined its molecular formula as C6H6 [2].

Figure 1. Faraday
2.2. Source of benzene
The light tar produced in the coking process of coal contains large amounts of benzene. This is how benzene was originally produced. The generated coal tar and gas are passed through the washing and absorption equipment together, and the coal tar with a high boiling point is used as the washing and absorption agent to recover the coal tar in the gas. After distillation, crude benzene and other high boiling point fractions are obtained. Industrial-grade benzene can be obtained by refining crude benzene. The purity of benzene obtained by this method is relatively low, and the environmental pollution is serious, and the technology is relatively backward [3].
Small amounts of benzene are present in crude oil, and the extraction of benzene from petroleum products is the most widely used preparation method [3].
At 500-525℃, 8-50 atmospheres of pressure, a variety of boiling points between 60-200℃ aliphatic hydrocarbons, by platinum-rhenium catalyst, through dehydrogenation, cyclation into benzene and other aromatic hydrocarbons. After the aromatic hydrocarbon product is extracted from the mixture, benzene is separated by distillation. These fractions can also be used as high-octane gasoline [4].
Steam cracking is a process for producing alkenes from low molecular alkanes such as ethane, propane or butane, and petroleum components such as naphtha and heavy diesel. One of its byproducts, cracked gasoline, is rich in benzene and can be fractionated into benzene and other components. Cracked gasoline can also be mixed with other hydrocarbons as additives to gasoline.
About 40-60% of benzene in cracked gasoline, but also contains diolefin and styrene and other unsaturated components, these impurities in the storage process easy to further react to the formation of polymer gum. Therefore, first through the hydrogenation process to remove these impurities and sulfide in the cracked gasoline, and then the appropriate separation to obtain benzene products.
2.3. Toxicity of benzene
Because benzene is volatile, it disperses easily when exposed to air. Inhalation or skin contact with large amounts of benzene into the body can cause acute and chronic benzene poisoning in humans and animals. Some studies have reported that benzene poisoning is partly due to the formation of phenol from benzene in the body.
Benzene has a paralyzing effect on the central nervous system, causing acute poisoning. In severe cases, headache, nausea, vomiting, confusion, loss of consciousness, coma, convulsions, etc., and in severe cases, death due to central system paralysis. A small amount of benzene can also cause drowsiness, dizziness, rapid heart rate, headache, shaking, confusion, confusion and other phenomena. Ingesting food containing too much benzene can cause vomiting, stomach pain, dizziness, insomnia, convulsions, rapid heart rate and even death. Breathing 20‰ benzene vapor for 5-10 minutes can be fatal.
Long-term exposure to benzene can cause great damage to the blood, causing chronic poisoning. Causes neurasthenic syndrome. Benzene can damage bone marrow, reduce the number of red blood cells, white blood cells, and platelets, and cause chromosome aberration, which can lead to leukemia, and even aplastic anemia. Benzene can cause excessive bleeding, which suppresses the immune system and allows disease to take hold. Studies have reported that the incubation period of benzene in the body can be as long as 12 to 15 years.
3. The discovery of benzene molecular structure
3.1. Conjectures on the structure of benzene
Benzene was discovered in 1825, and for decades its structure was unknown. Everything about benzene showed that its molecules were very symmetrical, but at the time it was hard to imagine how six carbon and six hydrogen atoms could be arranged in perfect symmetry to form a stable molecule.
Since 1825, many chemical researchers have speculated that benzene should have its own special structure based on the different properties of benzene and aliphatic compounds found at that time, and should propose new structure or new valence bond explanations for benzene.
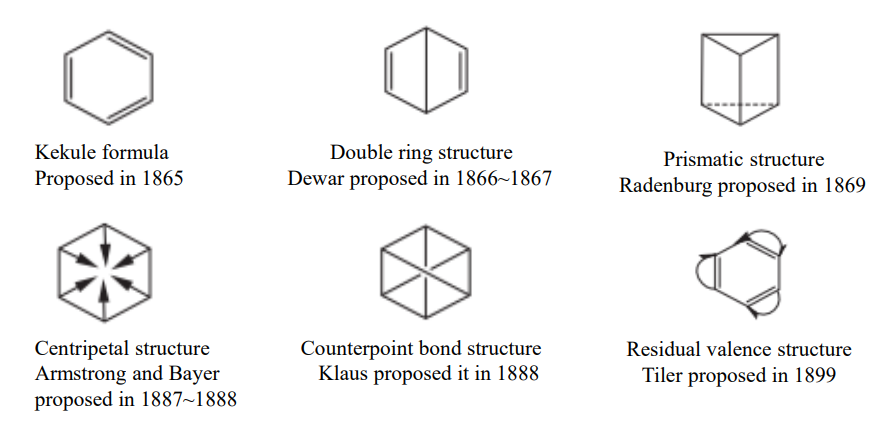
Figure 2. Several molecular structures of benzene
Figure 2 shows the molecular structure formula proposed by different scientists in different ages. In 1857, the German chemist Kekule proposed the theory of tetravalent carbon. In 1858, he proposed that benzene molecules have a ring structure. Suppose a benzene molecule’s six-carbon chain is connected to each other in a ring, and each carbon is connected to a hydrogen, so that the six hydrogens occupy the same positions. In 1890, at a ceremony commemorating the 25th anniversary of the benzene ring structure, he described his scientific discovery as a “dream” inspired by a moment’s inspiration.
The double-ring structure was proposed by Dewar and was historically known as Dewar benzene, which is now known to be a reactive cycloolefin - dicyclo-2, 5-hexadiene [2.2.0].
The prismatic structure is called prism alkane, which is also very active.
Armstrong and Baeyer proposed the centripetal formula and argued that: in benzene molecules, the fourth valence of each carbon atom points to the center of the ring and is not connected to other atoms. This is called the central bond, and the six central bonds balance each other, making the combined energy of each bond a potential force. The central bond does not exist in aliphatic compounds, so the aromatic properties can be considered to be due to the special symmetrical arrangement of the fourth valence of the carbon atoms in the ring.
Tiler’s theory of residual valence holds that the double bond in the structural formula of residual valence cannot use all the single valence, so a part of the unused valence is left, which is called residual, and the residual valence combines with each other to form a new bond. According to this assumption, the bonds between C and C in the benzene ring are roughly equal. There is no difference between single and double bonds, and the covalence of every two adjacent carbon atoms in the six carbon atoms are combined with each other, becoming a new system.
The covalent or central bond theory proposed new solutions to the structural formulations of benzene. Essentially, they are in general agreement with the modern methods of describing the structural formulations of benzene. But at that time, it was impossible to elucidate the nature of the central bond and covalent, and there were no suitable experimental methods for further confirmations. Therefore, these two structures contrary to the classical valence bond theory have not been universally accepted by chemists.
As shown in Figure 3 and Figure 4, modern studies have shown that rosin and dewar bene are also related to benzene. It is proved that benzene can be changed into benzene and dewar bene by light excitation. Although benzene and Dewar bene conform to classical valence bond theory, this structure is inconsistent with the related properties of benzene.
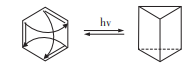
Figure 3. Benzene is converted to prism
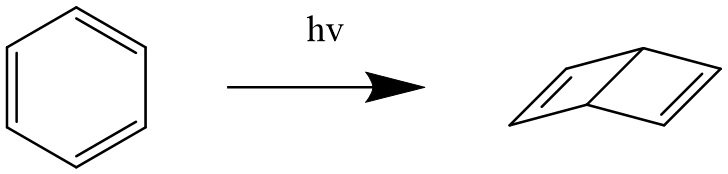
Figure 4. Benzene is converted to dewar benzene.
As shown in Figure 5 and Figure 6. Kekule’s formula reveals that benzene can have only one unary substitution and three binary substitutions.
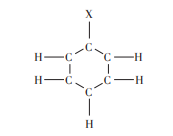
Figure 5. One unary substitute of benzene
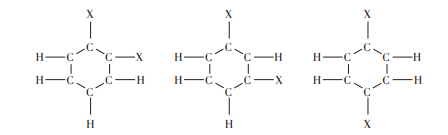
Figure 6. Three binary substituents for benzene
After many studies, it is found that the number of isomers of benzene substitutes is always consistent with Kekule’s ring structure, and Kekule’s form has achieved great success. According to the hexagonal ring structure of benzene, each carbon only needs to remove the trivalent, and the remaining one is combined with each other to form three double bonds. Every single bond has a double bond, so each carbon becomes quadrivalent and meets the theoretical requirements of the quadrivalent carbon. However, Kekule’s formula has two major disadvantages: first, it does not explain why benzene molecules, since they have double bonds, do not normally react with the addition of reagents of unsaturated hydrocarbons; Secondly, according to such a structure, there should be two isomers of the ortho-binary substitution of benzene, but this is inconsistent with experience, and various tests have shown that there is only one ortho-binary substitution.
To solve this contradiction, Kekule proposed an alternative explanation, which was to assume that the double bonds in benzene were not fixed in position, but could move back and forth so fast that it would not be possible to tell the difference between single and double bonds. This was the so-called oscillating double bond theory [2].
3.2. Confirmation of modern experiments
In the 20th century, due to the progress of theoretical physics and physical methods, people verified the structure conjectures of benzene.
Modern physical methods such as x-ray method and spectrum method have proved that the benzene molecule is a planar regular hexagon configuration. The bond Angle of benzene and the bond length of the carbon-carbon bond in benzene have been determined.
As shown in Figure 7, the scanning tunneling microscope gave people their first glimpse of benzene, and an image of benzene molecules was published in the journal Chemistry Today, making the hexagonal structure of benzene molecules clearly visible to people.
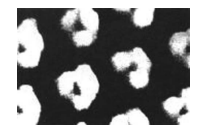
Figure 7. Images of benzene molecules were obtained using a scanning tunneling microscope
3.3. Theoretical explanation of benzene molecular structure
It is an accepted fact that the shape of the benzene molecule space is hexagonal. Is the Kekule formula of benzene molecule alternating single and double bonds correct? This has been a nagging topic.
As the research progressed, it was found that three compounds, butanedione, acetone aldehyde and glyoxal, could be formed by the interaction of o-xylene and ozone.
As shown in Figure 8, this suggests that benzene and benzene derivatives may indeed have two structures with different double bond arrangements, as Kekule suggested, because if there is only one structure, either one should produce only two compounds. However, benzene can not discolor bromate water and acid potassium permanganate solution, and it proves that benzene does not have the characteristic of unsaturated hydrocarbon, that is, it does not have the structure of carbon-carbon double bond, so people have doubts about the Kekule formula.
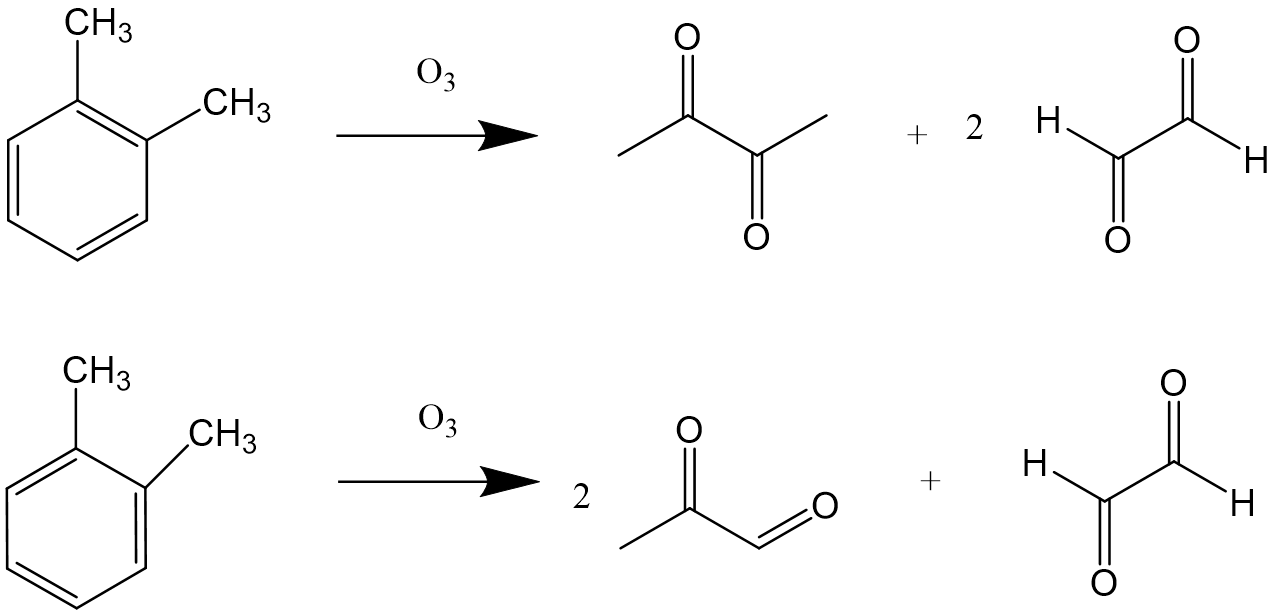
Figure 8. Two ozonolysis products of o-xylene
So what is the structure of benzene?
With the development of chemical theory, some chemists have put forward some theoretical explanations for the structure of benzene, which seem to accord with the relevant chemical properties of benzene. The typical ones are as follows:
(1) Hybrid orbital theory
Hybrid Orbital Theory was proposed in 1931 by Pauling L and others on the basis of the valence bond theory. The theory of hybrid orbitals holds that the electrons of the six carbon atoms in the benzene molecule overlap each other with sp2 hybrid orbitals to form six σ bonds of carbon and carbon, and each of them overlaps the 1s orbital of hydrogen atom with 1 sp2 hybrid orbital to form six σ bonds of carbon and hydrogen. Because sp2 hybridized, the bond Angle is 120. And all six carbon atoms and six hydrogen atoms are connected to each other on the same plane, forming a regular hexagonal structure. The six carbon atoms on the benzene ring do not participate in the hybrid 2p orbital. They are perpendicular to the ring plane, their sides overlap each other and form a closed large π bond, which is evenly distributed above and below the ring plane. The large π bond of benzene makes the property of benzene more stable than that of unsaturated hydrocarbons, and it is generally not easy to occur the addition reaction of general unsaturated hydrocarbons [4].
(2) Molecular orbital theory
Molecular orbital theory, also known as molecular orbital theory (Molecular orbital theory) or MO method, was proposed by American chemist R.S. Mulliken (R.S. Mulliken) and German physicist F. Hundt (F. Hundt) in 1932. The molecular orbital theory states that six p orbitals are linearly combined into six π molecular orbitals, three bonding orbitals and three antibonding orbitals. The ground state is six p electrons in pairs filling three bonding orbitals, so all of the low energy bonding orbitals, which are all lower energy than the original atomic orbitals, are full of electrons, so the benzene molecule is stable, and the system is low energy. The large π bond of the benzene molecule can be seen as the result of the superposition of three π-bonding orbitals. After the superposition of orbitals, the electron cloud density between each adjacent carbon atom is equal, and the bond length of the benzene carbon-carbon bond is completely average [5].
(3) Resonance theory
As shown in Figure 9, resonance theory is a theory of molecular structure formulated by the American chemist L.C. Pauling in 1931 for the discussion of molecules that cannot be described in terms of valence bond structures. Resonance theory holds that benzene mainly resonates between the two Kekule structures, and the energy of the whole system is reduced. Resonance makes the carbon-carbon bond neither single nor double, and the six bonds are the same, and the benzene ring is stable. Resonance makes the hydrogenation energy of benzene 121.8kJ·mol-1 lower than the theoretical calculated hydrogenation energy of 1, 3, 5-cyclohexatriene, which is called the resonance energy of benzene. It is because of the resonance energy that the benzene ring is more stable and can replace the addition [2].
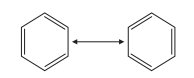
Figure 9. Resonance of benzene
(4) Molecular structure of modern benzene
In recent decades, some people have raised questions about “the π electron of benzene is delocalized” and “the delocalization of the π electron in benzene makes benzene stable”. The new idea is that the symmetrical hexagonal structure of benzene depends only on the σ electron, and that the π system of benzene does not favor a delocalized “aromatic hexagon”, but rather a structure with three localized π bonds. Cop⁃per et al. put forward the spin coupling valence bond theory in “Electronic Structure of benzene molecules” published in 1986. According to this theory, the two localized Kekule structures are a pair of “electron tautomers”, which represent the microstructure of the compound molecule and cannot be separated. From a microscopic point of view, compounds can be multi structural, that is, a compound may have several microscopic structures. We usually talk about the molecular structure is the macro-structure of molecules. A compound molecule can only have one macro-structure, so the macro-structure is a balanced structure mixed with several micro-structures. Benzene is actually a balanced mixture of two microscopic structures (Kekule structure). Spin coupling valence bond theory differs from resonance theory, which holds that benzene has a variety of molecular structures, and benzene is a mixture of structures. According to the spin coupling valence bond theory, benzene has only one molecular structure, but one pair of internal microstructure is inseparable [6].
The structure of benzene and its expression have been discussed for more than 170 years. So far, no satisfactory conclusion has been reached. The study of benzene molecular structure is far from over, and it still needs to be explored by aspiring chemists. Now people commonly used benzene molecular structure formula is: one is the Kekule formula; The other is represented by a regular hexagon with an inner circle representing the π electron cloud in the benzene ring as a whole.
4. Products made from benzene
4.1. Friedel-Crafts alkylation reaction
As shown in Figure 10, Friedel-Crafts alkylation is the connection of an alkyl group to an electron-rich benzene ring or derivative catalyzed by Lewis acid. First, halogenated hydrocarbons are ionized by Lewis acid to form a carbocation electrophilic body. The aromatic ring then attacks the carbocation, forming a carbon-carbon bond and a new carbocation intermediate. Finally, cyclic deprotonation restores aromaticity, while Lewis acid is regenerated.

Figure 10. Friedel-Crafts alkylation
4.2. Friedel-Crafts acylation reaction
The electrophilic substitution reaction of an aromatic compound with an acyl halide or anhydride catalyzed by a protic acid or Lewis acid, such as aluminum trichloride, is a modified electrophilic substitution reaction. Figure 11 shows the Acylation mechanism.
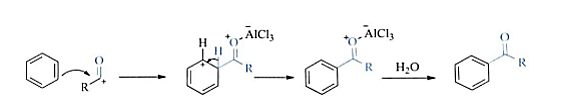
Figure 11. Acylation mechanism
Benzene is a very important chemical raw material. Many chemical products are prepared from benzene.
5. Epilogue
The benzene molecule is a kind of highly unsaturated molecule, but it is easy to replace and difficult to add. This is a time to create new ideas, and to create new ideas requires establishing a logical relationship between evidence and conclusion. Many guesses have been put forward about the molecular structure of benzene, but the Kekule formula is proven to meet the requirements based on the fact that benzene has only one unary substitution and three binary substitutions. The evidence of ozonation and reduction of ortho-xylene-produced products proved that the Kekule formula did not fully meet the requirements, and scientists made new theoretical hypotheses for the structure of benzene.
The structure of benzene and its expression has been discussed for 170 years. Although various hypotheses have been put forward, no satisfactory conclusion has been reached. This is still a problem that needs further investigation. Research is continuing on the structure and properties of benzene.
6. Conclusion
This paper mainly introduces the basic properties of benzene and its background, and tells about the structure of the benzene exploration process. From different periods of different scientists put forward the structure analysis. The Kekule structure formula is widely recognized as one of the structures. However, the Kekule structure formula also has some limitations, so from the analysis of modern research theory, the structure of benzene still needs to be explored. At the same time, benzene as one of the most basic chemical raw materials, according to Friedel-Crafts reaction can produce many chemical products.
In the future, the exploration of benzene structure will continue, there will be more appropriate theories to describe the structure of benzene, and in chemical synthesis, benzene as a basic chemical raw material, there will be more synthesis routes using benzene as raw material will be developed.
References
[1]. Jiazhi Zhang, History of chemistry course (in Chinese)[M].Tai Yuan: Shanxi Education Press, 2005: 87.
[2]. Qiyi Xing, Basic Organic Chemistry (in Chinese)[M]. Bei Jing: Peking University Press, 2017: 707.
[3]. Hongbin Gao, Organic Chemistry [M]. Bei Jing: High Education Press,2005: 195-196.
[4]. Hancock, E.G, Benzene and its industrial derivatives, Chemical Industry Press, 1982.11
[5]. Chemistry Society of People’s Education Press, High school chemistry reader (in Chinese)[M]. Bei Jing: Chemistry Society of People’s Education Press, 2006: 197.
[6]. Shaoqiong Zeng. Organic Chemistry (in Chinese)[M]. Bei Jing: High Education Press, 1993: 150.
Cite this article
Li,J. (2023). Overview of benzene and exploration of benzene structure. Theoretical and Natural Science,21,245-252.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Jiazhi Zhang, History of chemistry course (in Chinese)[M].Tai Yuan: Shanxi Education Press, 2005: 87.
[2]. Qiyi Xing, Basic Organic Chemistry (in Chinese)[M]. Bei Jing: Peking University Press, 2017: 707.
[3]. Hongbin Gao, Organic Chemistry [M]. Bei Jing: High Education Press,2005: 195-196.
[4]. Hancock, E.G, Benzene and its industrial derivatives, Chemical Industry Press, 1982.11
[5]. Chemistry Society of People’s Education Press, High school chemistry reader (in Chinese)[M]. Bei Jing: Chemistry Society of People’s Education Press, 2006: 197.
[6]. Shaoqiong Zeng. Organic Chemistry (in Chinese)[M]. Bei Jing: High Education Press, 1993: 150.









