1. Introduction
Alzheimer’s disease (AD) is a prevalent neurodegenerative disorder that affects memory and cognitive function, characterized by cognitive dysfunction such as memory loss and the formation of new memory difficulties. Currently, there is no mature, clinically certified treatment for AD. However, over the last decade, dietary therapies involving Omega-3 polyunsaturated fatty acids (n-3 PUFA) supplementation have gained acceptance due to significant laboratory test results indicating a slower cognitive decline rate. The deficiency of n-3 fatty acids in neural tissue cell membranes has been linked to dementia [1–2]. N-3 fatty acids are found in various food sources, mainly in fatty deep-sea fish and seeds. α-linolenic acid (ALA) is the primary component of seed-based n-3 PUFAs, while docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are the main components of fish-based n-3 PUFAs. A small portion of ALA can naturally convert into DHA or EPA in the human body [3]. Therefore, it is important to study the effects of different n-3 PUFA on memory preservation individually. Seed oil is rich in polyunsaturated fatty acids and contains a significant amount of ALA, which plays a crucial role in brain development, learning, and memory [4]. DHA, one of the most important n-3 PUFAs found in the brain, is essential for brain function and development [5]. Seed oil is abundant in ALA, which is the precursor of DHA. ALA can be converted into DHA through a series of reactions in humans and most mammals. Consuming a certain amount of DHA may enhance learning and memory abilities and potentially prevent AD [6]. Fish oil is rich in DHA and EPA, which can regulate gene expression related to neuron production and signaling pathways. For instance, they promote the production of endothelial nitric oxide and brain acetylcholine, which support proper blood flow in the brain [7]. They also influence cellular responses, affect brain cell-signaling pathways, and support lipid synthesis, potentially preventing brain degeneration.
In theory, since both seed and fish-based n-3 PUFA influence memory retention [8], the research results from both sides suggest that regular intake of n-3 PUFA may decrease the rate of developing AD. Therefore, our study aims to investigate whether seed oil and fish oil can serve as potential dietary supplements for AD patients. We analyzed the effects of seed oil and fish oil on memory deterioration separately. We hypothesized that seed oil intake may be an effective method for reducing memory degeneration and preventing AD due to its high content of n-3 PUFAs and ALA. Furthermore, we hypothesized that dietary fish oil may also be effective in preventing AD. In summary, this paper uses meta-analysis to collect studies containing experimental results related to Alzheimer’s performance, memory degeneration, and spatial memory problems with interventions involving n-3 fatty acid intake. We aim to determine whether regular n-3 fatty acid intake, particularly in the early stages of memory decline, can help mitigate the development of Alzheimer’s disease.
2. Methods
2.1. Seed oil
2.1.1. Literature research. To identify the studies that explore the effects of seed oil on Alzheimer’s diseases, we searched the electronic databases PubMed, Web of Science, and Biomed Central from 2010 to 2022. Text words “Alzheimer OR memory determination OR cognitive impairment” AND “seed oil OR fatty acid from seed OR alpha arachidonic acid (ALA)” were searched. As a result, 43 articles were obtained after the research and were further filtered.
2.1.2. Study selection. All the studies chosen have to fulfill the following five criteria: animal experiments with simulated AD states were available; animal type was provided; at least 3 animals were examined in each trial; experiment results showed 95% confidence interval; and the type, duration, and amount of consumption of seed oil were provided. Data from various memory-related efficiency experiments were acceptable, for example, Morris water maze test (MWMT), Y-maze test, etc. In total, five studies that met the final filter options were analyzed.
2.1.3. Data synthesis. We calculated the memory efficiency increase rate of each experiment through dividing the data difference between the experimental group and control group by the control group data. The memory efficiency increase rate helped to elaborate on the significance of data more clearly and meaningfully. Then we conducted a regression analysis to explore the relationship between memory efficiency increase rate and seed oil intake dosage. In addition, 4 out of 5 articles conducted MWMT experiments. Thus, we analyzed the data from the MWMT experiment and utilized the t test for comparison between the control group and the experimental group. Differences between experimental groups were considered significant at p < 0.05 (not significant at p > 0.05), very significant at p < 0.01, and extremely significant at p < 0.001.
2.2. Fish oil
2.2.1. Literature research. PubMed, ScienceDirect, and Web of Science were searched for terms “fish oil OR DHA OR omega-3 fatty acids” AND “Alzheimer’s disease OR cognitive impairment OR dementia” from 2010 to 2022. 270 studies were obtained and further filtered.
2.2.2. Study selection. Focusing on the prevention of AD, articles that measured the AD incidence rate, the number of AD cases, and Mini-Mental State Exam (MMSE) score metrics are selected. All the studies chosen have to fulfill the following criteria: clinically diagnosed Alzheimer’s patients, prospective observational studies or trials, and certified cognitive tests with evaluation. In view of the memory loss problem alongside aging, the studied participants’ ages are above 60. There is no specific requirement for other human being characteristics such as race, sex, or nationality because the more randomized the objects are, the more generalized the result can be. The abstracts of the retrieved citations were reviewed, and the five studies that met the final filter options were analyzed.
2.2.3. Data synthesis. For the AD incidence rate, the value is either calculated from AD cases over the total sample size or directly obtained. The daily amount of dosage for both DHA and placebo was converted into the total amount of dosage to account for the uncertainty from the studies’ durations. Similarly, to account for the uncertainty of AD incidence rates due to sample size differences, the changes in the AD incidence rate are calculated for both treatments. This is calculated as the after-trial’s period AD incidence rate minus the before-trial AD incidence rate.
The relationship between DHA intakes and the AD incidence rate of the DHA group, as well as the relationship between placebo intakes and the AD incidence rate of the placebo group, are constructed by the regression test. T-test is carried to see if there’s a significant difference between the change in AD incident rate of DHA and placebo. Both t-test and regression tests are performed by Vassarstats.
3. Results
3.1. Seed oil
Based on figure 1, the T-test analysis conducted via Vassar Stats showed that the escape latency of the experimental treatment (mice with seed oil intake) is significantly lower than the escape latency of control treatment (p value < 0.0001). Based on figure 2, the regression analysis showed that the relationship between the dosage of seed oil intake and memory efficiency increase rate was nearly a linear regression (p value =0.0172). With the increase in seed oil intake dosage, the memory efficiency rate increased.
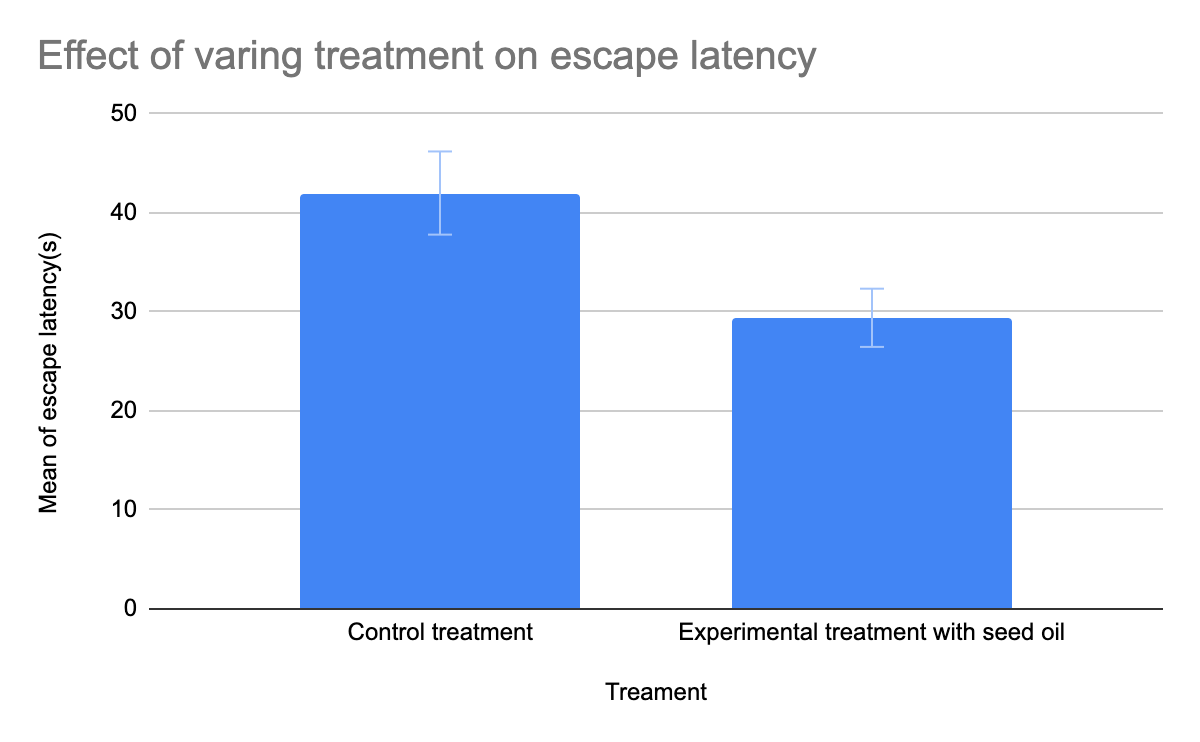
Figure 1. The comparison on escape latency jbetween control group (without seed oil intake) and experimental group (with seed oil intake).
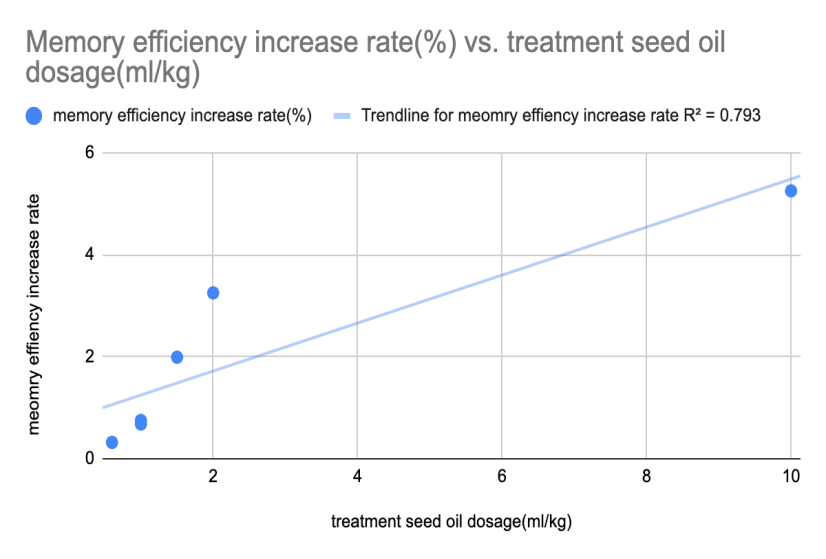
Figure 2. The relationship between memory efficiency increase rate and seed oil intake dosage.
3.2. Fish oil
In terms of changes in Alzheimer’s disease (AD) incidence ratios, Figure 3 illustrates the relationship between total DHA intake and the change in AD incidence rate within the DHA group. This relationship is represented by the equation y = -6*10^-6x - 0.0337, with an R^2 value of 0.291. Figure 4, on the other hand, showcases the connection between placebo intake and the change in AD incidence ratio within the placebo group. This relationship is described by the equation y = -5E-07x + 0.0049, with an R^2 value of 0.019. Both figures exhibit negative correlations with changes in AD incidence rates, although neither group demonstrates a strong, negative correlation with the AD incidence ratio. The correlation in the DHA group can be considered moderate, while the correlation in the placebo group is relatively weak. In t-tests, assuming that the data for both AD incidence ratio and MMSE score are independent, we obtained a two-tailed p-value of 0.0016 for the AD incidence rate section.
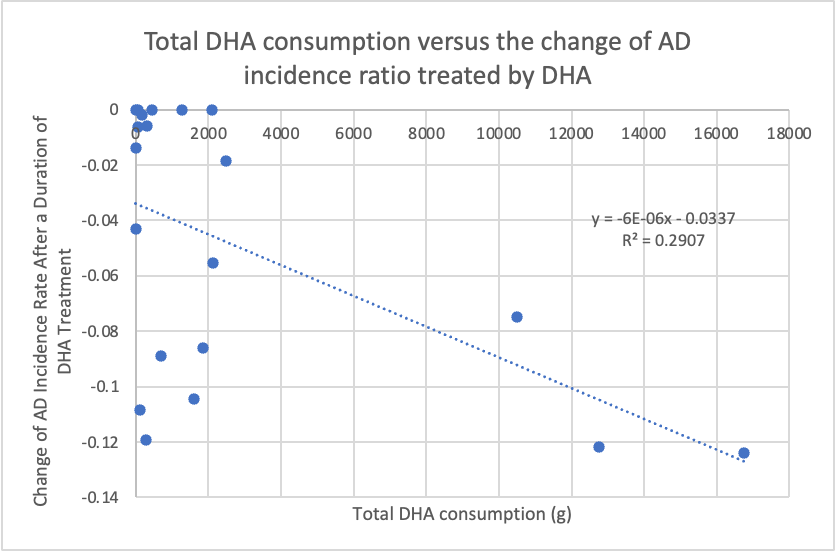
Figure 3. The relationship between DHA total intake and the change of AD incidence rate treated by DHA
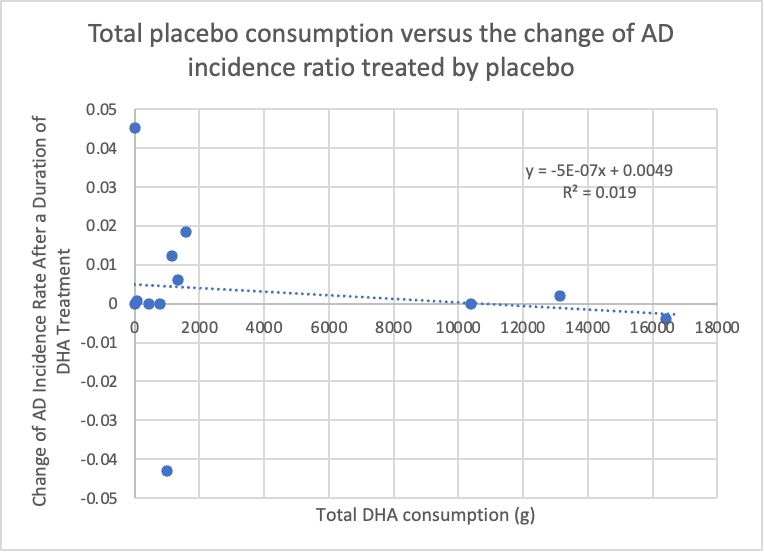
Figure 4. The relationship between total placebo intake and the change of AD incidence rate treated by placebo
4. Discussion& Limitation
4.1. Seed oil
Overall, the data from all these experiments showed seed oil could improve the memory deficit. There was a significant difference between the control group and the experiential group (mice with seed oil intake) (p<0.0001). The escape latency time of the experimental group with seed oil intake was less than that of the control group (Figure 1). The shorter escape latency time indicates that rats have better spatial memory, illustrating that the intake of seed oil has a positive effect on ameliorating spatial memory and short-term memory in Alzheimer’s disease. Also, the relationship between the dosage of seed oil intake and memory efficiency increase rate was a linear regression (p = 0.0172). A higher dosage of seed oil intake had a greater memory efficiency increase rate (Figure 2), indicating that the seed oil intake can improve memory ability.
The studies included in this meta-analysis varied in sample size, experimental methods, as well as the duration of supplementation, yet the effect of seed oil intake on AD was significant. Examining and comparing the results of the selected articles allowed for the observation of the role of seed oil in memory improvement. From the selected 5 articles, the Morris water maze test (MWMT) and the Y-maze test were conducted to test the effect of seed oil intake on memory ability. The Y-maze test is utilized to measure the short-term memory of the mice, and 2 of 4 articles had significant results. The Morris water maze test (MWMT) is used to evaluate the behavioral memory of AD rats, and 3 out of 4 articles had significant results on MWMT. Even though some experiments did not show significant results between seed oil intake and memory improvement, by combining and comparing results from various experiments, we could illustrate that seed oil may serve as an effective supplement for reducing memory degeneration and preventing AD [9].
The meta-analysis by Abubakari and Yurko-Mauro focused mainly on DHA and analyzed various cognitive functions related to DHA intake [10–11]. DHA is a well-known long-chain PUFA. The meta-analysis concentrated on the function of seed oil, which is rich in PUFAs and contains ALA, a precursor of DHA. The result of this study, combined with the results from Abubakari and Yurko-Mauro, provided a solid foundation to elaborate that seed oil can enhance memory ability [10–11]. In addition, Abubakari combined studies of healthy adults with disease populations besides AD, like depression, and thus included numerous cognitive tests [10]. Yurko-Mauro analyzed the clinical trials and found that the subjects of various experiments were humans [11]. The subjects of our study on seed oil were mainly animals, therefore, converging the results of clinical studies from other meta-analyses provided a more comprehensive conclusion. We can infer that seed oil is an efficient dietary supplement for humans to retard cognitive detorioration in AD patients.
The articles related to seed oil intake effect on AD were not abundant. This meta-analysis only focused on the animal experiments of seed oil effect on AD due to the lack of data support. Future analyses could contain various data based on human-subjective experiments. Another drawback was that there were numerous cognitive experiments in our study, but the data analysis did not include all the experiments separately because the data of the similar experiments were not enough to compare. A future study could focus on it when more experiment data is available.
4.2. Fish oil
In summary, there is a significant difference between the DHA and placebo groups regarding the change in AD incidence rate, as indicated by the two-tailed p-value of 0.0016, which is lower than the threshold of 0.05. Based on Figure 3, it can be concluded that DHA has a protective effect on AD prevention, although the effect is moderate. The negative change in AD incidence percentage suggests a reduction in new AD cases in the sample population, indicating that fewer people are diagnosed with AD after being treated with DHA. In contrast, Figure 4 shows that without DHA, the number of AD diagnoses remains unchanged when exposed to other fatty acid alternatives, such as corn oil. Therefore, DHA appears to have a relatively pronounced protective effect on AD prevention compared to other types of fatty acids.
However, it’s important to note that the relationship between the reduction in the number of new AD cases and DHA intake was not very strong, likely due to differences in study duration and the characteristics of the selected study populations. Firstly, variations in the duration of the studies led to significant differences in the total DHA intake. For example, the study by Kröger [12] was nine times longer than the study by Barberger-Gateau [13], resulting in a wide range of x-values, with the highest DHA intake significantly higher than the lowest DHA intake. This led to a large x-axis range but a concentrated y-axis range, impacting the estimation of the correlation trendline and resulting in a lower R^2 value. After removing an outlier, the R^2 increased to about 0.6. Secondly, in a group of healthy individuals, the preventive effect of DHA should be more pronounced than in a population with pre-existing mild cognitive impairment, as cognitive dysfunction due to brain atrophy is irreversible. For example, one of the studies conducted by Morris involved a follow-up case for the AD group [14]. Additionally, the number of studies in the selected articles varies, and smaller sample sizes are more likely to produce fluctuating results. Nevertheless, compared to the nearly flat trendline in the placebo group, long-term adherence to DHA intake is expected to have a mild preventive effect on AD prevalence.
Since the clinical criteria for Alzheimer’s disease remain somewhat vague and lack direct clinical evidence, cognitive ability tests are the primary means of predicting an individual’s mental status. Given that memory decline is irreversible, and Alzheimer’s is not solely a genetic disease, identifying potential high-risk groups for developing Alzheimer’s disease remains challenging. Future analyses could focus on obtaining clinical validation for Alzheimer’s assessment to better identify suitable experimental populations. By recording the diets of high-risk groups and analyzing their data, future studies can further explore the association between DHA and memory loss in AD patients.
5. Conclusion
This paper focuses on the effects of seed oil and fish oil intake, respectively, on the prevention of Alzheimer’s disease. After analyzing the results, it is known that supplementation with omega-3 fatty acids (including seed oil and fish oil) is associated with the improvement of memory. The results of the meta-analysis showed that seed oils and fish oils had a protective effect against attention deficit disorder. Observational data support the broad role of omega-3 fatty acid intake and its positive impact on improving memory capacity and, thus, on the prevention of Alzheimer’s disease. Regular intake of n-3 fatty acids in the early stages of memory loss may help mitigate the progression of Alzheimer’s disease. However, there are not enough articles on the effects of seed oil intake on AD. This article focuses only on animal experiments on the effects of seed oils on AD, and there is no data based on human subjective experiments as an analysis, and the amount of data is rather lacking. In addition, the duration of the studies and the populations in the selected articles may also lead to differences in total DHA intake, which may affect the results of the experiments. Future analyses will focus on obtaining clinical trials for Alzheimer’s assessment to explore the association between DHA and memory loss in patients with AD by recording the diets of people at high risk for Alzheimer’s and analyzing the data.
References
[1]. Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE (2001), Essential fatty acids in visual and brain development. Lipids, 36, 885-895. -DOI:0.1007/s11745-001-0798-1
[2]. Hashimoto M, Hossain S, Shimada T, Sugioka K, Yamasaki H, Fujii Y, Ishibashi Y, Oka JI, Shido O (2002) Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J Neurochem 81, 1084-1091.
[3]. Pawlosky RJ, Hibbeln JR, Novotny JA, Salem NJ. Physiological com- partmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res 2001; 42:1257–1265. PMID: 11483627.
[4]. Wei, G., Zhang, Z., Fu, D., Zhang, Y., Zhang, W., Zu, Y., Zhang, L., & Zhang, Z. (2021). Enzyme-assisted Solvent Extraction of High-yield Paeonia suffruticosa Andr. Seed Oil and Fatty Acid Composition and Anti-Alzheimer’s Disease Activity. Journal of Oleo Science, 70(8), 1133–1146. https://doi.org/10.5650/jos.ess21040
[5]. Takeyama, E., Islam, A., Watanabe, N., Tsubaki, H., Fukushima, M., Mamun, M. A., Sato, S., Sato, T., Eto, F., Yao, I., Ito, T. K., Horikawa, M., & Setou, M. (2019). Dietary Intake of Green Nut Oil or DHA Ameliorates DHA Distribution in the Brain of a Mouse Model of Dementia Accompanied by Memory Recovery. Nutrients, 11(10), 2371. https://doi.org/10.3390/ nu11102371
[6]. Gao, J., Wang, L., Zhao, C., Wu, Y., Lu, Z., Gu, Y., Ba, Z., Wang, X., Wang, J., & Xu, Y. (2021). Peony seed oil ameliorates neuroinflammation-mediated cognitive deficits by suppressing microglial activation through inhibition of NF-κB pathway in presenilin 1/2 conditional double knockout mice. Journal of Leukocyte Biology, 110(6), 1005–1022. https://doi.org/10.1002/JLB.3MA0821-639RR
[7]. Calder, P. C. (2017). Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proceedings of the Nutrition Society, 77(1), 52–72. https://doi.org/10.1017/s0029665117003950
[8]. Witte AV, Kerti L, Hermannstädter HM, Fiebach JB, Schreiber SJ, Schuchardt JP, Hahn A, Flöel A. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb Cortex. 2014 Nov;24(11):3059-68. doi: 10.1093/cercor/bht163. Epub 2013 Jun 24. PMID: 23796946
[9]. Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “mini-mental state.” Journal of Psychiatric Research, 12(3), 189–198. https://doi.org/10.1016/0022-3956(75)90026-6
[10]. Abubakari, A. R., Naderali, M. M., & Naderali, E. K. (2014). Omega-3 fatty acid supplementation and cognitive function: are smaller dosages more beneficial?. International journal of general medicine, 7, 463–473. https://doi.org/10.2147/IJGM.S67065
[11]. Yurko-Mauro K, Alexander DD, Van Elswyk ME (2015) Docosahexaenoic Acid and Adult Memory: A Systematic Review and Meta-Analysis. PLOS ONE 10(3): e0120391. https://doi.org/10.1371/journal.pone.0120391
[12]. Kröger, E., Verreault, R., Carmichael, P.-H., Lindsay, J., Julien, P., Dewailly, É., Ayotte, P., & Laurin, D. (2009). Omega-3 fatty acids and risk of dementia: The Canadian Study of Health and Aging. The American Journal of Clinical Nutrition, 90(1), 184–192. https://doi.org/10.3945/ajcn.2008.26987
[13]. Barberger-Gateau, P. (2002). Fish, meat, and risk of dementia: Cohort study. BMJ, 325(7370), 932–933. https://doi.org/10.1136/bmj.325.7370.932
[14]. Morris, M. C., Evans, D. A., Bienias, J. L., Tangney, C. C., Bennett, D. A., Wilson, R. S., Aggarwal, N., & Schneider, J. (2003). Consumption of fish and N-3 fatty acids and risk of incident alzheimer disease. Archives of Neurology, 60(7), 940. https://doi.org/10.1001/archneur.60.7.940
Cite this article
Li,H.;Pan,Y. (2024). The effect of Omega-3 fatty acid– fish oil and seed oil–on the prevention of Alzheimer’s disease. Theoretical and Natural Science,33,12-19.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE (2001), Essential fatty acids in visual and brain development. Lipids, 36, 885-895. -DOI:0.1007/s11745-001-0798-1
[2]. Hashimoto M, Hossain S, Shimada T, Sugioka K, Yamasaki H, Fujii Y, Ishibashi Y, Oka JI, Shido O (2002) Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J Neurochem 81, 1084-1091.
[3]. Pawlosky RJ, Hibbeln JR, Novotny JA, Salem NJ. Physiological com- partmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res 2001; 42:1257–1265. PMID: 11483627.
[4]. Wei, G., Zhang, Z., Fu, D., Zhang, Y., Zhang, W., Zu, Y., Zhang, L., & Zhang, Z. (2021). Enzyme-assisted Solvent Extraction of High-yield Paeonia suffruticosa Andr. Seed Oil and Fatty Acid Composition and Anti-Alzheimer’s Disease Activity. Journal of Oleo Science, 70(8), 1133–1146. https://doi.org/10.5650/jos.ess21040
[5]. Takeyama, E., Islam, A., Watanabe, N., Tsubaki, H., Fukushima, M., Mamun, M. A., Sato, S., Sato, T., Eto, F., Yao, I., Ito, T. K., Horikawa, M., & Setou, M. (2019). Dietary Intake of Green Nut Oil or DHA Ameliorates DHA Distribution in the Brain of a Mouse Model of Dementia Accompanied by Memory Recovery. Nutrients, 11(10), 2371. https://doi.org/10.3390/ nu11102371
[6]. Gao, J., Wang, L., Zhao, C., Wu, Y., Lu, Z., Gu, Y., Ba, Z., Wang, X., Wang, J., & Xu, Y. (2021). Peony seed oil ameliorates neuroinflammation-mediated cognitive deficits by suppressing microglial activation through inhibition of NF-κB pathway in presenilin 1/2 conditional double knockout mice. Journal of Leukocyte Biology, 110(6), 1005–1022. https://doi.org/10.1002/JLB.3MA0821-639RR
[7]. Calder, P. C. (2017). Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proceedings of the Nutrition Society, 77(1), 52–72. https://doi.org/10.1017/s0029665117003950
[8]. Witte AV, Kerti L, Hermannstädter HM, Fiebach JB, Schreiber SJ, Schuchardt JP, Hahn A, Flöel A. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb Cortex. 2014 Nov;24(11):3059-68. doi: 10.1093/cercor/bht163. Epub 2013 Jun 24. PMID: 23796946
[9]. Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “mini-mental state.” Journal of Psychiatric Research, 12(3), 189–198. https://doi.org/10.1016/0022-3956(75)90026-6
[10]. Abubakari, A. R., Naderali, M. M., & Naderali, E. K. (2014). Omega-3 fatty acid supplementation and cognitive function: are smaller dosages more beneficial?. International journal of general medicine, 7, 463–473. https://doi.org/10.2147/IJGM.S67065
[11]. Yurko-Mauro K, Alexander DD, Van Elswyk ME (2015) Docosahexaenoic Acid and Adult Memory: A Systematic Review and Meta-Analysis. PLOS ONE 10(3): e0120391. https://doi.org/10.1371/journal.pone.0120391
[12]. Kröger, E., Verreault, R., Carmichael, P.-H., Lindsay, J., Julien, P., Dewailly, É., Ayotte, P., & Laurin, D. (2009). Omega-3 fatty acids and risk of dementia: The Canadian Study of Health and Aging. The American Journal of Clinical Nutrition, 90(1), 184–192. https://doi.org/10.3945/ajcn.2008.26987
[13]. Barberger-Gateau, P. (2002). Fish, meat, and risk of dementia: Cohort study. BMJ, 325(7370), 932–933. https://doi.org/10.1136/bmj.325.7370.932
[14]. Morris, M. C., Evans, D. A., Bienias, J. L., Tangney, C. C., Bennett, D. A., Wilson, R. S., Aggarwal, N., & Schneider, J. (2003). Consumption of fish and N-3 fatty acids and risk of incident alzheimer disease. Archives of Neurology, 60(7), 940. https://doi.org/10.1001/archneur.60.7.940









