1. Introduction
Stem cells can self-renew and differentiate into different types of cells within specific tissues, including embryonic stem cells and adult stem cells. They are known for their ability to undergo division and generate new cells in the next generation, which can either remain in their stem cell state or develop into specialized cell types like muscle cells, nerve cells, or blood cells. They are present in various tissues in the body, like blood, brain, skin, and liver. Embryonic stem cells located in the inner mass of developing embryos, has the unique potential to mature into any cell type found in the adult body. On the other hand, adult stem cells, also known as somatic stem cells. They are located in various tissues throughout the body and can differentiate into cell types that are specific to the particular tissue in which they are situated. Additionally, somatic stem cells hold significant potential in regenerative medicine and the treatment of injuries and diseases, including cancer.
Stem cells can control cell expression through various mechanisms. This regulation involves the utilization of transcription factors, which are proteins with the capacity to bind to precise DNA sequences, thereby influencing the activity of neighboring genes. Stem cells further employ epigenetic mechanisms like DNA methylation and histone modification to fine-tune gene expression. Through these techniques, DNA structure and chromatin bonded will be changed, which will influence their attachment and later binding to the transcription factor, leading to different consequences of the RNA transcription process. Besides, Micro RNA (miRNA), is also used during the process that somatic cells control gene expression. miRNA is mainly used to interfere with or prevent the expression of one particular part of genes(mRNAs). Through all these regulations, stem cells hold their ability of self-renewing, influencing the overall cell physiological process.
2. The stem cells self-renewal and their control
Regarding self-renewal, stem cells have the unique possibility to divide then produce more stem cells, while maintaining their undifferentiated state, and enabling them to endure throughout the organism’s lifetime and strike a crucial equilibrium between self-renewal and differentiation.
There are a few different pathways related to stem cell renewal, like the Notch pathway and the Sonic Hedgehog pathway. It is noticeable that most pathways were common in both stem cell regulation and oncogenesis because the formation of cancer is highly relevant to the stem cell mechanism. One particular pathway that is worth paying attention to is the wnt pathway. As an intercellular signalling molecule that is located mainly in bone marrow. Wnt proteins act as the role to regulate the cellular development in a few types of organisms. The chaos and error happen related to the Wnt protein will lead to cancer. Research has shown that the expression of wnt may also influence the regulation of HSCs. For example, the amount of TCF-4, which is a transcription factor in the wnt signalling pathway, will sensitively influence and get rid of the progenitors located in the gut epithelium crypts that have not been differentiated during the process of development [1]. Research indicates that by activating the downstream protein β-catenin in the Wnt signaling pathway, the pool of HSCs can be expanded, making them more readily transplantable. Nonetheless, when Axin, which acts as an inhibitor of the pathway, is inappropriately activated, it results in the inhibition of hematopoietic stem cell (HSC) proliferation, heightened HSC mortality in a laboratory setting, and a diminished capacity for reconstitution in live subjects. In addition, special environment culture will control the proliferation of hematopoietic progenitors in different organs of humans and mice. Human keratinocytes that are cultured and possess greater potential for proliferation exhibit higher levels of beta-catenin. All this evidence has shown that the Wnt pathways not only regulate the HSCs concentration and activities but also play an important role in promoting many other issues’ reproductions process.
3. Stem cells and cancer
One more important aspect that contribute to people’s attention to stem cell is their high similarity with cancer cell which implies the potential curing method. Firstly, the most special ability that cancer cells is the capacity of self-renewal and reproduce infinitely. The produce that cells reproduce and grow into tumors is quite the same as the one of the stem cell self-renewal. This implies that stem cell and cancer cell share a similar mechanism, so the research of the stem cell may also reveal the principle behind the cancer.
In the majority of human acute myeloid leukemia (AML) subtypes, the cells with the ability to initiate AML in NOD/SCID mice shows a CD34+ CD38, which closely mirrors that of normal hematopoietic stem cells (HSCs). On the contrary, CD34+ CD38+ leukemia cells, despite exhibiting a leukemia-like blast phenotype, usually cannot induce the disease in mice. This implies that leukemic transformation is more likely to target normal HSCs rather than committed progenitor cells. Different types of chromatin mutations focusing on HSCs exist, the most typical one is the AML involving the 8;21 translocations, leading to leukemic cells AML1–ETO chimeric transcripts. This suggests that the translocation process often originates in normal hematopoietic stem cells (HSCs), and that occurring in a part of these HSCs or their descendants subsequently lead to the development of leukemia. This idea is further supported by studies on lymphoid and chronic myeloid leukemias, in which specific leukemia-associated chromosomal rearrangements have been identified in CD34+ CD38– cells. This cell population is notably enriched in hematopoietic stem cells (HSCs) [2]. The malignant transformation of stem cell period HSCs will be the consequence of the accumulation of leukemias mutations.
However, although traditional cancer research has made significant progress in analyzing the gene mutation that occurs in the tumor cell, there still exists a barrier between the understanding of DNA sequence and what happens. At the application and clinical level, researchers often struggle to predict the effect of mutations on actual cancer cells showed specific cancer types, hindering the translation of these findings into new therapies. One approach is to consider a tumor as an abnormal organ that develops from a tumorigenic cancer cell. This cancer cell gains the ability for uncontrolled and indefinite proliferation as a result of accumulated mutations., which is somehow the same as the perspective of stem cells. Heterogeneity in tumors can result from both mutagenesis and aberrant differentiation of cancer cells. Some cancers exhibit diverse phenotypes reflecting the differentiation processes of the tissues they originate from. It’s worth noting that cancer cells frequently display functional and phenotypic characteristics that closely resemble those of the normal cells from which they initially derive. By considering a tumor as a deviant organ, we can utilize the fundamental principles of conventional stem cell biology to enhance our comprehension of the mechanisms underlying tumor development.
4. The cancer stem cell—the possible origin of cancer
Cancer stem cell (CSC) theory posits that within tumors, there are small populations of tumor stem cells. This theory explains various clinical observations, such as the almost certain reappearance of tumors following an initially successful round of chemotherapy. There are a few pieces of evidence building this theory, which originates from the fact that tumor was formed by some specific types of cancer cell that can extensively proliferate. Such similarity between the model that cancer cell growth follows and one of the stem cells give rise to the assumption of cancer stem cells. For example, studies on solid tumors have revealed that these tumors consist of diverse populations of cancer cells with varying abilities to initiate tumor growth. In breast cancer, for instance, it has been observed that as few as 100 CD44+CD24-/low cells have the capability to kickstart tumor growth, whereas other cell phenotypes are notably less effective in this regard. This pattern of heterogeneity within solid tumors has been documented across various cancer types.
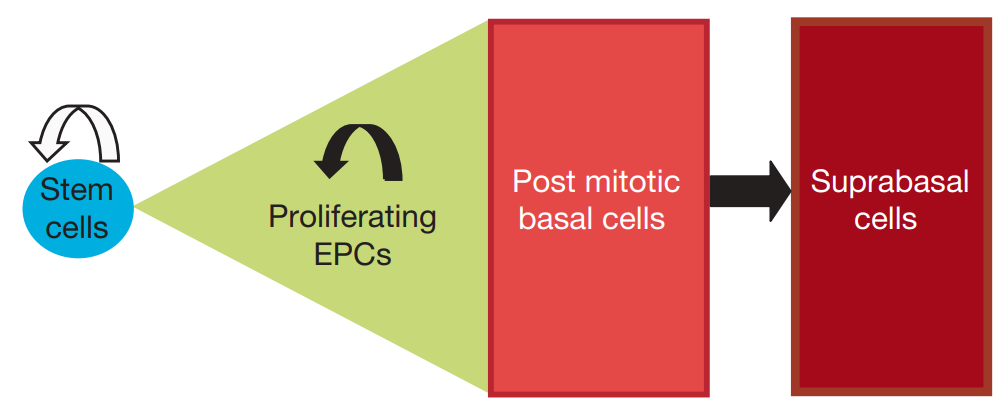
Figure 1. Single progenitor cell compartment model for epidermal homeostasis [3].
By now, the xenotransplantation method for studying the characteristics of cancer stem cells (CSCs) comes with certain inherent technical and conceptual constraints. There are also some lineage-tracing studies targeting human cancers. Studies used CRISPR-Cas9 technology to insert genetic markers into patient-derived colorectal cancer organoids and xenografts, allowing for lineage tracing and revealing insights into the behavior of cancer cells. Following this, grafts can be utilized to explore the behavior of human Lgr5+ cells within intact tumors. In addition, relevant research has emphasized the diversity and complexity of adult stem cell hierarchies as well as focusing on the plasticity of adult stem cells and their various roles in tissue maintenance and regeneration [3], as shown in Figure 1. The microenvironment plays a pivotal role in influencing the adaptability of CSCs. In the context of colorectal cancer, within an inflammatory environment, the activation of NF-κB signaling can boost the tumor-initiating capability of non-stem cells by prompting their transformation back into stem-like cells. Recent research into the plasticity of cancer stem cells involved experiments that involved removing cells in xenografted human cancers. By using CRISPR-Cas9, an inducible suicide gene (iCasp9) was introduced into the LGR5 gene of human colorectal cancer organoids. When apoptosis was triggered, the tumor size decreased, but it grew back after removing the inducer. What’s remarkable is that the regenerated tumor witnessed the proliferation of previously arrested cells. By conducting lineage-tracing experiments using KRT20-expressing differentiated cells, it was proven that these cells reacquired the ability to proliferate, thus restoring the LGR5+ CSC pool and underscoring the plasticity of CSCs.
Much research has focused on ‘plasticity’, which means It has been unveiled that committed cells can transition along the hierarchy of differentiation, either moving towards a more specialized state or reverting to a less specialized one. When progenitor cells are exposed to a combination of niche signals, such as WNT, NOTCH, EGFR ligands, and BMP/TGF-β inhibitors in an in vitro setting, these cells quickly regain their stem cell characteristics. This suggests that when committed cells are close to the niche located at the base of the crypt, they receive signals and can recover to a pluripotent stem cell state [4].
There is substantial evidence backing the presence of cancer stem cell hierarchies in different tumors, not all cancers adhere to the CSC model. For example, melanoma-initiating cells’ xenotransplantation frequency can vary significantly depending on the technology used, raising questions about the presence of a strict CSC hierarchy [5]. Furthermore, research suggests that both CSCs and non-CSCs are capable of displaying plasticity, undergoing changes in their characteristics in response to environmental signals., challenging the notion of fixed CSC and non-CSC states [6].
Epithelial cancer cells have the capacity to adopt a mesenchymal genetic profile, which promotes their ability to migrate and invade surrounding tissues. This change is termed “epithelial-to-mesenchymal transition” (EMT). The connection between CSCs and EMT has attracted considerable attention, and it has been suggested that EMT may be transient, as epithelial tumor cells can transition into intermediate mesenchymal states [7]. In many types of carcinoma, metastatic cells often preserve their epithelial characteristics and do not exhibit mesenchymal traits. This suggests that tumor cells capable of migration can revert to an epithelial state once they have reached a foreign organ., and in some cases, undergoing an EMT might not be necessary for metastasis. In fact, cells that are trapped in a permanent EMT state exhibit reduced metastatic potential. For efficient metastatic growth, they must revert to an epithelial state.
Cancer stem cells exhibit a distinctive metabolism that differs from non-CSCs. Metabolism reliant on oxidative phosphorylation (OxPhos) is essential for generating the energy needed to sustain complex tissues. However, it also leads to the formation of potentially harmful reactive oxygen species (ROS), which have the possibility to interfere with the functioning of stem cells. It’s commonly believed that stem cells avoid OxPhos in favor of glycolysis to protect against ROS. However, recent research has unveiled that CSCs have a unique metabolism distinct from non-CSCs. For instance, particular disseminated tumor cells derive their energy from fatty acids, and this metabolic process is regulated by the expression of the fatty acid receptor CD36. This distinction in energy requirements, particularly in CSCs during metastasis, may offer new avenues for treating the advanced stages of the disease [8].
5. Mesenchymal stem cells
Mesenchymal stem cells (MSCs) have become commonly used terms to describe a single cell and a population of versatile stem/progenitor cells [9]. MSCs exhibit good anti-inflammatory and immune regulatory abilities, rendering them valuable in a range of clinical applications [10]. They have been the subject of investigation in more than 950 clinical trials for conditions like lung injury. The clinical benefits of MSC therapy primarily revolve around their capacity to modulate immune system and improve damaged tissues function. It’s worth noting, however, that not all patients respond to MSC therapy, and MSCs can be able to produce significant effects in only around 40-50% of patients., underscoring the need for further understanding of MSC therapy in patients.
6. Conclusion
Stem cell, being considered as the origin of all cells who own the full potential ability, has now been well studied and still owns infinite possibility. However, it still hasn’t got a complete understanding of the principle lying behind, especially since it is difficulties in the application level of putting it into practical use. Advancements in technology are reshaping our view of cancer stem cells. CSCs may not always be rare, quiescent, or hardwired. The idea of eliminating CSCs to cure cancer may need rethinking. Tumor cell plasticity poses a challenge. Instead of solely targeting intrinsic CSC features, modulating stem cell-niche interactions appears promising. Understanding how the tumor microenvironment controls CSC states is essential. This evolving understanding raises questions and complexities in cancer therapy design. MSCs have been under study for nearly three decades and are currently undergoing diverse clinical trials, with some showing strong support from animal model studies. Their potential therapeutic applications are vast, especially in areas with significant patient needs, and they possess unique properties. However, there are areas requiring further research to advance our understanding and harness the full potential of MSCs, emphasizing their complex biology and therapeutic capabilities. Overall, MSCs have emerged as a powerful cellular entity with substantial promise for regenerative medicine. In conclusion, the relationship between stem cells and cancer is marked by several key propositions. These encompass the fundamental property of self-renewal, the propensity of certain stem cell types to accumulate mutations that trigger malignancies, and the role of shared signalling pathways in regulating self-renewal. Additionally, it is essential to target CSCs with their potential for indefinite self-renewal to effectively combat cancer. The intersections between these two fields offer rich opportunities for advancing cancer research and treatment, presenting novel pathways for therapy development and deeper insights into the mechanisms underpinning cancer progression. Understanding the dynamic interplay between stem cells and cancer holds significant promise for improving cancer outcomes and ultimately finding a cure for this complex disease.
References
[1]. Korinek V, Barker N, Moerer P, et al. 1998 Nature genetics 19(4) 379-383
[2]. George A A, Franklin J, Kerkof K, et al. 2001 Blood, The Journal of the American Society of Hematology 97(12) 3925-3930
[3]. Clayton E, Doupé D P, Klein A M, et al. 2007 Nature 446(7132) 185-189
[4]. Sato T, Van Es J H, Snippert H J, et al. 2011 Nature 469(7330) 415-418
[5]. Quintana E, Shackleton M, Sabel M S, et al. 2008 Nature 456(7222) 593-598
[6]. Gupta P B, Fillmore C M, Jiang G, et al. 2011 Cell 146(4) 633-644
[7]. Cano A, Pérez-Moreno M A, Rodrigo I, et al. 2000 Nature cell biology 2(2) 76-83
[8]. Suda T, Takubo K, Semenza G L. 2011 Cell stem cell 9(4) 298-310
[9]. Rodríguez-Fuentes D E, Fernández-Garza L E, Samia-Meza J A, et al. 2021 Archives of medical research 52(1) 93-101
[10]. Sheng G. 2015 BMC developmental biology 15 1-8
Cite this article
Hu,Y. (2024). Gene expression controlled by stem cells and its relationship with cancer. Theoretical and Natural Science,32,52-56.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Korinek V, Barker N, Moerer P, et al. 1998 Nature genetics 19(4) 379-383
[2]. George A A, Franklin J, Kerkof K, et al. 2001 Blood, The Journal of the American Society of Hematology 97(12) 3925-3930
[3]. Clayton E, Doupé D P, Klein A M, et al. 2007 Nature 446(7132) 185-189
[4]. Sato T, Van Es J H, Snippert H J, et al. 2011 Nature 469(7330) 415-418
[5]. Quintana E, Shackleton M, Sabel M S, et al. 2008 Nature 456(7222) 593-598
[6]. Gupta P B, Fillmore C M, Jiang G, et al. 2011 Cell 146(4) 633-644
[7]. Cano A, Pérez-Moreno M A, Rodrigo I, et al. 2000 Nature cell biology 2(2) 76-83
[8]. Suda T, Takubo K, Semenza G L. 2011 Cell stem cell 9(4) 298-310
[9]. Rodríguez-Fuentes D E, Fernández-Garza L E, Samia-Meza J A, et al. 2021 Archives of medical research 52(1) 93-101
[10]. Sheng G. 2015 BMC developmental biology 15 1-8









