1. Introduction
Bioactive compounds, naturally occurring chemical substances found in plants, animals, and microorganisms, have the potential to induce specific physiological or pharmacological effects on living organisms. Among these compounds, phytosterols, also known as plant sterols, represent a class of bioactive molecules structurally similar to cholesterol but exclusively derived from plants. These compounds are commonly found in cell membranes of plants and are present in varying concentrations in a wide range of foods, especially in vegetable oils, nuts, seeds, and whole grains. The chemical structure of phytosterols is characterized by a tetracyclic cyclopenta(α)phenanthrene structure, consisting of four rings (A, B, C, D), with an aliphatic R group side chain located at carbon 17 (C17) of ring D. Phytosterols exhibit an amphiphilic nature, featuring both hydrophobic and hydrophilic domains. The polar hydroxyl group (OH) imparts polar attributes to the compound, while the rest of the structure remains non-polar. In some instances, double bonds may exist between carbon atoms, and the inclusion of methyl or ethyl groups adds to the structural diversity. The spatial orientation and proximity of these double bonds influence their functional properties. Additionally, the biochemical activity of phytosterols is shaped by the stereochemistry and degree of saturation within the alkyl side chain [1].
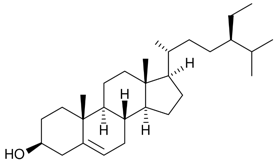
Figure 1. Chemical structure of phytosterol
Phytosterols play a pivotal role in promoting human health and well-being due to their diverse effects, including antimicrobial, anti-inflammatory, and antioxidant properties. Notably, phytosterols are renowned for their capacity to reduce blood cholesterol levels, making them invaluable in preventing and managing cardiovascular diseases [2]. In recent years, there has been a surge of activity in phytosterol research across various scientific domains. However, many experiments and clinical studies tend to focus on specific properties, creating a notable gap in the field. To address this gap, this article comprehensively explores the extraction process, a crucial factor in obtaining high yields of phytosterols. Among the various extraction methods, Ultrasonic-assisted extraction (UAE) and Enzyme-assisted extraction processing (EAEP) emerge as frequently employed techniques. Additionally, the detection of phytosterols assumes a paramount role in scientific and industrial applications. Various methods, such as Gas Chromatography (GC) and Fourier Transform Infrared Spectroscopy (FTIR), are instrumental in detecting and analyzing phytosterols, advancing our understanding of these compounds [3].
The application of phytosterols in the medical field, cosmetics, and the food industry is noteworthy. In the medical context, phytosterols are recognized for their ability to reduce low-density lipoprotein (LDL) cholesterol levels, making them effective in managing hypercholesterolemia and preventing cardiovascular diseases. Through competition with cholesterol absorption in the intestine, phytosterols can lower LDL cholesterol levels while leaving high-density lipoprotein (HDL) cholesterol levels unaffected [1]. This unique mechanism has led to the incorporation of phytosterols into various treatments and medicines. In the cosmetics realm, plant-based oils rich in polyunsaturated and fatty acids serve as antioxidants and moisturizers, countering the detrimental effects of free radicals [4]. Notable advancements have also been made in utilizing phytosterols within the food industry, where researchers have employed conventional lipids and free phytosterols to construct nanostructured carriers (NLCs) designed to preserve the bioavailability of bioactive compounds [5]. Providing a comprehensive summary and review of existing research on phytosterols not only facilitates future investigations but also serves to streamline research efforts. Additionally, review articles have the capacity to illuminate the limitations and gaps present in current studies.
2. Source of Phytosterols
Phytosterols have garnered considerable attention in recent years, with numerous investigations unveiling their diverse biological functions. This bioactive compound, sharing physiological purposes and a structure akin to human cholesterol, is abundantly present in our daily diet, though the human body cannot synthesize it [6]. Classified under triterpenes, phytosterols encompass over 100 different forms, with primary variants including β-Sitosterol, campesterol, stigmasterol, brassicasterol, and ∆5-avenasterol [7]. Unprocessed foods stand out as the primary natural sources of phytosterols, such as vegetable oils, nuts, vegetables, and fruits. For example, corn oil contains approximately 746 mg/100 g of phytosterols, while sesame seeds consist of 714 mg/100 g. Nuts exhibit relatively lower amounts, ranging from 30-220 mg/100 g compared to vegetable oil. Vegetables offer phytosterol supplies ranging from 4-40 mg/100 g, while fruits provide only 4-24 mg/100g of phytosterol [8].
Table 1. Various Sources of Phytosterol and Their Content [9]
Source of phytosterol | Phytosterol content mg / 100g of oil |
Brazil Nuts | 95 |
Pumpkin seed kernel | 265 |
Pistachio & Sunflower seed | 270-289 |
Sesame seed | 400-413 |
Corn oil | 746 |
In addition to naturally occurring compounds, recent discoveries in phytosterol production pathways have generated significant waste, yet studies reveal that these by-products possess higher value and exhibit biochemical functions. As depicted in Figure 2, crab apples from the genus Malus have emerged as a potential source of five phytosterols: β-sitosterol, isofucosterol, campesterol, stigmasterol, and ∆7-avenasterol. Notably, β-sitosterol dominates apple oil extract, constituting as much as 82.1% to 97.5%. Variations in phytosterol ratios may be attributed to different environment and agricultural techniques employed throughout the growing season [10].
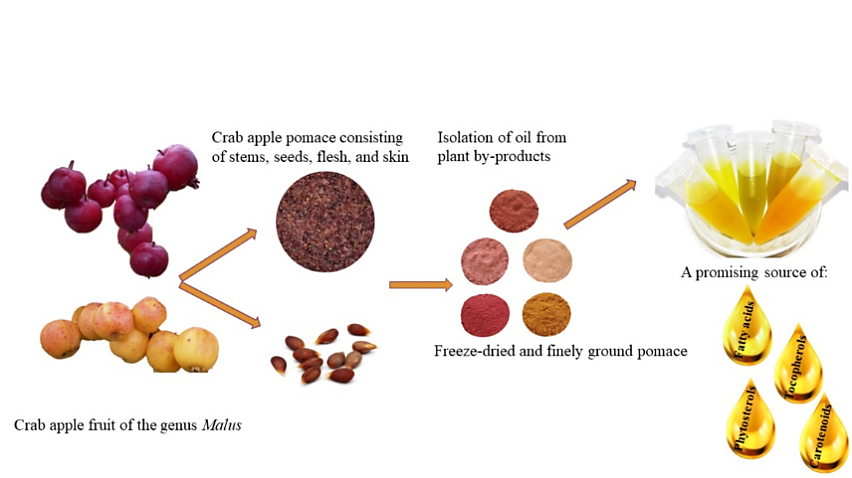
Figure 2. By-product of Crab Apple (Malus spp.) as a Source of Phytosterols [10]
Moreover, research indicates that pumpkin seeds can effectively produce phytosterols through specific extraction methods. Given the substantial consumption of pumpkins, a significant amount of their seeds goes to waste. Scientists have found that the phytosterols within these seeds can be extracted and repurposed. In a study, ultrasound-assisted solvent extraction (UAE) yielded the highest oil content and phytosterol concentration, with results of 95.46±0.06% and 2017.5±100.1 mg/100 ml of oil, respectively [11].
3. Preparation Method of Phytosterols
In the exploration of bioactive compounds, the extraction process plays a pivotal role as the yield of phytosterols is highly dependent on the extraction conditions and processes. Among the methods utilized by contemporary scientists, Ultrasonic-Assisted Extraction (UAE) stands out as a more commonly employed technique. This method is favored over others due to its expedited process, energy efficiency, and its ability to maintain the quality of the extracted substance under low-temperature conditions. Ultrasounds, as mechanical waves with a frequency above 20 kHz, can propagate through solid, liquid, or gas mediums, displacing molecules in the process. The application of ultrasound waves induces cavitation in the samples, leading to the formation and implosion of vacuum bubbles, resulting in elevated pressures and temperatures. The primary mechanism behind ultrasound-assisted extraction is acoustic cavitation, which also induces turbulence and shear forces within the fluid, facilitating the breakdown of cell walls and promoting the release of bioactive substances. This rapid fragmentation expedites the dissolution process of bioactive compounds in the solvent by increasing mass transfer rates at the boundary of the plant matrix and reducing particle sizes.
UAE presents numerous advantages over other extraction systems, including reduced time, energy consumption, temperature, and chemical requirements. The extraction process can be further optimized by designing equipment that prevents direct contact between specific components, such as ultrasonic horns. This enhancement holds the potential to improve the efficiency and effectiveness of UAE [12].
Enzyme-Assisted Extraction Processing (EAEP) emerges as a promising and advantageous method when compared with conventional solvent extraction. The use of an aqueous medium renders it a safer and eco-friendly approach. Moreover, EAEP provides flexible and secure operation, resulting in a greater yield and improved nutritional value. The process minimizes oil loss by neutralizing under alkaline conditions, leading to oil with a low concentration of phospholipids and excellent antioxidant stability. However, some drawbacks of EAEP are evident in the industry, such as a longer processing time and higher production costs, primarily due to steps like centrifugation for separation and emulsification [13].
Enzymes play a crucial role as a pre-treatment step, acting as catalysts for the subsequent release of oil by hydrolytically reacting with the cell membrane. Major components of the cell wall, namely cellulose, pectin, and hemicellulose, are degraded by enzymes like cellulases, hemicellulases, and pectinases. In a study focusing on corn oil extraction, Celluclast and Multifect GC cellulase enzymes were employed, resulting in improved oil yields of 91% and 93%, respectively [13]. The flexibility of this method lies in the ability to use various combinations of enzymes tailored to the specific extraction sample. In another study concentrating on the extraction of bioactive compounds from bay leaves, cellulase, hemicellulase, and xylanase enzymes were utilized [14]. After enzyme treatment, the treated samples underwent hydrodistillation for around 2 hours to separate essential oils. Subsequently, the extracted oils were weighed, dried, and stored in airtight containers at -20 degrees Celsius until analysis [13].
4. Methods for the Detection of Phytosterols
A gas chromatography-mass spectrometry (GC-MS) technique was developed as a straightforward method for analyzing the composition of sterols in milk and palm oil within milk fat. Iron magnetic nanoparticles were modified using hydrophobic nanoparticle synthesis, achieved through the addition of octadecyltrimethoxysilane (ODTMS). In this study, the milk sample was homogenized, and a smaller portion was placed into a flask. Subsequently, 1 ml of 1M methanolic KOH liquid was added to induce saponification, ensuring the conversion of linked sterols to free sterols. The mixture was heated at 60 °C for 45 minutes and then cooled to 35 °C. To prepare the test solution, 5 ml was taken and diluted to 50 ml. The modified magnetic adsorbent was added to the prepared mixture, facilitating sterol absorption. After the separation of the absorbed target analytes, GC-MS was employed for examination [15]. The peak retention time from the chromatogram of the sample was compared with the sterol standards from a known spectrum to identify the substances [16]. GC-MS is the preferred technique for determining phytosterols due to its sensitivity and specificity. However, given the unique physical properties and low concentrations of certain sterols, a derivative is typically required before analyzing these substances [17].
The determination of sterol concentration in samples requires additional procedures, such as Fourier Transform Infrared Spectroscopy (FTIR). FTIR utilizes an infrared spectrometer to measure the infrared spectrum produced by the sample substance’s absorbance on both horizontal and vertical axes [18]. This technique allows for the quantification of compounds by analyzing their specific reactions to the infrared beam, as different substances absorb infrared light at distinct frequencies based on their molecular bonds. The absorption of infrared radiation by molecules leads to the excitation of their fundamental vibrations, causing them to transition from a ground state to a vibrational state. Through the analysis of data obtained from FTIR spectroscopy, valuable information regarding the structural and functional properties of the examined substance can be acquired [19].
5. Application of Phytosterols in Medicine
Phytosterols, essential bioactive compounds, exhibit applications across various domains, notably in medical interventions. In 2000, an interim ruling by the Food and Drug Administration (FDA) sanctioned the use of health claims for phytosterol-containing products, attributing them to the reduction of cardiovascular disease risk. Research indicates that phytosterols have the potential to optimize blood lipid profiles and mitigate susceptibility to cardiovascular illnesses. Noteworthy studies emphasize that a daily intake of 2 grams of phytosterols can result in up to a 10% reduction in LDL cholesterol levels. Clinical investigations have shown even more pronounced cholesterol level reductions in patients concurrently administered with anti-hypercholesterolemic medications such as fibrates and statins [1]. Due to their constrained intestinal absorption, plasma concentrations of phytosterols generally lag behind those of cholesterol. This is due to a shared absorption pathway where phytosterols and cholesterol compete for entry into micelles and, subsequently, enterocytes. The amplified presence of phytosterols in the gut precipitates diminished cholesterol absorption, accentuating the reciprocal relationship [20].
Phytosterol compounds, under intensive investigation, have demonstrated their potential as protective agents against diverse cancer types. Notably, their capacity to impede the proliferation of cancer cells has been established in various anatomical sites, including the liver, prostate, breast, and lung. However, the precise mechanistic underpinnings of these compounds in cancer therapy remain elusive, owing to the limited body of corroborative research. Among cancers prevalent in women, breast cancer stands as a prominent concern. Its pathogenesis, potentially linked to multifarious factors including cholesterol levels, necessitates comprehensive exploration. Lof et al. uncovered a correlation between breast cancer risk and excess weight, underscoring its intricate interplay. Furthermore, Qadir and Malik’s investigation highlighted elevated serum levels of total cholesterol, triglycerides, and LDL cholesterol in cancer-afflicted women. Significantly, beyond its inhibitory impact on breast cancer progression, phytosterol consumption holds promise in ameliorating the cancer-induced disruptions in lipid profiles [21].
The escalating interest in harnessing phytosterols as functional supplements has culminated in the emergence of novel products meticulously crafted to address metabolic disorders. The condition of obesity is often accompanied by a perturbed energy equilibrium stemming from elevated energy intake compared to expenditure. This dichotomy results in lipid accrual within diverse tissues, including the liver and adipose tissue, and concurrently fosters a low-grade inflammatory milieu characterized by the generation of specific pro-inflammatory agents. Additionally, it incites the proliferation of macrophages within adipose tissue, thereby exacerbating metabolic aberrations such as insulin resistance and hyperglycemia. Phytosterols have shown a capacity to ameliorate these obesity-associated metabolic dysfunctions. Pioneering studies delving into the interplay between phytosterols and adipose tissue have elucidated the vital role of adipose tissue in orchestrating systemic metabolism. Comprising a heterogeneous amalgamation of constituents, including pre-adipocytes, fibroblasts, and macrophages, adipose tissue assumes a pivotal role in response to negative energy balance. This engagement entails the generation of energy-dense fatty acids, which, when requisite, are dispatched to targeted tissues to mitigate lipid accumulation. Predominantly conducted using 3T3L1 cells, murine-derived preadipocyte lines, in vitro investigations have unveiled noteworthy insights. Notably, saringosterol, a steroid derived from a brown alga indigenous to Korea, has exhibited the propensity to augment the expression of adipogenic genes in 3T3L1 cells. Among these pivotal genes are adiponectin, resistin, and fatty acid synthase, collectively substantiating its anti-obesity effects [22].
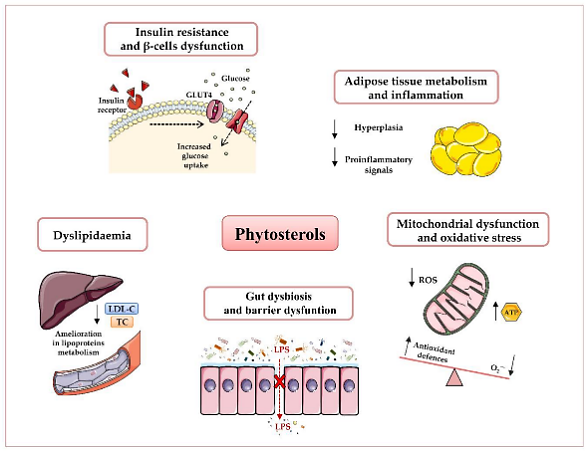
Figure 3. Potential beneficial effects of phytosterols in obesity and type 2 diabetes T2D prevention and therapy [22]
The burgeoning multitude of clinical trials substantiating the therapeutic employment of phytosterols in addressing obesity underscores the manifold advantages inherent in this compound. An investigative endeavor conducted in Japan unveiled a notable reduction in adipose tissue across the abdomen, subcutaneous, and visceral regions among male participants administered with phytosterols. Echoing these findings, a congruent study executed in China proffered evidence of a negative correlation between heightened phytosterol consumption and body mass index (BMI) in a cohort comprising 503 Chinese women and 409 men [22].
Notwithstanding these revelations, the precise mechanisms through which phytosterols exert their influence on adipose tissue expansion remain enigmatic, necessitating further meticulous inquiry to unequivocally validate the efficacy of this compound under clinical circumstances. As only a marginal fraction, ranging from 0.4% to 3.5%, of ingested phytosterols undergoes viable absorption within the intestinal milieu, the administration of elevated dosages is improbable to engender an escalation in their uptake. Notably, a measured and judicious protraction of phytosterol consumption over a sustained temporal expanse has been conjectured to elicit propitious outcomes [22].
6. Application of Phytosterols in Cosmetics
Fatty acids and polyunsaturated fatty acids, inherent in plant-based oils, serve as natural moisturizers and antioxidants, effectively countering the deleterious effects of free radicals. Various skin stressors, including chemical exposure, alcohol consumption, tobacco use, and UV radiation, can provoke the formation of these free radicals. At a molecular level, free radicals possess formidable chemical reactivity and exhibit remarkable instability, enabling them to engage with a gamut of biological molecules, including DNA, proteins, and lipids. Such interactions result in cellular damage and disrupt the delicate balance of homeostasis. The unchecked proliferation of free radicals can trigger lipid oxidation, thereby giving rise to oxidative stress. Although our biological system is endowed with endogenous antioxidants, augmenting DNA repair mechanisms and mitigating oxidative stress necessitates the topical application of exogenous antioxidants. A wealth of scientific studies supports the role of antioxidants in fortifying the skin’s natural defenses against free radical onslaught. Notably, research suggests that the maintenance of natural skin color can be achieved through the use of Moringa oleifera seed oil, which exhibits mild sun-protective properties. In this study, the antioxidant activity of Moringa seed oil cream was evaluated employing the DPPH method. The observed decline in DPPH absorption signifies the compound’s adeptness at efficiently neutralizing free radicals. The findings unveiled a dose-dependent increment in free-radical scavenging activity; at a Moringa oleifera seed oil concentration of 121.9 mg/mL, it effectively inhibited the activity of 50% of the free radicals. Intriguingly, skin hydration levels exhibited a significant increase, surging from 16% to 85% over a span of four weeks. In contrast, conventional creams had a less pronounced impact on skin hydration compared to the Moringa seed oil-based counterpart. Nevertheless, it is worth noting that the study’s results revealed that the use of Moringa seed oil for four weeks did not yield discernible effects on skin elasticity [4].
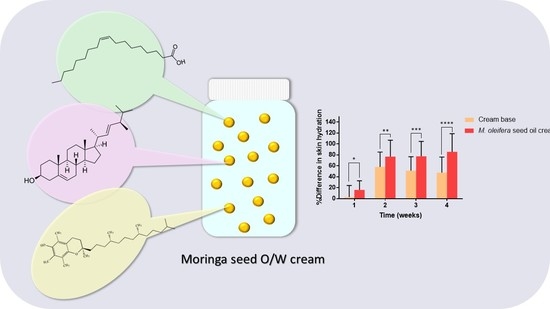
Figure 4. The components and effects of Moringa seed cream [4]
Regarding cosmetic properties, phytosterols are recognized for their similarity to the constituents of the stratum corneum’s intercellular cement. Consequently, they exhibit a remarkable ability to permeate and establish an occlusive layer on the skin’s surface (shown in Figure 5). Argan oil, categorized within this group of plant oils, primarily comprises spinasterol and schottenol. Argan oil finds extensive application in various cosmetic products related to both skincare and haircare. It is believed to play a pivotal role in averting skin dehydration and offering protection against ultraviolet-B radiation, chickenpox scars, and pimples. Remarkably, postmenopausal women who applied argan oil for a duration of 60 days reported a noticeable improvement in skin elasticity. The oil forms a protective barrier on the skin’s surface, thereby reducing transepidermal water loss (TEWL) and impeding water from escaping the stratum corneum and evaporating into the surrounding environment. Furthermore, owing to its inhibitory effect on melanin production, argan oil is equipped to address hyperpigmentation concerns. It additionally nourishes the skin by replenishing essential lipids, aiding in the reconstruction of the intercellular cement and sustaining the hydro-lipid film. Following the hydrolysis of polyunsaturated fatty acids into simpler lipid forms, they penetrate deeper into the epidermal layers. A clinical trial involving ten women who applied argan oil to their skin for 28 days demonstrated a reduction in skin melanin concentration. This discovery suggests the potential use of argan oil in dermo-cosmetic products designed to mitigate dark pigmentation. Notably, none of the subjects experienced skin irritation, and the researchers also observed a slight reduction in skin swelling, suggesting the oil’s applicability in products with anti-inflammatory properties. Moreover, argan oil’s capacity to regulate sebum production renders it suitable for both mixed and oily skin types [23].
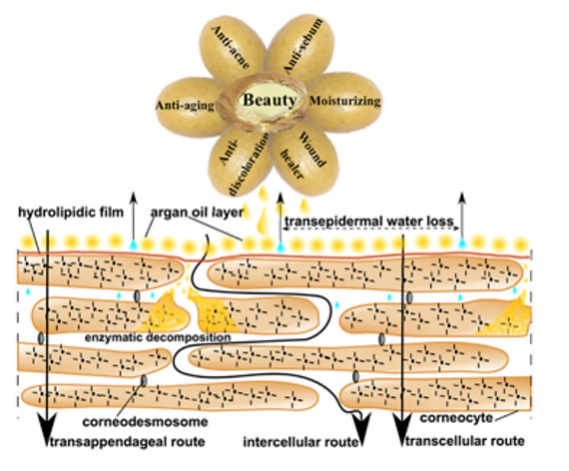
Figure 5. Cosmetic activity of argan oil on skin [23]
7. Application of Phytosterols in the Food Industry
Significant progress has been achieved in the utilization of phytosterols within the food industry. Researchers have ingeniously employed traditional fats and lipids to engineer nanostructured lipid carriers (NLCs) containing free phytosterols. These lipid carriers play a pivotal role in transporting bioactive substances within food products, enabling the creation of functional, nutritious, and health-promoting foods for consumers. While the potential health benefits of bioactive compounds are well-recognized, their incorporation into various food items often faces challenges. Direct extraction from natural sources and integration into food products can lead to a loss of functional properties during the manufacturing process or storage, resulting in reduced bioavailability and chemical stability. NLCs have emerged as a solution to safeguard bioactive compounds against chemical degradation, thereby preserving their functional attributes. In a recent investigation, scientists explored NLCs incorporated with grape seed oil, showcasing remarkable stability and several other advantages. The substantial contact surface area of lipid nanoparticles was noted to significantly enhance the absorption of bioactive substances within the gastrointestinal tract. Conventional oils, such as canola and crambe oil, supplemented with oleic sunflower oil, were employed to optimize the characteristics of the NLCs. The sunflower oil used boasted an unsaturated fatty acid content of approximately 90%, with 11% being linoleic acid and 79% oleic acid. Two variants of NLCs were crafted using 30% and 50% free phytosterols. The results unequivocally favored the NLCs containing 30% free phytosterols, showcasing superior stability and homogenous particle sizes. Conversely, counterparts comprising 50% free phytosterols displayed greater polydispersity. The study also revealed that the properties of natural raw materials, such as plant oils and fats used in NLC formulation, can exhibit variances contingent upon their source. This suggests the need for further investigations to explore combinations of diverse vegetable oils and fats, aiming to craft NLCs possessing varying thermal stability and crystalline characteristics [5].
The therapeutic attributes of aloe vera have been extensively documented in numerous studies, primarily owing to its capacity to bolster immune responses. Recent clinical investigations have unveiled its substantial impact on skin health, particularly through aloe sterols derived from aloe vera gel as phytosterols. Research indicates that aloe sterols can infiltrate peripheral tissues post-ingestion, actively modulating lipid and glucose metabolism. Encompassing lophenol and cycloartenol, aloe sterols underwent scrutiny in an experiment involving Japanese women with dry skin. Over an 8-week duration, participants were administered aloe vera gel powder tablets, each containing approximately 40 μg of aloe sterols. Within the skin’s dermal layer reside dermal fibroblasts, responsible for synthesizing crucial connective tissues such as collagen, elastin, and hyaluronic acid (HA). Collagen constitutes the foundational structural framework of the skin, elastin preserves elasticity, and HA regulates moisture retention. The study’s outcomes demonstrated that the presence of aloe sterols effectively elevated collagen levels and heightened the synthesis of both type III and type I collagen. Furthermore, the production of HA saw a significant increase, attributable to gene expression modifications catalyzed by aloe sterols, particularly enhancing HAS3 and HAS2 levels. Notably, daily oral supplementation with aloe vera sterol-enriched powder yielded a remarkable reduction in facial wrinkles among women aged 40 and above. The depth of these wrinkles exhibited a marked decrease, suggesting that daily oral consumption of aloe vera gel powder exerts a targeted effect on wrinkle reduction. Moreover, aloe vera sterols were found to stimulate the production of hyaluronic acid (HA) and collagen by human dermal fibroblasts, further underscoring their potential in promoting skin health [24].
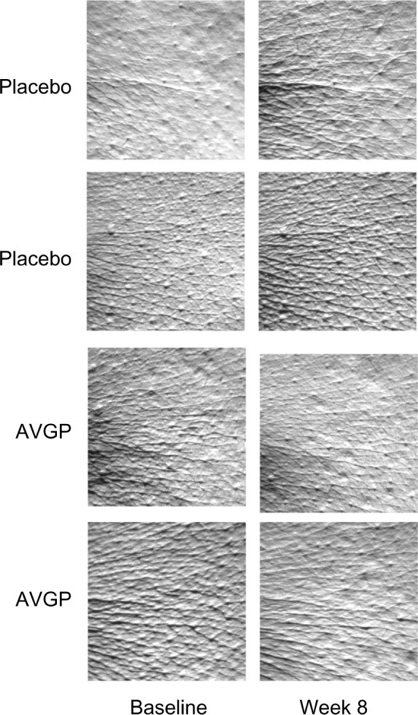
Figure 6. Effects of oral AVGP therapy facial skin hydration [24]
8. Conclusion
In summary, this review article has elucidated the structural properties and biological functions of phytosterols, encompassing contemporary biotechnological procedures for extraction and analysis. Additionally, it delineates diverse applications in the medical, cosmetic, and food industries. Phytosterols present promising prospects, particularly in the medical field. Future scientific endeavors could prioritize exploring the full potential of phytosterols, delving into the mechanisms behind their LDL cholesterol reduction. Through comprehensive studies, researchers have the opportunity to broaden the therapeutic scope of phytosterols, extending their application to the treatment of various metabolic disorders. Furthermore, ongoing research endeavors can contribute to an enhanced understanding of genetic manipulation and metabolic engineering, fostering a deeper comprehension of phytosterols’ functional properties and unlocking possibilities for tailoring them for specific applications.
References
[1]. Luo, X., Su, P., & Zhang, W. (2015). Advances in microalgae-derived phytosterols for functional food and pharmaceutical applications. Marine drugs, 13(7), 4231-4254.
[2]. Pavani, M., Singha, P., Dash, D. R., Asaithambi, N., & Singh, S. K. (2022). Novel encapsulation approaches for phytosterols and their importance in food products: A review. Journal of Food Process Engineering, 45(8), e14041.
[3]. Chen, Z., Shen, N., Wu, X., Jia, J., Wu, Y., Chiba, H., & Hui, S. (2023). Extraction and Quantitation of Phytosterols from Edible Brown Seaweeds: Optimization, Validation, and Application. Foods, 12(2), 244. https://doi.org/10.3390/foods12020244
[4]. Athikomkulchai, S., Tunit, P., Tadtong, S., Jantrawut, P., Sommano, S. R., & Chittasupho, C. (2020). Moringa oleifera seed oil formulation physical stability and chemical constituents for enhancing skin hydration and antioxidant activity. Cosmetics, 8(1), 2.
[5]. da Silva Santos, V., Braz, B. B., Silva, A. Á., Cardoso, L. P., Ribeiro, A. P. B., & Santana, M. H. A. (2019). Nanostructured lipid carriers loaded with free phytosterols for food applications. Food chemistry, 298, 125053.
[6]. Makhmudova, U., Schulze, P. C., Lütjohann, D., & Weingärtner, O. (2021). Phytosterols and cardiovascular disease. Current atherosclerosis reports, 23(11), 68.
[7]. Witkowska, A. M., Waśkiewicz, A., Zujko, M. E., Mirończuk-Chodakowska, I., Cicha-Mikołajczyk, A., & Drygas, W. (2021). Assessment of phytosterols in the diet of adult Polish population with the use of a newly developed database. Nutrients, 13(8), 2722.
[8]. Yang, R., Xue, L., Zhang, L., Wang, X., Qi, X., Jiang, J., ... & Li, P. (2019). Phytosterol contents of edible oils and their contributions to estimated phytosterol intake in the Chinese diet. Foods, 8(8), 334.
[9]. Phillips, K. M., Ruggio, D. M., & Ashraf-Khorassani, M. (2005). Phytosterol Composition of Nuts and Seeds Commonly Consumed in the United States. Journal of Agricultural and Food Chemistry, 53(24), 9436–9445. https://doi.org/10.1021/jf051505h
[10]. Radenkovs, V., Kviesis, J., Juhnevica-Radenkova, K., Valdovska, A., Püssa, T., Klavins, M., & Drudze, I. (2018). Valorization of wild apple (Malus spp.) by-products as a source of essential fatty acids, tocopherols and phytosterols with antimicrobial activity. Plants, 7(4), 90.
[11]. Ganchovska, V., Bosakova-Ardenska, A., Panayotov, P., & Boyanova, P. (2021). Food Science and Applied Biotechnology.
[12]. Kumar, K., Srivastav, S., & Sharanagat, V. S. (2021). Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrasonics sonochemistry, 70, 105325.
[13]. Liu, J. J., Gasmalla, M. A. A., Li, P., & Yang, R. (2016). Enzyme-assisted extraction processing from oilseeds: Principle, processing and application. Innovative Food Science & Emerging Technologies, 35, 184-193.
[14]. Boulila, A., Hassen, I., Haouari, L., Mejri, F., Amor, I. B., Casabianca, H., & Hosni, K. (2015). Enzyme-assisted extraction of bioactive compounds from bay leaves (Laurus nobilis L.). Industrial Crops and Products, 74, 485-493.
[15]. Bigdelifam, D., Hashemi, M., Zohrabi, P., Sadeghpour, M., & Radaee, E. (2017). Sensitive magnetic dispersive solid-phase extraction using hydrophobic magnetic nanoparticles and GC-MS analysis for the determination of sterol composition in milk samples for the detection of palm oil. Analytical Methods, 9(14), 2211-2219.
[16]. Lluveras-Tenorio, A., Mazurek, J., Restivo, A., Colombini, M. P., & Bonaduce, I. (2012). Analysis of plant gums and saccharide materials in paint samples: comparison of GC-MS analytical procedures and databases. Chemistry Central Journal, 6, 1-16.
[17]. Marcos, J., & Pozo, O. J. (2015). Derivatization of steroids in biological samples for GC–MS and LC–MS analyses. Bioanalysis, 7(19), 2515-2536.
[18]. Dutta, A. (2017). Fourier transform infrared spectroscopy. Spectroscopic methods for nanomaterials characterization, 73-93.
[19]. Berthomieu, C., & Hienerwadel, R. (2009). Fourier transform infrared (FTIR) spectroscopy. Photosynthesis research, 101, 157-170.
[20]. Poli, A., Marangoni, F., Corsini, A., Manzato, E., Marrocco, W., Martini, D., ... & Visioli, F. (2021). Phytosterols, cholesterol control, and cardiovascular disease. Nutrients, 13(8), 2810.
[21]. Ramprasath, V. R., & Awad, A. B. (2015). Role of phytosterols in cancer prevention and treatment. Journal of AOAC International, 98(3), 735-738.
[22]. Vezza, T., Canet, F., de Marañón, A. M., Bañuls, C., Rocha, M., & Víctor, V. M. (2020). Phytosterols: nutritional health players in the management of obesity and its related disorders. Antioxidants, 9(12), 1266.
[23]. Goik, U., Goik, T., & Załęska, I. (2019). The properties and application of argan oil in cosmetology. European Journal of Lipid Science and Technology, 121(4), 1800313.
[24]. Tanaka, M., Misawa, E., Yamauchi, K., Abe, F., & Ishizaki, C. (2015). Effects of phytosterols derived from Aloe vera gel on human dermal fibroblasts in vitro and on skin condition in Japanese women. Clinical, cosmetic and investigational dermatology, 95-104.
Cite this article
Xinyue,L. (2024). Advances in the source, preparation, and application of phytosterols. Theoretical and Natural Science,32,221-230.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Luo, X., Su, P., & Zhang, W. (2015). Advances in microalgae-derived phytosterols for functional food and pharmaceutical applications. Marine drugs, 13(7), 4231-4254.
[2]. Pavani, M., Singha, P., Dash, D. R., Asaithambi, N., & Singh, S. K. (2022). Novel encapsulation approaches for phytosterols and their importance in food products: A review. Journal of Food Process Engineering, 45(8), e14041.
[3]. Chen, Z., Shen, N., Wu, X., Jia, J., Wu, Y., Chiba, H., & Hui, S. (2023). Extraction and Quantitation of Phytosterols from Edible Brown Seaweeds: Optimization, Validation, and Application. Foods, 12(2), 244. https://doi.org/10.3390/foods12020244
[4]. Athikomkulchai, S., Tunit, P., Tadtong, S., Jantrawut, P., Sommano, S. R., & Chittasupho, C. (2020). Moringa oleifera seed oil formulation physical stability and chemical constituents for enhancing skin hydration and antioxidant activity. Cosmetics, 8(1), 2.
[5]. da Silva Santos, V., Braz, B. B., Silva, A. Á., Cardoso, L. P., Ribeiro, A. P. B., & Santana, M. H. A. (2019). Nanostructured lipid carriers loaded with free phytosterols for food applications. Food chemistry, 298, 125053.
[6]. Makhmudova, U., Schulze, P. C., Lütjohann, D., & Weingärtner, O. (2021). Phytosterols and cardiovascular disease. Current atherosclerosis reports, 23(11), 68.
[7]. Witkowska, A. M., Waśkiewicz, A., Zujko, M. E., Mirończuk-Chodakowska, I., Cicha-Mikołajczyk, A., & Drygas, W. (2021). Assessment of phytosterols in the diet of adult Polish population with the use of a newly developed database. Nutrients, 13(8), 2722.
[8]. Yang, R., Xue, L., Zhang, L., Wang, X., Qi, X., Jiang, J., ... & Li, P. (2019). Phytosterol contents of edible oils and their contributions to estimated phytosterol intake in the Chinese diet. Foods, 8(8), 334.
[9]. Phillips, K. M., Ruggio, D. M., & Ashraf-Khorassani, M. (2005). Phytosterol Composition of Nuts and Seeds Commonly Consumed in the United States. Journal of Agricultural and Food Chemistry, 53(24), 9436–9445. https://doi.org/10.1021/jf051505h
[10]. Radenkovs, V., Kviesis, J., Juhnevica-Radenkova, K., Valdovska, A., Püssa, T., Klavins, M., & Drudze, I. (2018). Valorization of wild apple (Malus spp.) by-products as a source of essential fatty acids, tocopherols and phytosterols with antimicrobial activity. Plants, 7(4), 90.
[11]. Ganchovska, V., Bosakova-Ardenska, A., Panayotov, P., & Boyanova, P. (2021). Food Science and Applied Biotechnology.
[12]. Kumar, K., Srivastav, S., & Sharanagat, V. S. (2021). Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrasonics sonochemistry, 70, 105325.
[13]. Liu, J. J., Gasmalla, M. A. A., Li, P., & Yang, R. (2016). Enzyme-assisted extraction processing from oilseeds: Principle, processing and application. Innovative Food Science & Emerging Technologies, 35, 184-193.
[14]. Boulila, A., Hassen, I., Haouari, L., Mejri, F., Amor, I. B., Casabianca, H., & Hosni, K. (2015). Enzyme-assisted extraction of bioactive compounds from bay leaves (Laurus nobilis L.). Industrial Crops and Products, 74, 485-493.
[15]. Bigdelifam, D., Hashemi, M., Zohrabi, P., Sadeghpour, M., & Radaee, E. (2017). Sensitive magnetic dispersive solid-phase extraction using hydrophobic magnetic nanoparticles and GC-MS analysis for the determination of sterol composition in milk samples for the detection of palm oil. Analytical Methods, 9(14), 2211-2219.
[16]. Lluveras-Tenorio, A., Mazurek, J., Restivo, A., Colombini, M. P., & Bonaduce, I. (2012). Analysis of plant gums and saccharide materials in paint samples: comparison of GC-MS analytical procedures and databases. Chemistry Central Journal, 6, 1-16.
[17]. Marcos, J., & Pozo, O. J. (2015). Derivatization of steroids in biological samples for GC–MS and LC–MS analyses. Bioanalysis, 7(19), 2515-2536.
[18]. Dutta, A. (2017). Fourier transform infrared spectroscopy. Spectroscopic methods for nanomaterials characterization, 73-93.
[19]. Berthomieu, C., & Hienerwadel, R. (2009). Fourier transform infrared (FTIR) spectroscopy. Photosynthesis research, 101, 157-170.
[20]. Poli, A., Marangoni, F., Corsini, A., Manzato, E., Marrocco, W., Martini, D., ... & Visioli, F. (2021). Phytosterols, cholesterol control, and cardiovascular disease. Nutrients, 13(8), 2810.
[21]. Ramprasath, V. R., & Awad, A. B. (2015). Role of phytosterols in cancer prevention and treatment. Journal of AOAC International, 98(3), 735-738.
[22]. Vezza, T., Canet, F., de Marañón, A. M., Bañuls, C., Rocha, M., & Víctor, V. M. (2020). Phytosterols: nutritional health players in the management of obesity and its related disorders. Antioxidants, 9(12), 1266.
[23]. Goik, U., Goik, T., & Załęska, I. (2019). The properties and application of argan oil in cosmetology. European Journal of Lipid Science and Technology, 121(4), 1800313.
[24]. Tanaka, M., Misawa, E., Yamauchi, K., Abe, F., & Ishizaki, C. (2015). Effects of phytosterols derived from Aloe vera gel on human dermal fibroblasts in vitro and on skin condition in Japanese women. Clinical, cosmetic and investigational dermatology, 95-104.









