1. Introduction
Natural products have long been recognized as a significant source of inspiration for drug discovery, and because of their immense structural diversity and wide range of biological activity, they play a vital role in drug development [1]. Among them, the bioactivity of plant-derived compounds has many functions to treat and overcome a wide range of diseases and fatalities, including diabetes and cancer [2,3].
People with diabetes mellitus (DM) experience many different types of effects, ranging from the immediate danger of a severe hyperglycemic hyperosmolar coma, ketoacidosis, or metabolic disturbances caused by severe hypoglycemia to serious long-term complications in the large and small arterial vessels, as well as lifelong challenges to quality of life due to a variety of psychosocial issues [4]. Type 1 diabetes (T1DM), type 2 diabetes (T2DM), and gestational diabetes (GDM) are the three subtypes of DM [5]. Absolute insulin shortage and pancreatic cell death are hallmarks of T1DM, whereas insulin resistance (IR) and inadequate insulin production are the main causes of T2DM [6,7]. Blood glucose levels in GDM can be raised during pregnancy (though this is most likely to happen after week 24), and this usually subsides after delivery [5]. People with diabetes are mainly T2DM, who represent approximately around 90% of the total population [8]. Previous research has established that common disease of middle age and the elderly, T2DM diagnosis rates are getting older every year, with a definite tendency towards younger age groups [9].
Currently, insulin, insulin analogs, non-insulin oral hypoglycemic medicines, and other newly developed therapy methods are the most often used medications in the clinical treatment of diabetes [5].
For overall glycemic control, insulin therapy is the most effective treatment, reducing HbA1c concentrations by 1.5-2% [10]. Insulin analogs are synthetically manufactured by modifying the amino acid sequence in human insulin in order to mimic as closely as possible the normal endogenous insulin secretion and action [11]. The class of medications known as oral hypoglycemics includes insulin secretagogues, biguanides, insulin sensitizers, etc [12]. Other emerging treatment strategies include nano-based diabetes therapy, insulin pump, pancreatic islet cell transplantation, and more [5]. Because of the shortcomings of the medications themselves as well as the restrictions placed on the methods of administration, such as the negative side effects of prolonged subcutaneous injections and the various challenges posed by oral administration, there is no effective treatment for diabetes mellitus. Therefore, it is crucial to create effective new medications and investigate full treatment approaches based on the characteristics of medications and diabetes mellitus [13].
Although many articles have been published on the antidiabetic potential of natural products, there are very few examples of molecules based on natural products that have been approved for use as antidiabetic drugs. Metformin, discovered based on a natural compound, galegine from the plant Galega officinalis, is one of the few examples of an antidiabetic drug developed from a natural compound and clinically approved. It is a first-line treatment option for patients with T2DM unless there are specific contraindications, such as for patients with impaired renal function [14]. And it has been demonstrated that metformin has a significant effect on the cell survival of HepG2 cells and high glucose-induced IR-HepG2 cells [15]. It can be used as a positive control group to evaluate the anti-glycemic activity of substance.
Nigella glandulifera Freyn et Sint (N. glandulifera) is frequently added to naan as a spice. Alopecia and hair-blacking, urethral calculi, hypogalactia, heat stranguria, amnesia, amenorrhea, edema, and bronchial asthma were all can be treated with the water decoction of N.glandulifera in traditional Uighur medicine [16]. The binding affinity of two compounds, α-hederin and negillicine, isolated from Nigella glandulifer, to the RNA-dependent RNA polymerase (RdRp) enzyme active site of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) could be considered for use in a sustained drug development strategy against SARS-CoV-2 [17].
A additional constituent of it, 5,7-dihydroxy-6-(3-methybut-2-enyl)-isobenzofuran-(13H)-one, was isolated in 2006 from Fungus Stereum sp. by Li’s group [18]. Additionally, the IR-HepG2 cell model was used to assess its hypoglycemic activity. To develop an IR model using human hepatoblastoma HepG2 cells, metformin was chosen as a positive control because it could encourage hepatic glucose consumption in IR HepG2 cells. The results showed that it had higher glucose consumption at different concentrations than the positive control of metformin. These findings illustrate the antidiabetic activity of it [19]. However, although the compound has been isolated over 16 years ago, and in 2022, its function of promoting glucose consumption by IR-HepG2 cells for the treatment of T2DM has been demonstrated, little attention has been paid to the total synthesis of it. Herein, we attempted to disclose a new method for the total synthesis of 5,7-dihydroxy-6-(3-methybut-2-enyl)-isobenzofuran-(13H)-one, shown in Figure 1.
2. Method
We first synthesized 5-bromo-3-methylene-1,3-dihydroisobenzofuran-4,6-diol (4) and (3-methylbut-2-en-1-yl) boronic acid (6) and planned to couple the two known compounds by Suzuki-Miyaura Cross Coupling reaction [20].
Product 4 was prepared in three steps from resorcinol (1). Initially 1 was subjected to an electrophilic bromination reaction with NBS to give 2-bromobenzene-1,3-diol (2). Then, under high pressure ambient circumstances, a Kolbe-Schmitt type reaction was achieved by combining an organic base with resorcinols [21]. The equivalent salicylic acid derivatives, 3-bromo-2,4-dihydroxybenzoic acid (3), were produced by nucleophilic addition to carbon dioxide after hydroxyphenol derivatives 2 were treated with DBU in a carbon dioxide environment. Subsequently the cyclic palladium intermediate obtained by carboxyl-directed carbon-hydrogen bond activation could be oxidized by the solvent CH2Br2 to give the o-alkylated product, followed by the SN2 reaction to give the cyclic lactone 4. [22]. Product 6 was then obtained from compound 3-methylbut-2-en-1-ol (5) by a boronization reaction [23]. Finally, the total synthesis of 5,7-dihydroxy-6-(3-methybut-2-enyl)-isobenzofuran-(13H)-one (7) was achieved by taking advantage of the high functional group selectivity of Suzuki-Miyaura Cross Coupling reaction.
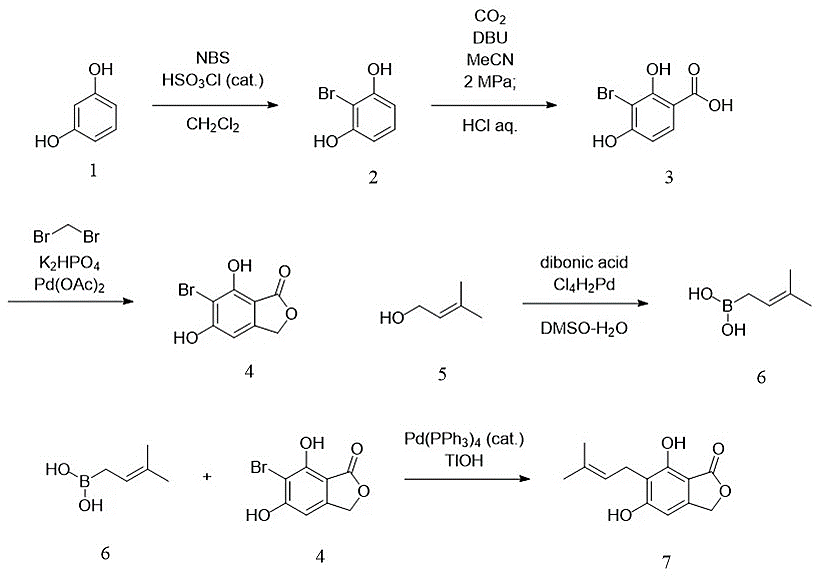
Figure 1. Proposed total synthesis of 5,7-dihydroxy-6-(3-methybut-2-enyl)-isobenzofuran-(13H)-one
3. Conclusion
The method successfully utilized existing structurally simple compounds and constructed the proposed total synthetic route by using Suzuki-miyaura cross-coupling reaction. To successfully present a new complete synthesis technique for 5,7-dihydroxy-6-(3-methybut-2-enyl)-isobenzofuran-(13H)-one. The synthesis method has a few steps and it is easy to follow. The conditions for all reaction types are relatively easy to control and the cost is also comparatively low, which makes it potential for industrialization in the future. It is expected to be applied to the synthesis of drugs based on this compound in the future, as well as the development of type 2 diabetes drugs, providing new ideas for type 2 diabetes drug development.
In future work, the proposed synthesis scheme needs to be studied experimentally to confirm its feasibility. After the product has been synthesized, it needs to be spectroscopically analyzed and compared with the natural sample to verify the identity of the synthesized product with the natural sample. After the complete synthesis of 5,7-dihydroxy-6-(3-methybut-2-enyl)-isobenzofuran-(13H)-one has been accomplished, additional research can be done to enhance the synthetic pathways and the reaction conditions in order to provide larger yields and more effective procedures.
In conclusion, the total synthesis method is highly feasible.
References
[1]. Li, Gang, and Hong‐Xiang Lou. “Strategies to Diversify Natural Products for Drug Discovery.” Medicinal research reviews 38, no. 4 (2018): 1255–1294.
[2]. Asma, Syeda Tasmia, Ulas Acaroz, Kálmán Imre, Adriana Morar, Syed Rizwan Ali Shah, Syed Zajif Hussain, Damla Arslan-Acaroz, et al. “Natural Products/Bioactive Compounds as a Source of Anticancer Drugs.” Cancers 14, no. 24 (2022): 6203–.
[3]. Blahova, Jana, Monika Martiniakova, Martina Babikova, Veronika Kovacova, Vladimira Mondockova, and Radoslav Omelka. “Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus.” Pharmaceuticals 14, no. 8 (2021): 806.
[4]. Thomas, Celeste C., and Louis H. Philipson. “Update on Diabetes Classification.” The Medical clinics of North America 99, no. 1 (2015): 1–16.
[5]. Mishra, Vijay, Pallavi Nayak, Mayank Sharma, Aqel Albutti, Ameen S. S. Alwashmi, Mohammad Abdullah Aljasir, Noorah Alsowayeh, and Murtaza M. Tambuwala. “Emerging Treatment Strategies for Diabetes Mellitus and Associated Complications: An Update.” Pharmaceutics 13, no. 10 (2021): 1568–.
[6]. Chillarón, Juan J, Juana A Flores Le-Roux, David Benaiges, and Juan Pedro-Botet. “Type 1 Diabetes, Metabolic Syndrome and Cardiovascular Risk.” Metabolism, clinical and experimental 63, no. 2 (2014): 181–187.
[7]. DeFronzo, Ralph A. “Pathogenesis of Type 2 Diabetes Mellitus.” Medical Clinics of North America 88, no. 4 (2004): 787–835.
[8]. Standl, Eberhard, Kamlesh Khunti, Tina Birgitte Hansen, and Oliver Schnell. “The Global Epidemics of Diabetes in the 21st Century: Current Situation and Perspectives.” European journal of preventive cardiology 26, no. 2_suppl (2019): 7–14.
[9]. Yeung, Roseanne O, Yuying Zhang, Andrea Luk, Wenying Yang, Leorino Sobrepena, Kun-Ho Yoon, S R Aravind, et al. “Metabolic Profiles and Treatment Gaps in Young-Onset Type 2 Diabetes in Asia (The Jade Programme): A Cross-Sectional Study of a Prospective Cohort.” The Lancet Diabetes & Endocrinology 2, no. 12 (2014): 935–943.
[10]. Chatterjee, Sudesna, Kamlesh Khunti, and Melanie J Davies. “Type 2 Diabetes.” The Lancet (British edition) 389, no. 10085 (2017): 2239–2251.
[11]. Griffin, Stacy. “Insulin Treatment of Type 2 Diabetes: Considerations When Converting from Human Insulin to Insulin Analogs.” Annals of medicine (Helsinki) 45, no. 2 (2013): 129–140.
[12]. Padhi, Santwana, Amit Kumar Nayak, and Anindita Behera. “Type II Diabetes Mellitus: a Review on Recent Drug Based Therapeutics.” Biomedicine & pharmacotherapy 131 (2020): 110708–110708.
[13]. Zhao, Ruichen, Zhiguo Lu, Jun Yang, Liqun Zhang, Yan Li, and Xin Zhang. “Drug Delivery System in the Treatment of Diabetes Mellitus.” Frontiers in bioengineering and biotechnology 8 (2020): 880–880.
[14]. Jugran, Arun K., Sandeep Rawat, Hari P. Devkota, Indra D. Bhatt, and Ranbeer S. Rawal. “Diabetes and Plant‐derived Natural Products: From Ethnopharmacological Approaches to Their Potential for Modern Drug Discovery and Development.” Phytotherapy research 35, no. 1 (2021): 223–245.
[15]. Lv, Yan, Na Tian, Junsong Wang, Minghua Yang, and Lingyi Kong. “Metabolic Switching in the Hypoglycemic and Antitumor Effects of Metformin on High Glucose Induced HepG2 Cells.” Journal of pharmaceutical and biomedical analysis 156 (2018): 153–162.
[16]. Zheng, Yunliang, Qiao Zhang, and Xingjiang Hu. “A Comprehensive Review of Ethnopharmacological Uses, Phytochemistry, Biological Activities, and Future Prospects of Nigella Glandulifera.” Medicinal Chemistry Research 29, no. 7 (2020): 1168–1186.
[17]. Mir, Shabir Ahmad, Ahmad Firoz, Mohammed Alaidarous, Bader Alshehri, Abdul Aziz Bin Dukhyil, Saeed Banawas, Suliman A Alsagaby, et al. “Identification of SARS-CoV-2 RNA-Dependent RNA Polymerase Inhibitors from the Major Phytochemicals of Nigella Sativa: An in Silico Approach.” Saudi journal of biological sciences 29, no. 1 (2022): 394–401.
[18]. Li, Guo-Hong, Lei Li, Meng Duan, and Ke-Qin Zhang. “The Chemical Constituents of the Fungus Stereum Sp.” Chemistry & biodiversity 3, no. 2 (2006): 210–216.
[19]. Li, Qingqing, Jing Xu, Yiyu Chen, Wenli Xie, Gui Mei, Xueni Li, Yu Chen, and Guangzhong Yang. “Chemical Constituents from the Seeds of Nigella Glandulifera and Their Hypoglycemic Activities.” RSC advances 12, no. 30 (2022): 19445–19451.
[20]. Miyaura, Norio, and Akira Suzuki. “Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds.” Chemical reviews 95, no. 7 (1995): 2457–2483.
[21]. Sadamitsu, Yuta, Akira Okumura, Kodai Saito, and Tohru Yamada. “Kolbe-Schmitt Type Reaction Under Ambient Conditions Mediated by an Organic Base.” Chemical communications (Cambridge, England) 55, no. 66 (2019): 9837–984.
[22]. Zhang, Yang-Hui, Bing-Feng Shi, and Jin-Quan Yu. “Palladium(II)-Catalyzed Ortho Alkylation of Benzoic Acids with Alkyl Halides.” Angewandte Chemie (International ed.) 48, no. 33 (2009): 6097–6100.
[23]. Alam, Rauful, Tobias Vollgraff, Lars Eriksson, and Kálmán J Szabó. “Synthesis of Adjacent Quaternary Stereocenters by Catalytic Asymmetric Allylboration.” Journal of the American Chemical Society 137, no. 35 (2015): 11262–11265.
Cite this article
Li,K. (2024). Proposed total synthesis of the potential antidiabetic compound 5,7-dihydroxy-6-(3-methybut-2-enyl)-isobenzofuran-(13H)-one. Theoretical and Natural Science,33,24-28.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Li, Gang, and Hong‐Xiang Lou. “Strategies to Diversify Natural Products for Drug Discovery.” Medicinal research reviews 38, no. 4 (2018): 1255–1294.
[2]. Asma, Syeda Tasmia, Ulas Acaroz, Kálmán Imre, Adriana Morar, Syed Rizwan Ali Shah, Syed Zajif Hussain, Damla Arslan-Acaroz, et al. “Natural Products/Bioactive Compounds as a Source of Anticancer Drugs.” Cancers 14, no. 24 (2022): 6203–.
[3]. Blahova, Jana, Monika Martiniakova, Martina Babikova, Veronika Kovacova, Vladimira Mondockova, and Radoslav Omelka. “Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus.” Pharmaceuticals 14, no. 8 (2021): 806.
[4]. Thomas, Celeste C., and Louis H. Philipson. “Update on Diabetes Classification.” The Medical clinics of North America 99, no. 1 (2015): 1–16.
[5]. Mishra, Vijay, Pallavi Nayak, Mayank Sharma, Aqel Albutti, Ameen S. S. Alwashmi, Mohammad Abdullah Aljasir, Noorah Alsowayeh, and Murtaza M. Tambuwala. “Emerging Treatment Strategies for Diabetes Mellitus and Associated Complications: An Update.” Pharmaceutics 13, no. 10 (2021): 1568–.
[6]. Chillarón, Juan J, Juana A Flores Le-Roux, David Benaiges, and Juan Pedro-Botet. “Type 1 Diabetes, Metabolic Syndrome and Cardiovascular Risk.” Metabolism, clinical and experimental 63, no. 2 (2014): 181–187.
[7]. DeFronzo, Ralph A. “Pathogenesis of Type 2 Diabetes Mellitus.” Medical Clinics of North America 88, no. 4 (2004): 787–835.
[8]. Standl, Eberhard, Kamlesh Khunti, Tina Birgitte Hansen, and Oliver Schnell. “The Global Epidemics of Diabetes in the 21st Century: Current Situation and Perspectives.” European journal of preventive cardiology 26, no. 2_suppl (2019): 7–14.
[9]. Yeung, Roseanne O, Yuying Zhang, Andrea Luk, Wenying Yang, Leorino Sobrepena, Kun-Ho Yoon, S R Aravind, et al. “Metabolic Profiles and Treatment Gaps in Young-Onset Type 2 Diabetes in Asia (The Jade Programme): A Cross-Sectional Study of a Prospective Cohort.” The Lancet Diabetes & Endocrinology 2, no. 12 (2014): 935–943.
[10]. Chatterjee, Sudesna, Kamlesh Khunti, and Melanie J Davies. “Type 2 Diabetes.” The Lancet (British edition) 389, no. 10085 (2017): 2239–2251.
[11]. Griffin, Stacy. “Insulin Treatment of Type 2 Diabetes: Considerations When Converting from Human Insulin to Insulin Analogs.” Annals of medicine (Helsinki) 45, no. 2 (2013): 129–140.
[12]. Padhi, Santwana, Amit Kumar Nayak, and Anindita Behera. “Type II Diabetes Mellitus: a Review on Recent Drug Based Therapeutics.” Biomedicine & pharmacotherapy 131 (2020): 110708–110708.
[13]. Zhao, Ruichen, Zhiguo Lu, Jun Yang, Liqun Zhang, Yan Li, and Xin Zhang. “Drug Delivery System in the Treatment of Diabetes Mellitus.” Frontiers in bioengineering and biotechnology 8 (2020): 880–880.
[14]. Jugran, Arun K., Sandeep Rawat, Hari P. Devkota, Indra D. Bhatt, and Ranbeer S. Rawal. “Diabetes and Plant‐derived Natural Products: From Ethnopharmacological Approaches to Their Potential for Modern Drug Discovery and Development.” Phytotherapy research 35, no. 1 (2021): 223–245.
[15]. Lv, Yan, Na Tian, Junsong Wang, Minghua Yang, and Lingyi Kong. “Metabolic Switching in the Hypoglycemic and Antitumor Effects of Metformin on High Glucose Induced HepG2 Cells.” Journal of pharmaceutical and biomedical analysis 156 (2018): 153–162.
[16]. Zheng, Yunliang, Qiao Zhang, and Xingjiang Hu. “A Comprehensive Review of Ethnopharmacological Uses, Phytochemistry, Biological Activities, and Future Prospects of Nigella Glandulifera.” Medicinal Chemistry Research 29, no. 7 (2020): 1168–1186.
[17]. Mir, Shabir Ahmad, Ahmad Firoz, Mohammed Alaidarous, Bader Alshehri, Abdul Aziz Bin Dukhyil, Saeed Banawas, Suliman A Alsagaby, et al. “Identification of SARS-CoV-2 RNA-Dependent RNA Polymerase Inhibitors from the Major Phytochemicals of Nigella Sativa: An in Silico Approach.” Saudi journal of biological sciences 29, no. 1 (2022): 394–401.
[18]. Li, Guo-Hong, Lei Li, Meng Duan, and Ke-Qin Zhang. “The Chemical Constituents of the Fungus Stereum Sp.” Chemistry & biodiversity 3, no. 2 (2006): 210–216.
[19]. Li, Qingqing, Jing Xu, Yiyu Chen, Wenli Xie, Gui Mei, Xueni Li, Yu Chen, and Guangzhong Yang. “Chemical Constituents from the Seeds of Nigella Glandulifera and Their Hypoglycemic Activities.” RSC advances 12, no. 30 (2022): 19445–19451.
[20]. Miyaura, Norio, and Akira Suzuki. “Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds.” Chemical reviews 95, no. 7 (1995): 2457–2483.
[21]. Sadamitsu, Yuta, Akira Okumura, Kodai Saito, and Tohru Yamada. “Kolbe-Schmitt Type Reaction Under Ambient Conditions Mediated by an Organic Base.” Chemical communications (Cambridge, England) 55, no. 66 (2019): 9837–984.
[22]. Zhang, Yang-Hui, Bing-Feng Shi, and Jin-Quan Yu. “Palladium(II)-Catalyzed Ortho Alkylation of Benzoic Acids with Alkyl Halides.” Angewandte Chemie (International ed.) 48, no. 33 (2009): 6097–6100.
[23]. Alam, Rauful, Tobias Vollgraff, Lars Eriksson, and Kálmán J Szabó. “Synthesis of Adjacent Quaternary Stereocenters by Catalytic Asymmetric Allylboration.” Journal of the American Chemical Society 137, no. 35 (2015): 11262–11265.









