1. Introduction
With global economic development, the prevalence of obesity has increased due to the spread of western-style diets and a lack of physical activity [1]. According to data from the U.S. National Health and Nutrition Examination Survey (NHNES), between 1988 and 2010, the average body mass index (BMI) of U.S. men and women increased by 0.37% per year, respectively. Metabolic syndrome, also known as syndrome X, is closely related to abdominal obesity and consists of several common metabolic abnormalities, including insulin resistance, dyslipidemia, decreased high-density lipoprotein (HDL)-cholesterol levels, hyperuricemia, and hypertension. Also, metabolic syndrome is known to be highly atherogenic and impacts patients’ health. Its incidence has increased over the past decade. Specifically, from 2011 to 2012, the estimated prevalence rate in the United States was 34.7%. Recently, diacylglycerol (DAG) has been widely researched as it is effective for treating absorptive and post-absorptive hyperlipidemia, thus preventing excess adiposity [2]. DAG is naturally found in vegetable oils with a percentage around 1–10 and is therefore recognised as a secure anti-obesity functional cooking oil. The US FDA also marked it as GRAS (generally recognised as safe). Enriched DAG oil can be obtained through structural improvements in traditional dietary triacylglycerol (TAG)-rich oils like sunflower oil, rice brain oil, etc. Synthesised DAG oil ought to contain 80% or greater DAG content. At present, many studies have shown that DAG oil consumption can improve metabolic diseases other than hyperlipidemia, such as obesity, diabetes, etc., and can be used for the prevention and control of the metabolic syndrome by lowering postprandial blood lipids and body mass, improving insulin sensitivity, and preventing atherosclerosis. The review will be to focus on the metabolic advantages of DAG oils over TAG oils by providing a theoretical basis. By exploring this topic, we could broaden the clinical possibilities for DAG oil and provide another pathway for treating metabolic patients.
2. Metabolic Pathways of Dag
Sn-1,2-DAG, sn-1,3-DAG and sn-2,3-DAG are the three existing isomers of DAG. Relying on the lipase cleavage of the esterified fatty acid (FA) at the sn-1 or sn-3 position of the TAG molecule, it is possible to generate a chiral center at the sn-2 position and produce one of the two DAG enantiomers, i.e., a DAG of sn-2,3 or sn-1,2, distinctly [3]. It is well defined that DAG can be produced by the enzyme 1,3-specific lipase through reverse reaction and it contains a larger proportion of 1,3-DAG since it is hemodynamically more stable than the 1,2-species or 2,3-species. The equilibrium ratio of the DAG oil is 7:3 for 1,3-DAG and 1,2-(2,3-) DAG [3].
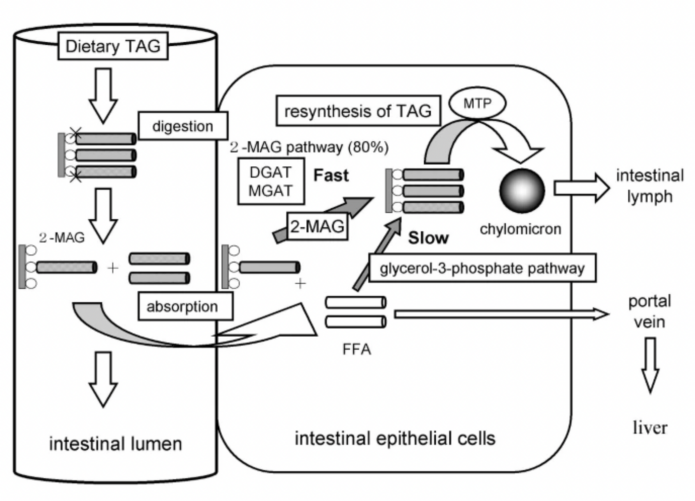
Figure 1. Digestion and absorption of dietary triacylglycerol (TAG) [4].
Figure 1 shows that TAG is typically hydrolyzed to intermediates during metabolism in the form of 1,2 or 2,3-DAG and is then hydrolyzed in the intestinal lumen to 2-monoacylglycerol (MAG), which is then re-esterified and passes into the intestinal lymph as chylomicrons (CM) by microsomal triglyceride transfer protein (MTP). Whereas edible DAG oils contain the majority of 1,3-DAG which has a different pathway for absorption. Dietary DAG is first hydrolyzed by lipase to 1-monoacylglycerol (1-MAG) and then further hydrolyzed to glycerol and FFA, and absorbed by intestinal epithelial cells. As it is shown in Figure 2, 1,3-DAG is first hydrolyzed by lipase in the intestine to 1-monoacylglycerol (1-MAG), and then further hydrolyzed to glycerol and FFA, which is absorbed by intestinal epithelial cells. 1-MAG is not a substrate of DGAT and MGAT but 1-MAG can be re-synthesized into TAG through glycerol-3-phosphate pathway (which is lower than the activity of the 2-MAG pathway), or it may be combined with FFA for energy storage and enter the human body through the blood circulation. The unique metabolic profile of 1,3-DAG is thought to reduce body fat accumulation, reduce body mass, and inhibit postprandial lipid elevation [5].
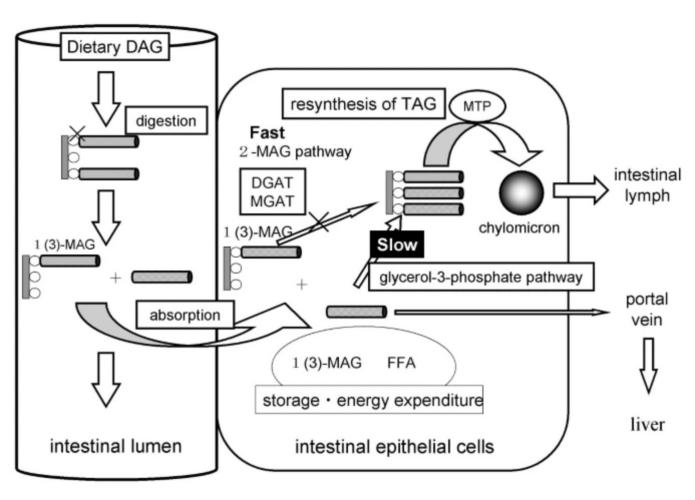
Figure 2. Digestion and absorption of dietary diacylglycerol (DAG) [6].
3. Effect of Dag Oil on Reduction of Postprandial Blood Lipids
Hyperlipidemia has been verified to link with a variety of health problems, including diabetes mellitus, obesity, and hypertension, which are all serious components of the metabolic syndrome [7]. Several clinical trials and studies have shown that a replacement of TAG oil with DAG oil is effective for postprandial hyperlipidemia. Taguchi, H., et al. recruited 40 normolipidemic males and studied the effect of dietary DAG on postprandial lipemia in healthy individuals by providing fat emulsions at various doses: 10g, 20g, and 44g. Investigators found out that post-absorptive lipemia induced by the DAG (6.54 ± 5.12 mmol·h/L, mean ± SD) was significantly (p < 0.05) less by 23% than that induced by the TAG (8.45 ± 7.54 mmol·h/L, mean ± SD) in the late postprandial phase (>4 h) after ingestion [8]. Tomonobu, K. et al. did a double-blind, randomized, crossover test on dietary DAG oil ingestion compared with TAG oil among 43 healthy Japanese men and 7 postmenopausal women. Results suggest that replacing 10 grams of dietary TAG with DAG daily can help prevent postprandial hyperlipidemia and related diseases by lowering postprandial TAG, remnant-like particle cholesterol (RLP-C), and chylomicron TAG [9].
Additionally, research on animals has demonstrated that dietary 1,3-DAG-enriched oil promotes the reversal of atherosclerosis. In diabetic ApoE-deficient mice, DAG has been shown to be related with a decrease in plasma cholesterol, notably in the bigger triglyceride-rich lipoproteins [10]. Long-term intake of DAG can also reduce postprandial lipemia by stimulating hepatic lipid catabolism and thereby modulating aortic lipid accumulation in New Zealand white rabbits [11].
4. Effect of Dag Oil on Obesity Prevention
The reason why obesity is one of the key factors in metabolic syndrome is because it can lead to chronic inflammation. Abdominal or central obesity, the most common manifestation of metabolic syndrome, is a sign of dysfunction in adipose tissue [12]. Clinical trials and studies indicated that DAG oil has certain fat-lowering and weight-loss effects, so long-term use can slow down the progression of metabolic abnormalities. Maki, K. C. et al. performed a 24-week randomised, double-blind, parallel intervention trial with intention-to-treat analysis that involved 131 overweight or obese subjects. During the trial, food products containing DAG or TAG oil for subjects were correlated into a reduced-energy diet. After twenty four weeks, mean body weight and fat mass among the group consuming DAG (p=0.025) were significantly lower than those in the TAG group(p=0.037). By the end of the trial, the average body weight in the DAG and TAG groups had decreased by 3.6% and 2.5%. Fat mass decreased by 8.3% and 5.6% in the DAG and TAG groups, respectively. As a result, it is concluded that foods containing diacylglycerols can promote weight loss and reduce body fat and can be used as an adjunct to dietary therapy in the treatment of obesity [13]. Thereafter, Kawashima, H., et al. conducted a randomisedcontrolled trial with intention-to-treat analysis across a 12-month period to measure the effects of ad libitum consumption of DAG at home on body weight and serum triglycerides in 312 overweight Japanese subjects (aged 22 to 73 years). To ensure the effectiveness of the experiment, there is no significant difference in total energy intake and physical activity between individuals. It was found that the reductions in body weight and BMI were 0.87 kilogram\ (P=0.002) in the DAG group and 0.32 kilogram (P=0.002) in the TAG group [14].
5. Effect of Dag Oil on Lowering Blood Sugar and Ameliorating Insulin Sensitivity
Insulin resistance is the causative link of various metabolic abnormalities in the metabolic syndrome. Consuming a high-energy fat diet is strongly associated with being overweight, which in turn decreases insulin sensitivity, especially if there is excess abdominal fat. Consequently, choice and quantity of dietary fat are crucial for controlling metabolic syndrome [15]. Studies have demonstrated that DAG oil may play a role in monitoring blood glucose and improving insulin sensitivity. Li, D., et al. designed a double-blind, controlled parallel study involving 112 Chinese type 2 diabetes mellitus patients (aged 40–65). It is noticed that body weight, body mass index (BMI), waist circumference, Homeostatic modelling assessment of insulin resistance (HOMA-IR), serum insulin, and leptin levels were all significantly lower in the dietary DAG group (with no adverse effects), whereas not in the dietary TAG group [16]. Based on the analysis, fasting insulin was significantly lower in the DAG group among normal weight subjects (BMI<=25). Therefore, people with type 2 diabetes can benefit from DAG oil since it does improve insulin sensitivity, but only if they are not overweight (BMI>=25). Besides that, a meta-analysis of five randomised controlled studies with 200 participants found that consumption of DAG can reduce fasting blood glucose and insulin concentrations by Tongcheng, X. et al. This may be related to a decrease in dihydroxyacetone phosphate, an intermediate product of lipid metabolism and gluconeogenesis during DAG metabolism. Accordingly, DAG oil may have the ability to manage insulin resistance, which slows the course of disease in patients with type 2 diabetes.
6. Conclusion
DAG oil has the effect of lowering postprandial lipids, reducing body fat and body mass, lowering blood glucose, and improving insulin sensitivity. It can better prevent and control metabolic syndrome. This effect is achieved by regulating relevant risk factors, and it is also a safe and emerging dietary fat. However, at present, the physiological and pathological effects of DAG oil in preventing and controlling metabolic syndrome have not yet been fully elucidated, the preparation process is not yet mature, and the market price is high, which makes it difficult to popularize. More high-quality research is needed to provide conclusive theoretical and technical support for the popularisation and application of DAG. This article did not include the side effects of consuming DAG oil. In order to better serve the public as a replacement for traditional TAG oil, more research could be done on the clinical safety of dietary DAG.
Acknowledgment
First of all, I would like to express my gratitude to the teachers at Zhongshan Hospital, China, especially Dr. Gao. He has provided me with instructive advice during the completion of this review and throughout my internship at the hospital. I am also grateful to all my professors at University of California, Davis. They provided me with a solid foundation of knowledge. Further, I would like to thank my parents and all my friends for the encouragement. They gave me valuable mental support and helped me overcome bottlenecks. Without all their guidance, I could not have achieved this paper.
References
[1]. Saklayen, M. G. The Global Epidemic of the Metabolic Syndrome. Current Hypertension Reports, 20(2), 12. (2018).
[2]. Yanai, H., Tomono, Y., Ito, K., Furutani, N., Yoshida, H., & Tada, N. Diacylglycerol oil for the metabolic syndrome. Nutrition Journal, 6(1), 43. (2007).
[3]. Eichmann, T. O., & Lass, A. DAG tales: The multiple faces of diacylglycerol — stereochemistry, metabolism, and signaling. Cellular and Molecular Life Sciences, 72(20), 3931–3952. (2015).
[4]. Lee, Y.-Y., Tang, T.-K., Phuah, E.-T., Tan, C.-P., Wang, Y., Li, Y., Cheong, L.-Z., & Lai, O.-M. Production, safety, health effects and applications of diacylglycerol functional oil in food systems: A review. Critical Reviews in Food Science and Nutrition, 60(15), 2509–2525. (2020).
[5]. Takase, H. Metabolism of diacylglycerol in humans. Asia Pacific Journal of Clinical Nutrition, 16 Suppl 1, 398–403. (2007).
[6]. Lai, M., Peng, H., Wu, X., Chen, X., Wang, B., & Su, X. IL-38 in modulating hyperlipidemia and its related cardiovascular diseases. International Immunopharmacology, 108, 108876. (2022).
[7]. Taguchi, H., Watanabe, H., Onizawa, K., Nagao, T., Gotoh, N., Yasukawa, T., Tsushima, R., Shimasaki, H., & Itakura, H. Double-Blind Controlled Study on the Effects of Dietary Diacylglycerol on Postprandial Serum and Chylomicron Triacylglycerol Responses in Healthy Humans. Journal of the American College of Nutrition, 19(6), 789–796. (2000).
[8]. Tomonobu, K., Hase, T., & Tokimitsu, I. Dietary diacylglycerol in a typical meal suppresses postprandial increases in serum lipid levels compared with dietary triacylglycerol. Nutrition, 22(2), 128–135. (2006).
[9]. Fujii, A., Allen, T. J., & Nestel, P. J. A 1,3-diacylglycerol-rich oil induces less atherosclerosis and lowers plasma cholesterol in diabetic apoE-deficient mice. Atherosclerosis, 193(1), 55–61. (2007).
[10]. Ota, N., Soga, S., Hase, T., Tokimitsu, I., & Murase, T. Dietary Diacylglycerol Induces the Regression of Atherosclerosis in Rabbits ,2. The Journal of Nutrition, 137(5), 1194–1199. (2007).
[11]. Després, J.-P., & Lemieux, I. Abdominal obesity and metabolic syndrome. Nature, 444(7121), 881–887. (2006).
[12]. Kawada, T. Food-derived regulatory factors against obesity and metabolic syndrome. Bioscience, Biotechnology, and Biochemistry, 82(4), 547–553. (2018).
[13]. Maki, K. C., Davidson, M. H., Tsushima, R., Matsuo, N., Tokimitsu, I., Umporowicz, D. M., Dicklin, M. R., Foster, G. S., Ingram, K. A., Anderson, B. D., Frost, S. D., & Bell, M. Consumption of diacylglycerol oil as part of a reduced-energy diet enhances loss of body weight and fat in comparison with consumption of a triacylglycerol control oil. The American Journal of Clinical Nutrition, 76(6), 1230–1236. (2002).
[14]. Kamphuis, M. M., Mela, D. J., & Westerterp-Plantenga, M. S. Diacylglycerols affect substrate oxidation and appetite in humans. The American Journal of Clinical Nutrition, 77(5), 1133–1139. (2003).
[15]. Zheng, J. S., Wang, L., Lin, M., Yang, H., & Li, D. BMI status influences the response of insulin sensitivity to diacylglycerol oil in Chinese type 2 diabetic patients. Asia Pacific journal of clinical nutrition, 24(1), 65–72. (2015).
[16]. Tongcheng, X., Min, J., Xia, L., Bin, Q., Yuan, Z., Wei, L., Aizhen, Z., Lina, L., & Fangling, D. Intake of Diacylglycerols and the Fasting Insulin and Glucose Concentrations: A Meta-Analysis of 5 Randomized Controlled Studies. Journal of the American College of Nutrition, 37(7), 598–604. (2018).
Cite this article
Lyu,Y. (2024). Diacylglycerol oil on metabolic syndrome: A Review. Theoretical and Natural Science,33,1-5.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Saklayen, M. G. The Global Epidemic of the Metabolic Syndrome. Current Hypertension Reports, 20(2), 12. (2018).
[2]. Yanai, H., Tomono, Y., Ito, K., Furutani, N., Yoshida, H., & Tada, N. Diacylglycerol oil for the metabolic syndrome. Nutrition Journal, 6(1), 43. (2007).
[3]. Eichmann, T. O., & Lass, A. DAG tales: The multiple faces of diacylglycerol — stereochemistry, metabolism, and signaling. Cellular and Molecular Life Sciences, 72(20), 3931–3952. (2015).
[4]. Lee, Y.-Y., Tang, T.-K., Phuah, E.-T., Tan, C.-P., Wang, Y., Li, Y., Cheong, L.-Z., & Lai, O.-M. Production, safety, health effects and applications of diacylglycerol functional oil in food systems: A review. Critical Reviews in Food Science and Nutrition, 60(15), 2509–2525. (2020).
[5]. Takase, H. Metabolism of diacylglycerol in humans. Asia Pacific Journal of Clinical Nutrition, 16 Suppl 1, 398–403. (2007).
[6]. Lai, M., Peng, H., Wu, X., Chen, X., Wang, B., & Su, X. IL-38 in modulating hyperlipidemia and its related cardiovascular diseases. International Immunopharmacology, 108, 108876. (2022).
[7]. Taguchi, H., Watanabe, H., Onizawa, K., Nagao, T., Gotoh, N., Yasukawa, T., Tsushima, R., Shimasaki, H., & Itakura, H. Double-Blind Controlled Study on the Effects of Dietary Diacylglycerol on Postprandial Serum and Chylomicron Triacylglycerol Responses in Healthy Humans. Journal of the American College of Nutrition, 19(6), 789–796. (2000).
[8]. Tomonobu, K., Hase, T., & Tokimitsu, I. Dietary diacylglycerol in a typical meal suppresses postprandial increases in serum lipid levels compared with dietary triacylglycerol. Nutrition, 22(2), 128–135. (2006).
[9]. Fujii, A., Allen, T. J., & Nestel, P. J. A 1,3-diacylglycerol-rich oil induces less atherosclerosis and lowers plasma cholesterol in diabetic apoE-deficient mice. Atherosclerosis, 193(1), 55–61. (2007).
[10]. Ota, N., Soga, S., Hase, T., Tokimitsu, I., & Murase, T. Dietary Diacylglycerol Induces the Regression of Atherosclerosis in Rabbits ,2. The Journal of Nutrition, 137(5), 1194–1199. (2007).
[11]. Després, J.-P., & Lemieux, I. Abdominal obesity and metabolic syndrome. Nature, 444(7121), 881–887. (2006).
[12]. Kawada, T. Food-derived regulatory factors against obesity and metabolic syndrome. Bioscience, Biotechnology, and Biochemistry, 82(4), 547–553. (2018).
[13]. Maki, K. C., Davidson, M. H., Tsushima, R., Matsuo, N., Tokimitsu, I., Umporowicz, D. M., Dicklin, M. R., Foster, G. S., Ingram, K. A., Anderson, B. D., Frost, S. D., & Bell, M. Consumption of diacylglycerol oil as part of a reduced-energy diet enhances loss of body weight and fat in comparison with consumption of a triacylglycerol control oil. The American Journal of Clinical Nutrition, 76(6), 1230–1236. (2002).
[14]. Kamphuis, M. M., Mela, D. J., & Westerterp-Plantenga, M. S. Diacylglycerols affect substrate oxidation and appetite in humans. The American Journal of Clinical Nutrition, 77(5), 1133–1139. (2003).
[15]. Zheng, J. S., Wang, L., Lin, M., Yang, H., & Li, D. BMI status influences the response of insulin sensitivity to diacylglycerol oil in Chinese type 2 diabetic patients. Asia Pacific journal of clinical nutrition, 24(1), 65–72. (2015).
[16]. Tongcheng, X., Min, J., Xia, L., Bin, Q., Yuan, Z., Wei, L., Aizhen, Z., Lina, L., & Fangling, D. Intake of Diacylglycerols and the Fasting Insulin and Glucose Concentrations: A Meta-Analysis of 5 Randomized Controlled Studies. Journal of the American College of Nutrition, 37(7), 598–604. (2018).









