1. Introduction
Based on the statistics by centers for disease control and prevention (CDC) in 2018, about 20 percent of the United States population had STI. In other words, it is approximately that one in five people in US has STI. STIs remains high occurrence rate not only in United States, but also in a global scale. STIs can result in short-term health issues, long-term effects, or even cancers in both women and men. Virus, bacteria, and chlamydia are all the causes of STIs. Among them, chlamydia trachomatis is the most common sexually transmitted disease. Since some patients showed few symptoms, chlamydia trachomatis is extremely widespread. And without immediate treatments, it can infect initially cervix in women or rectum in men, and then other parts of urogenital system and genital system, leading to serious health issues, such as pelvic inflammatory diseases (PID), low sperm quality and so on. The severity guides a lot of researchers to focus on developing better treatments for chlamydia trachomatis infection. The most commonly applied conventional treatment is antibiotics, mainly including doxycycline and azithromycin. Both of them used to be effective to treat chlamydia trachomatis infection. However, the efficiency is gradually decreasing as a result of the resistance. Therefore, new therapies are in strong demand. Among them, gene therapy and peptide therapy are optimal choices.
2. Sextually transmitted infection (STI)
2.1. The clinical characteristics of STIs
There are various symptoms of sexually transmitted infection. Most of symptoms appear in urogenital system and genital system, including unusual vaginal, penile or anal discharge, pain when urinating, nodules or skin growths around the genitals or private parts, rash, abnormal vaginal bleeding, genital or anal itching, ulcers and sores around the genitals or anus, warts around the genitals or anus. However, some of STIs, such as gonorrhea, can also lead to warts in the mouth or throat.
STIs have been linked to a number of long-lasting issues in women. Pelvic inflammatory disease (PID), infertility, tubal or ectopic pregnancies, cervical cancer, and perinatal or congenital infections in babies born to infected mothers are among STI-related consequences [1]. In addition, women STIs also happen in men. Male fertility can be significantly impacted by sexually transmitted illnesses depending on where those infections are most prevalent. In western countries, STIs have little influence on male fertility, whereas it shows strong effect in other regions, especially in Africa or South East Asia. Urinary strictures and epididymal orchitis can be brought on by long-term infections. They are susceptible to contracting chlamydia trachomatis and Neisseria gonorrhoeae as well. Sperm quality may be harmed by Mycoplasma urealyticum (viability, DNA clotting). Unresolved is the link between viral infections and male infertility. The spread of the human immunodeficiency virus (HIV) is more likely to occur in cases of any sexually transmitted illness. HIV infection raises the possibility of the virus spreading through sperm. As immunodeficiency progresses, sperm quality declines [2].
Therefore, STIs extremely affected the health of both women and men in a worldwide scope and leaded to serious sequelae. It is obvious that STIs are not only severely affecting human health, especially urogenital system and genital system but also show a feature of universal spreading with no exclusion of sex.
2.2. Common pathogens of STIs
STIs can be caused by different pathogens, including chlamydia, gonorrhea, trichomoniasis, genital warts, genital herpes, pubic lice, scabies, syphilis, and human papillomavirus (HPV). Among them chlamydia trachomatis is the one leading to the most prevalent STI in many countries.
3. Chlamydia trachomatis
3.1. Characteristics of chlamydia trachomatis infection
The primary prevalent bacterial sexually transmitted infection in the UK is chlamydia trachomatis infection. Because of the characteristics of its main membrane proteins, chlamydia trachomatis has a number of serovariants. Conjunctivitis, urethritis, cervicitis, and proctitis are caused by the infection of columnar epithelial cells in the ocular, genital, and rectal regions by serovars D to K, separately. These serovars induce pneumonitis in the children and infect respiratory epithelial cells as well. Lymphogranuloma venereum (LGV), a sexually transmitted infection that mainly affects nations in the tropics, is brought on by serovars L1eL3 [3]. It can result in serious inflammation and invasive infection with clinical manifestation, such as genital ulcer diseases, lymphadenopathy, or proctocolitis. However, LGV can infect people without symptoms. Without treatment immediately, chronic colorectal fistulas and strictures or reactive arthropathy may happen to patients.
3.2. Comparison with other STIs
Since some patients infected by chlamydia trachomatis show few symptoms, they are often not treated immediately. In this case, although it infects initially the cervix, it can eventually spread to other parts of genital system, such as uterus or fallopian tubes in women. As a result, short-term and long-term health issues and sequelae can appear in patients, and the risks of getting other STIs can increase. Unlike HPV, it seldom causes cancers. Furthermore, comparing with gonorrhea, in most cases it doesn’t cause pharyngitis, even though it can spread to the throat. In addition, it can influence infants and children. Neonatal conjunctivitis or pneumonia can occur because of either intrauterine infection with chlamydia trachomatis or infection during passage through the birth canal. Intrauterine and infant infection can cause severe consequences, including otitis media, sudden infant death, apnoea, asthma, and chronic obstructive lung disease (COL) [4].
4. Conventional therapies: antibiotic
4.1. Introduction
For a long time, the first-line therapy of chlamydia trachomatis is antibiotics. And doxycycline is most recommended one by CDC, and azithromycin is alternative regimen. Both of them are effective in treating chlamydia trachomatis infection.
4.2. Doxycycline
Doxycycline is a type of tetracycline antibiotic, binding to ribosomal subunit 30s in order to achieve the inhibitory effect against chlamydia trachomatis. In randomized controlled trial, the efficiency of doxycycline for treating rectal chlamydia even reached 100% [5]. In another trial, researchers focused on female patients with acute urethral syndrome caused by chlamydia trachomatis. In the bacteriological evaluation, the eradication of doxycycline on chlamydia trachomatis achieved 94.7% within 7 days, and reached 100% in 14 days [6]. These studies show the high efficiency of doxycycline, which explains the reason why doxycycline is first-line therapy for chlamydia trachomatis infection. It is suggested to take doxycycline 100mg orally 2 times each day for 7 days as a regular therapy by CDC.
4.3. Azithromycin
As the alternative regimen, azithromycin is less effective than doxycycline. Meta-analysis shows that doxycycline was at most 3% more effective in treating urogenital chlamydia trachomatis infection and about 7% more effective in treating symptomatic urethral infection in men, even though azithromycin remains high efficiency in women [7]. Azithromycin is an azalide antibiotic that has an inhibitory effect on chlamydia trachomatis and other STIs at the same time. In a vitro trial, azithromycin could inhibit six strains of chlamydia trachomatis at concentrations less than 0.25 mg/l. This vitro models show that azithromycin is effective in inhibiting chlamydia trachomatis [8]. And the suggestion from CDC is to take azithromycin 1g orally in a single dose.
Despite the antibiotics discussed above, levofloxacin is another alternative regimen. Unfortunately, although it remains high efficiency as well, it is too expensive to be used as first-line therapy.
4.4. Therapeutic strategies of chlamydia trachomatis for vulnerable populations
There are some special considerations for treating certain vulnerable population, including patients during pregnancy and patients among infants and children.
4.4.1. Pregnant women. Women during pregnancy are more vulnerable and sensitive, so their treatment differs from the others. Considering the fact that the doxycycline can increase the risk for tooth discoloration during pregnancy and the fact that levofloxacin may lead to toxicity in breast milk, erythromycin or amoxicillin is advised. CDC recommendation shows that erythromycin at 500 mg orally four times each day for seven days or amoxicillin at 500 mg 3 times each day for seven days are applicable for chlamydial infection in pregnant women. However, a randomized trial of amoxicillin and erythromycin shows that amoxicillin is more tolerated and more suitable for treating pregnant women. In the trial, the overall cure rates were similar, 84.6% for amoxicillin and 84.2% for erythromycin. Nonetheless, the side effects of erythromycin that were reported by patients was 31.6%, whereas those of amoxicillin were 12.8% [9].
4.4.2. Infants and children. Infants and children are vulnerable and sensitive population as well, and their treatments for chlamydia trachomatis are based on weights and ages. Erythromycin or ethylsuccinate is suggested when the weight is less than 45 kg. Erythromycin is effective in prevention of chlamydia conjunctivitis. Compared to silver nitrate, infants who received erythromycin did not have chlamydia conjunctivitis [10]. While for children who weigh over 45 kg but are younger than 8 years old, azithromycin is recommended according to the CDC. As far as children aged more than 8 years, both azithromycin and doxycycline are advised. In fact, infant morbidity can be reduced by treating pregnant women. Only four out of 59 babies of mothers who received treatment were infected, whereas there were 12 out of 24 babies of mothers who did not receive treatment [11]. Therefore, treating pregnant females is a cost-effective way to preventing infant infection.
4.5. Resistance to antibiotics
However, there is a shortcoming of antibiotics, the resistance. Due to the resistance, the efficiency of this therapy decreases. As more and more reports of high recurrence rates in sexually active people, researchers gradually realized the importance of re-infection. Study indicated that azithromycin therapy is less effective for chlamydial infections in females than before [12]. Chlamydia trachomatis strains are resistant to some antibiotics, such as doxycycline, azithromycin, and ofloxacin [13]. Novel strategies such as gene therapy and peptide therapy with higher effectiveness and sensitivity are becoming a requirement for future treatments of chlamydial infections.
5. Gene therapy
5.1. Introduction
The goal of human gene therapy is to change the expression of genes or the genetic composition of live cells for medical purposes. Genetic diseases, cancer and infectious diseases are the targets of gene therapy. As a large number of infectious disorders are resistant to conventional therapeutic treatment, gene treatment becomes one of the alternative solutions. There are already a large number of studies about HIV gene therapy. It is conceivable that these studies can be extrapolated to other infectious agents and it shows an advantage of blocking at different phases of infectious agent’s life cycle [14]. This indicates that gene therapy can be a future option for treating chlamydia trachomatis infection as typical infectious disease.
5.2. Mechanism
Gene therapy can function in a variety of ways, including replacing a morbific gene with a normal duplication, abandoning a morbific gene that is malfunctioning, or adding a new or treated gene so as to achieve a condition. In detail, aiming at chlamydia trachomatis, infectious gene treatment should be focused. There are three main ways based on nucleic acid moieties, protein, immunotherapy, respectively. The first approach, the nucleic acid moieties method, changes antisense DNA or RNA, RNA decoys, and ribozymes. The second approach, the protein method, uses single-chain antibodies and transdominant negative proteins. The last approach, the immunotherapeutic method, uses pathogen-specific lymphocytes or genetic vaccines [14].
5.3. IRF5 and IL-10RA and their feasibility in chlamydial infection
Research indicates that IRF5(Interferon regulatory factor 5) and IL-10RA (Interleukin 10 receptor α) play a key role in macrophage-chlamydia interactions, a vital discovery for treating chlamydia trachomatis [15]. IRF5 is a member of the interferon regulatory factor (IRF) family. IRF has a variety of functions, including modulating cell proliferation, differentiation, apoptosis, and immune system activity. IL-10RA produces a protein that functions as an IL-10 receptor. This protein has been demonstrated to mediate the immunosuppressive signal of IL-10, which prevents the production of cytokines that cause inflammation. The important role of IRF5 in immune system and IL-10RA in preventing inflammation indicate that they can be promising targets for treating chlamydia trachomatis.
As unexplored targets, researches focused on IRF5 and IL-10RA were conducted. Biallelic knockouts in human induced pluripotent stem-cell (iPSC) using CRISPR/Cas technology were generated. Compared to the control group, human induced pluripotent stem-cell derived macrophage (iPSCdMs) with chlamydia trachomatis mutated by IRF5 shows less pro-inflammatory cytokines production (IL-1β, IL-6, and TNF-α), whereas anti-inflammatory cytokine production (IL-10) increases. It is obvious that IRF5 contributes to limiting intracellular development of chlamydia trachomatis in macrophages. As for the IL-10RA, in the process with chlamydia trachomatis infection, it increased for three times. IL-10, a vital immune regulator for the infection, increased for 150 times with chlamydia infection. Nonetheless, when researchers block IL-10 signaling through removing IL-10RA in human iPSdMs, its susceptibility to chlamydia trachomatis was upregulated (FIG. 1) [15]. This evidence supports the crucial function of IL-10 in limiting chlamydia infections. Consequently, despite the therapeutic function of IRF5 and IL-10RA, these data are all derived from vitro models. More clinical trials and research are needed for future application.
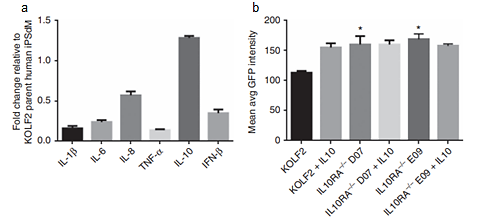
Figure 1. Human iPSdM CRISPR/ Cas9 mutants with chlamydia trachomatis infection. (A) Production of cytokines form iPSdMs with IRF5. (B) Level of GFP-tagged chlamydia trachomatis infection of human iPSdM CRISPR/ Cas9 mutants after 48 h.
Regardless of above discussion, ethical concern technical barrier are big problems. For gene therapy, adding gene in human, especially in the infants who are infected by chlamydia trachomatis, is still controversial so far. Besides, gene is a relatively novel and difficult field. It may be a long time to put the gene therapy for chlamydia trachomatis infection into market.
6. Peptide therapy
6.1. Introduction
Besides gene therapy, more and more novel strategies for chlamydia trachomatis infection are being developed. Peptide therapy is one of them and it is considered a substitute treatment for infectious diseases especially for those resistant to conventional therapeutic approaches. Therapeutic peptides are a special type of pharmacological drug that is made up of a string of carefully arranged amino acids. Peptide therapeutics imitate natural peptides, serving as hormones, growth factors, neurotransmitters, ion channel ligands, and anti-infectives. High tolerance is their advantage over conventional treatments of chlamydia trachomatis infection since they can be metabolized by the body. Even though there are some drawbacks, such as membrane impermeability, poor stability in vivo, and other limitations caused by amino acids, researchers keep on optimizing by modifying or recombination [16]. Thus, peptide therapies have huge potential for the treatment of chlamydia trachomatis infection.
6.2. Antimicrobial peptide and their feasibility in chlamydial infection
Antimicrobial peptides can be found in various creatures and can be metabolized by bodies, which are two advantages to be used in potential treatments. Pep-1, LL-37, and melittin are related to treatment of chlamydia trachomatis infection.
6.2.1. Cell-penetrating peptide: Pep-1. Cell-penetrating peptides (CPPs) are short peptides that help cells take in molecules of all sizes, from large DNA pieces to nanoscale particles. Through chemical coupling with covalent bonds or non-covalent interactions, the target part is connected to the peptides [17]. Membrane translocation is crucial in this process. The main methods to achieve this step include direct penetration, the usage of endocytosis, and the formation of a brief construction. Even though nucleic acid delivery, protein delivery, and contrast agent transport are three main applications, researchers found that pep-1 itself has an ability against intracellular chlamydia by chance [18].
With growing interests in antimicrobial peptides as a topical microbicide, especially for genital infections, researchers pay attention to reticulate bodies (RBs), the intracellular and active form of chlamydia. In the experiment, compared with the normal group, the number of chlamydial inclusions decreased dramatically with 4 mg/L Pep-1. The percentage of reduction even reached 82%. Chlamydial inclusions completely disappeared with higher concentrations of the peptide (no less than 8 mg/L). By contrast, Pep-1 showed no antimicrobial activity against progeny elementary bodies (EBs). Synthesized Pep-1 in this experiment showed an effect specifically in inhibiting the growth and replication of intracellular chlamydia [18]. Therefore, it can use for the treatment of chlamydia trachomatis infection.
6.2.2. LL-37. LL-37 is another potential peptide to treat chlamydia trachomatis infection. It is the only member of the cathelicidin family of peptides to be detected in the human body. Cathelicidins play a crucial part in the innate immune system of mammals. The basic mechanism of cathelicidin activity includes the disintegration (damage and puncturing) of cell membranes. Viral, bacterial, and fungal infections have all been shown to be susceptible to antimicrobial actions. After fusing with macrophage lysosomes, cathelicidins quickly degrade the lipoprotein membranes of microorganisms contained in phagosomes. LL-37 can thereby prevent the development of bacterial biofilms [19].
In order to examine the role of antimicrobial peptides against chlamydia trachomatis, researchers compared various human antimicrobial peptides. Among them, LL-37 showed an outstanding ability in inhibiting chlamydia trachomatis by destructing organisms exposed to extracellular environment. Infection of chlamydia trachomatis serovar L2 decreased about 50% with 5μg/ml LL-37 (FIG. 2). For other chlamydia trachomatis serovar D, LL-37 significantly controlled it as well. With minimal concentration 10μg/ml, LL-37 achieved inhibiting 50% chlamydia infection [20]. These observations support the crucial role of LL-37 against chlamydia trachomatis infection. Thus, it is possible for LL-37 to be a therapy for chlamydia trachomatis.
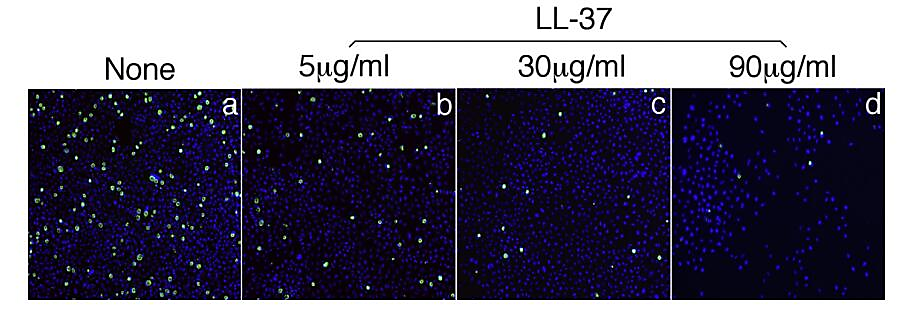
Figure 2. LL-37 against chlamydia trachomatis serovar L2. (A) Without LL-37. (B) With 5μg/ml. (C) With 30μg/ml. (D) With 90μg/ml.
6.2.3. Melittin. Melittin is another one of potential therapeutic peptides for chlamydia trachomatis infection. It is composed of 26 amino acids, the primary component and source of pain in honeybee venom. Although the principal function of melittin exhibits in bee venom, it is one of the most powerful anti-inflammatory substances. There has been research suggested that synthesized melittin can inhibit chlamydia trachomatis [21].
Researches showed that melittin's direct cytotoxic impact on bacteria are the primary mode of action that results in the suppression of chlamydia trachomatis infection in cell culture. In the experiment, the transmembrane possibility of the transfected cell was reduced by greater than a half as a result of the melittin. The transmembrane potentials differ among transfected and untransfected cells, the depolarization was also noticed, a phenomenon that equimolar K+ ion replaces for Na- in incubation buffer. It is supposed that the expression of melittin gene could reduce a transfected cell's transmembrane potential, which would disrupt mycoplasma and chlamydia's adhesion process and disrupt the usual cycle of their intracellular growth. With the synthesized melittin, chlamydia trachomatis decreased 60% in 24h and 75% in 48h [21]. In this circumstance, it can inhibit chlamydia trachomatis, therefore showing potential therapeutic effects for future treatments.
Although peptide therapy showed great potential for future treatment of STIs. Both the cost and the way to deliver the peptide are challenges. Chlamydia trachomatis, as a type of STIs, mainly infect genital system or urogenital system. In this case, aerosol is an intriguing future trend for topical applications. While proper attention should be put into its potential side effects regarding the systemic and oral use [22].
7. Conclusion
STIs can cause a serious sequalae both in urogenital system and in genital system, and remain a high worldwide appearance. Because of the high occurrence and the serious sequalae of STIs, researchers are paying more attention on them. As the most common sexually transmitted infection, chlamydia draws a lot of attention. Chlamydia trachomatis is the cause of the chlamydia, one of STIs. Clinically, antibiotics like doxycycline, azithromycin, and other are commonly used. Nevertheless, with more and more reports of high recurrence rates in sexually active people, the real cause, the failure of antibiotics, was discovered instead of re-infection. Chlamydia trachomatis strains show resistance to antibiotics especially azithromycin. In this situation, new therapies are strongly required to address this problem. Gene therapy and peptide therapy emerge at a historic moment.
Recently researchers discovered the key role of IRF5 (limit the intracellular growth of chlamydia trachomatis) and IL-10RA (relate to the susceptibility of chlamydia trachomatis) in macrophage-chlamydia interactions, which is crucial for treating chlamydia trachomatis infection. Furthermore, peptide therapy is novel and popular as well. Various peptides, including natural antimicrobial peptides and peptides with recombinant plasmid vector, show ability against chlamydia trachomatis. First, the cell-penetrating peptide. An effect specifically in inhibiting the growth and replication of intracellular chlamydia is shown by synthesized pep-1. Besides, LL-37 has a potent ability in inhibiting chlamydia trachomatis serovar L2 and D. In addition, melittin shows ability to inhibit chlamydia trachomatis as well. However, challenges remain since both therapies stay in vitro trials as a result of novelty. A lack of clinical trials or animal models is a problem for them. Considering the specificity issues, the technical difficulty and the ethical concern are the other two main issues for gene therapy. While for the peptide therapy, the cost and the way to deliver is an issue worth considering.
References
[1]. C. Deal, W. Cates, R. Peeling, and A. Wald, Emerg Infect Dis 10, e2 (2004).
[2]. F.R. Ochsendorf, C.R. Falk Ochsendorf, Z. Dermatologie Venerologie, and K.J. Goethe-, Andrologia 40, 72–75 (2008).
[3]. K. Manavi, Best Pract Res Clin Obstet Gynaecol 20, 941–951 (2006).
[4]. P.A. Mårdh, Best Pract Res Clin Obstet Gynaecol 16, 847–864 (2002).
[5]. J.C. Dombrowski, M.R. Wierzbicki, L.M. Newman, J.A. Powell, A. Miller, D. Dithmer, O.O. Soge, and K.H. Mayer, Clinical Infectious Diseases 73, 824–831 (2021).
[6]. V. Škerk, S. Schönwald, Z. Strapač, A. Beus, I. Francetić, I. Krhen, V. Lesko, and J. Vuković, Journal of Chemotherapy 13, 176–181 (2013).
[7]. F.Y.S. Kong, S.N. Tabrizi, M. Law, L.A. Vodstrcil, M. Chen, C.K. Fairley, R. Guy, C. Bradshaw, and J.S. Hocking, Clinical Infectious Diseases 59, 193–205 (2014).
[8]. L. Slaney, H. Chubb, A. Ronald, and R. Brunham, Journal of Antimicrobial Chemotherapy 25, 1–5 (1990).
[9]. N.S. Silverman, M. Sullivan, M. Hochman, M. Womack, and D.L. Jungkind, Am J Obstet Gynecol 170, 829–832 (1994).
[10]. M.R. Hammerschlag, J.W. Chandler, E.R. Alexander, M. English, W.-T. Chiang, L. Koutsky, D.A. Eschenbach, and J.R. Smith, JAMA 244, 2291–2293 (1980).
[11]. J. Schachter, R.L. Sweet, M. Grossman, D. Landers, M. Robbie, and E. Bishop, N Engl J Med 314, 276–279 (2009).
[12]. B.E. Batteiger, W. Tu, S. Ofner, B. van der Pol, D.R. Stothard, D.P. Orr, B.P. Katz, and J.D. Fortenberry, J Infect Dis 201, 42–51 (2010).
[13]. J. Somani, V.B. Bhullar, K.A. Workowski, C.E. Farshy, and C.M. Black, J Infect Dis 181, 1421–1427 (2000).
[14]. B.A. Bunnell and R.A. Morgan, Clin Microbiol Rev 11, 42–56 (1998).
[15]. A.T.Y. Yeung, C. Hale, A.H. Lee, E.E. Gill, W. Bushell, D. Parry-Smith, D. Goulding, D. Pickard, T. Roumeliotis, J. Choudhary, N. Thomson, W.C. Skarnes, G. Dougan, and R.E.W. Hancock, Nature Communications 2017 8:1 8, 1–12 (2017).
[16]. L. Wang, N. Wang, W. Zhang, X. Cheng, Z. Yan, G. Shao, X. Wang, R. Wang, and C. Fu, Signal Transduction and Targeted Therapy 2022 7:1 7, 1–27 (2022).
[17]. E.C.L. de Oliveira, K. Santana, L. Josino, A.H. Lima e Lima, and C. de Souza de Sales Júnior, Scientific Reports 2021 11:1 11, 1–15 (2021).
[18]. N. Park, K. Yamanaka, D. Tran, P. Chandrangsu, J.C. Akers, J.C. de Leon, N.S. Morrissette, M.E. Selsted, and M. Tan, Journal of Antimicrobial Chemotherapy 63, 115–123 (2009).
[19]. S. Dosler and E. Karaaslan, Peptides (N.Y.) 62, 32–37 (2014).
[20]. L. Tang, J. Chen, Z. Zhou, P. Yu, Z. Yang, and G. Zhong, Microbes Infect 17, 402–408 (2015).
[21]. V.N. Lazarev, T.M. Parfenova, S.K. Gularyan, O.Y. Misyurina, T.A. Akopian, and V.M. Govorun, Int J Antimicrob Agents 19, 133–137 (2002).
[22]. R.E.W. Hancock and H.G. Sahl, Nature Biotechnology 2006 24:12 24, 1551–1557 (2006).
Cite this article
Qin,J. (2023). Gene and peptide in the treatment of chlamydia trachomatis infections: beginning of an era?. Theoretical and Natural Science,4,18-25.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Biological Engineering and Medical Science (ICBioMed 2022), Part II
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. C. Deal, W. Cates, R. Peeling, and A. Wald, Emerg Infect Dis 10, e2 (2004).
[2]. F.R. Ochsendorf, C.R. Falk Ochsendorf, Z. Dermatologie Venerologie, and K.J. Goethe-, Andrologia 40, 72–75 (2008).
[3]. K. Manavi, Best Pract Res Clin Obstet Gynaecol 20, 941–951 (2006).
[4]. P.A. Mårdh, Best Pract Res Clin Obstet Gynaecol 16, 847–864 (2002).
[5]. J.C. Dombrowski, M.R. Wierzbicki, L.M. Newman, J.A. Powell, A. Miller, D. Dithmer, O.O. Soge, and K.H. Mayer, Clinical Infectious Diseases 73, 824–831 (2021).
[6]. V. Škerk, S. Schönwald, Z. Strapač, A. Beus, I. Francetić, I. Krhen, V. Lesko, and J. Vuković, Journal of Chemotherapy 13, 176–181 (2013).
[7]. F.Y.S. Kong, S.N. Tabrizi, M. Law, L.A. Vodstrcil, M. Chen, C.K. Fairley, R. Guy, C. Bradshaw, and J.S. Hocking, Clinical Infectious Diseases 59, 193–205 (2014).
[8]. L. Slaney, H. Chubb, A. Ronald, and R. Brunham, Journal of Antimicrobial Chemotherapy 25, 1–5 (1990).
[9]. N.S. Silverman, M. Sullivan, M. Hochman, M. Womack, and D.L. Jungkind, Am J Obstet Gynecol 170, 829–832 (1994).
[10]. M.R. Hammerschlag, J.W. Chandler, E.R. Alexander, M. English, W.-T. Chiang, L. Koutsky, D.A. Eschenbach, and J.R. Smith, JAMA 244, 2291–2293 (1980).
[11]. J. Schachter, R.L. Sweet, M. Grossman, D. Landers, M. Robbie, and E. Bishop, N Engl J Med 314, 276–279 (2009).
[12]. B.E. Batteiger, W. Tu, S. Ofner, B. van der Pol, D.R. Stothard, D.P. Orr, B.P. Katz, and J.D. Fortenberry, J Infect Dis 201, 42–51 (2010).
[13]. J. Somani, V.B. Bhullar, K.A. Workowski, C.E. Farshy, and C.M. Black, J Infect Dis 181, 1421–1427 (2000).
[14]. B.A. Bunnell and R.A. Morgan, Clin Microbiol Rev 11, 42–56 (1998).
[15]. A.T.Y. Yeung, C. Hale, A.H. Lee, E.E. Gill, W. Bushell, D. Parry-Smith, D. Goulding, D. Pickard, T. Roumeliotis, J. Choudhary, N. Thomson, W.C. Skarnes, G. Dougan, and R.E.W. Hancock, Nature Communications 2017 8:1 8, 1–12 (2017).
[16]. L. Wang, N. Wang, W. Zhang, X. Cheng, Z. Yan, G. Shao, X. Wang, R. Wang, and C. Fu, Signal Transduction and Targeted Therapy 2022 7:1 7, 1–27 (2022).
[17]. E.C.L. de Oliveira, K. Santana, L. Josino, A.H. Lima e Lima, and C. de Souza de Sales Júnior, Scientific Reports 2021 11:1 11, 1–15 (2021).
[18]. N. Park, K. Yamanaka, D. Tran, P. Chandrangsu, J.C. Akers, J.C. de Leon, N.S. Morrissette, M.E. Selsted, and M. Tan, Journal of Antimicrobial Chemotherapy 63, 115–123 (2009).
[19]. S. Dosler and E. Karaaslan, Peptides (N.Y.) 62, 32–37 (2014).
[20]. L. Tang, J. Chen, Z. Zhou, P. Yu, Z. Yang, and G. Zhong, Microbes Infect 17, 402–408 (2015).
[21]. V.N. Lazarev, T.M. Parfenova, S.K. Gularyan, O.Y. Misyurina, T.A. Akopian, and V.M. Govorun, Int J Antimicrob Agents 19, 133–137 (2002).
[22]. R.E.W. Hancock and H.G. Sahl, Nature Biotechnology 2006 24:12 24, 1551–1557 (2006).









