1. Introduction
1.1. Research Background
Research on the modification and water solubility of plant sterols holds significant potential. To address the current issue of the oil-water insolubility of plant sterols, modification is necessary without compromising their physiological functions, aiming to expand their applications in the food industry. Existing studies on plant sterol modification predominantly target increasing their solubility in fats, with limited focus on improving their water solubility. However, current research indicates that enhancing the water solubility of plant sterols is crucial for broadening their applications in food, reducing cholesterol levels, and minimizing toxicity. In recent years, there has been a growing number of studies internationally on improving the oil solubility of plant sterols, with substantial progress achieved. However, existing research faces challenges, such as the poor stability of physical methods to enhance water solubility, leading to a short shelf life. Additionally, there is limited research on the chemical synthesis of hydrophilic plant sterol compounds, and those available face issues of inadequate improvement in water solubility and low product conversion rates. Based on these challenges, this project proposes the use of acidic functionalized ionic liquids as catalysts and PEG with different molecular weights as hydrophilic modifiers to synthesize water-soluble derivatives of plant sterols.
1.2. Significance of the Study
Natural plant sterols have a high melting point (130-180 °C). They are white, powdery, or flaky solids that are nearly insoluble in water but soluble in anhydrous ethanol and organic solvents such as benzene and chloroform. Due to their unique chemical structure, plant sterols exhibit poor solubility in both fats and water, posing a bottleneck in their application in industries such as food, pharmaceuticals, and cosmetics. Scholars have attempted structural modifications to enhance their solubility, broaden their application areas, and achieve better industrial utilization. Plant sterol ester compounds possess various physiological functions, including cholesterol reduction, antioxidant properties, and anti-inflammatory effects. Despite some existing reports, enzymatic reactions face challenges such as low efficiency, high costs, and stringent conditions, making industrialization difficult. Currently, there is limited research on the chemical synthesis of plant sterol ester compounds, with only a few types reported. This project aims to use chemical methods to bond plant sterols and sugar compounds in two steps, synthesizing a series of structurally novel plant sterol compounds and expanding their application in aqueous food environments.
1.3. Development Process of the Study
Currently, methods to improve oil solubility are well-established, resulting in significant advancements in the oil solubility of plant sterols. However, research on enhancing their water solubility is limited. Current studies mainly focus on physical methods, with minimal exploration of structural modifications to plant sterols. Physical methods, such as emulsification, micro-emulsification, and spray modification, are employed to improve water solubility but face stability issues. International research has explored the use of cyclodextrin, a cyclic oligosaccharide, for inclusion of various small molecules. Researchers have successfully increased the water solubility of plant sterols using cyclodextrin-based compounds. Despite the prevalent use of plant sterol emulsions and microemulsions in current research, their stability is poor, limiting their application in food preservation. Structural modifications have been made to the reactive hydroxyl group at the 3-position to enhance water solubility. Previous studies have successfully synthesized water-soluble plant sterol analogs, such as ascorbic acid-plant sterol phosphate esters. This project aims to contribute to the field by using chemical methods to synthesize water-soluble derivatives of plant sterols.
1.3.1. Foreign Research Development. Cycloid, a cyclic oligosaccharide with seven sugar units, exhibits a unique barrel-shaped structure with hydrophobic interiors and hydrophilic exteriors, making it suitable for the inclusion of various small molecules [1]. FengS successfully encapsulated phytosterols using β-cyclodextrin, achieving encapsulation rates of 92-98% and producing products with good antioxidant properties [2]. HeW compounded cyclodextrin with plant sterol esters, increasing their solubility in water to 8.68g/L and significantly improving their water solubility [3]. Currently, plant sterol emulsions and microemulsions are the main raw materials, but their stability is poor, limiting their application in food preservation. To improve their water solubility, structural modifications have been made to the reactive hydroxyl group at the 3-position. YuyuanH successfully synthesized a water-soluble plant sterol analog - ascorbic acid-plant sterol phosphate ester, using ascorbic acid as a hydrophilic modifier [4]. XueX synthesized a novel plant sterol-phosphatidylserine compound using phospholipase D catalysis. Pang et al. achieved good esterification results by synthesizing L-glutamate salts, obtaining a 92% esterification rate. In previous studies, the applicant found that by using DCC/DMAP as acyl donors and 10 N-phosphorylated amino acids as acyl acceptors, structural modifications resulted in compounds with good lipid and water solubility [5].
1.3.2. Domestic Research Development. Li Huan et al. (2022) [6-7] conducted experiments using Sprague-Dawley rats as subjects, studying the lipid-lowering effects of equal amounts of water-soluble phospholipid emulsified plant sterols and free plant sterols through feeding. They found that water-soluble emulsified plant sterols were superior to free plant sterols due to their high melting point, insolubility in water, poor oil solubility, easy crystallization in food leading to low bioavailability, restricting their application in food processing. Jing et al. [8-9] used three water-soluble amino acids, glycine, aspartic acid, and glutamic acid, as primary materials to prepare three amino acid-type soy sterol esters, all of which are water-soluble. The solubilities of glycine-soy sterol ester salt, aspartic acid-soy sterol ester, and glutamic acid-soy sterol ester salt in aqueous solution are 1.1 g/L, 0.5 g/L, and 0.5 g/L, respectively. Existing literature reports that although the water solubility of hydrophilic plant sterol compounds synthesized through chemical bonding methods has been improved, there still exists a significant gap compared to their oil solubility. Wu Shanshan [10-11] and others prepared deep eutectic solvents using a grinding method, mixing compounds at room temperature and grinding them in a mortar until a clear liquid is formed. Considering optimization of time and energy consumption, Ren Mingxing et al. [12-13] proposed a more environmentally friendly microwave-assisted method, allowing the preparation of natural deep eutectic solvents in a few seconds. Liu Ping et al. [14-15] introduced an ultrasonic-assisted method for synthesizing natural deep eutectic solvents, using vortex homogenization for about 1 minute, and then synthesizing in a microwave reactor at a running temperature of 80 °C, microwave power of 850 W, and constant air flow (approximately 10 bar pressure), followed by storage at room temperature in a desiccator.
2. Experimental Materials and Methods
2.1. Experimental Equipment and Materials
2.1.1. Experimental Equipment.
RE-2000 rotary evaporator from Shanghai Yarong Biochemical Equipment Co., Ltd.
LC-20ABHPLC from Shimadzu Corporation, Japan.
ZAM4000 evaporation-scattering detector from Shimadzu Corporation, Germany.
N2000 chromatography workstation from Zhejiang University Zhida Automation Engineering Co., Ltd.
2.1.2. Experimental Materials. 1-Sulfonic acid-butyl-3-methylimidazole-sulfonic acid-3-methylimidazole (HSF4), 1-sulfonic acid-butyl-3-methylimidazole-p-toluenesulfonic acid (BSO3HMim), [BSO3HMim]Cl (>95%) produced by Shanghai Chengjie Chemical Co., Ltd.
2.2. Experimental Methods
2.2.1. Chemical Reaction Equation
(1) Synthesis of plant sterol succinic acid monoester
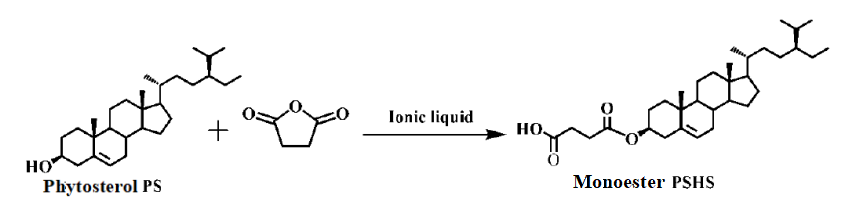
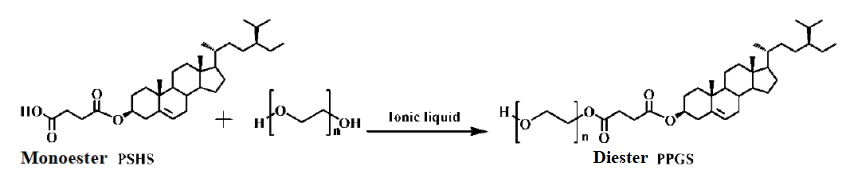
(2) Synthesis of plant sterol polyethylene glycol succinic acid diester
2.2.2. Synthesis of Intermediate Product Monoesters
Weigh 0.312 grams of plant sterol and 0.094 grams of adipic acid, placing them into a 50 mL round-bottom flask. Add 10 mL of petroleum ether (90-120 °C) to the flask. The quantity of [BSO3HMim] HSO4 used is 4% of the reactant mass (w/w, %). Secure the round-bottom flask in a magnetic stirring oil bath heating apparatus equipped with a condenser tube. Conduct a reflux reaction at 100 °C for 2 hours.
2.2.3. Separation and Purification of Intermediate Product Monoesters. The reaction solvent was removed by rotary evaporation to obtain a crude monoester product. Chromatographic separation was performed using a mixture of petroleum ether (60-90 °C)/ethyl acetate/formic acid (5/5/0.01, v/v/v) as the eluent. The eluate was collected in clean tubes, each with a capacity of approximately 10 milliliters. Simultaneously, thin-layer chromatography was employed for the detection and analysis of the eluted compounds. The eluate containing only one type of monoester was evaporated, and the resulting solid was dried under vacuum at 50 °C for 24 hours to obtain pure monoester product.
2.2.4. Synthesis of Target Product Diesters. The diester synthesis took place in a solvent-free system in a special reaction tube (16 mm × 160 mm). The reaction process involved placing the reaction tube into an oil bath heater equipped with a magnetic stirrer. Polyethylene glycol (PEG) 0.5 grams, monoester pure product 0.1 grams, ionic liquid 0.06 grams, and a magnetic stirrer were added separately. Nitrogen gas was introduced, and the temperature was raised to a predetermined level, maintaining the reaction for 1 hour.
2.2.5. Separation and Purification of Target Product Diesters. Upon completion of the reaction, heating was stopped until complete cooling, and nitrogen gas injection was ceased. The reaction sample was removed, and extraction with saturated salt solution and acetic acid ethyl ester solution was performed to remove excess PEG2000. The upper layer contained diesters, while the lower layer contained unreacted monoesters. Rotary evaporation was carried out to remove the solvent, obtaining crude diester product. A single separation was performed using a silica gel column chromatography with ethyl acetate/methanol/formic acid (9/1/0.1, v/v/v) as the eluent. The eluate containing only diesters was collected, evaporated by rotary flow, and the resulting solid was dried under vacuum at 50 °C for 24 hours to obtain pure diester product.
2.2.6. Thin-Layer Chromatography Analysis. During the reaction, a small portion (approximately 0.02 grams) was periodically taken out for thin-layer chromatography to determine the presence of products.
(1) Thin-Layer Chromatography Analysis of Monoesters: The crude monoester was dissolved in anhydrous ethanol. The sample solution was spotted on a thin-layer plate using a capillary tube, and, as a control, an ethanol solution of succinic anhydride and plant sterol was used. After drying with a blower, the plate was developed in petroleum ether (60-90 °C)/ethyl acetate/formic acid (5/5/0.01, v/v/v). After complete development, the thin-layer plate was placed in a 50 °C oven for 5 minutes. In a closed beaker, coloration was performed for 5 minutes under conditions of sufficient volatilization, and the results were observed.
(2) Thin-Layer Chromatography Analysis of Diesters: Crude diester, monoester, and PEG2000 were dissolved in anhydrous ethanol. An appropriate amount of the sample solution was extracted using a capillary tube and uniformly coated on a thin-layer chromatography column. The silica plate was placed in the eluting liquid, consisting of ethyl acetate/methanol/formic acid (9/1/0.01, v/v/v). After completion, the silica disk was removed and placed in an oven for 5 minutes to completely evaporate the solvent. Subsequently, the silica gel plate was dipped in copper sulfate phosphoric acid salt coloration solution for about 5 seconds and then removed, and placed in the oven for coloration at high temperature. The coloration solution was prepared by extracting 4.7 mL of phosphoric acid in 45.3 mL of Wahaha distilled water, then mixing, and adding 7.5 grams of copper sulfate pentahydrate to the prepared phosphoric acid solution while stirring in an ultrasonic bath.
2.2.7. High-Performance Liquid Chromatography (HPLC) Analysis. The test sample was dissolved in anhydrous ethanol to maintain a concentration between 1 and 2 mg/mL. Using a 2.5 mL syringe, approximately 1 mL of the sample solution was drawn and filtered through a 0.45μm organic filter membrane before being injected into the sample bottle. Analysis was performed using a High-Performance Liquid Chromatography-Evaporative Light Scattering Detector (HPLC-ELSD). The chromatographic column used was Symmetry C18 (5 μm, 4.6×150 mm, Waters, USA). The HPLC parameters were set as follows: column temperature at 35 °C, mobile phase as a mixture of methanol and formic acid (volume ratio of 1000:1), flow rate at 1 mL/min, and injection volume at 10 μL. ELSD parameters were set with a temperature of 60 °C, pressure of 0.5 bar, and high-purity nitrogen gas used as the carrier gas.
2.2.8. Infrared Spectroscopy Analysis. The attenuated total reflection method was selected with a resolution of 4 cm-1, scanning 32 times, and a scanning range between 4000 and 600 cm-1.
2.2.9. Water Solubility of Products. Water solubility: Approximately 0.3 grams, 0.4 grams, and 0.5 grams of diesters were weighed, and 1 milliliter of deionized water was added to a 5 mL centrifuge tube. The sample was placed in a 50 °C oven, heated to a gel-like state, then taken out from the vortex, shaken, and subsequently subjected to ultrasonication for 1 hour to check for any undissolved sample. In the presence of undissolved material, 200 μL of water was added continuously, vortexed, and shaken until complete dissolution.
3. Results and Discussion
3.1. Preparation and Purification of Hydrophilic Plant Sterol Derivatives
In the chemical reaction, the obtained diester product is not a singular substance but a mixture with various other compounds. Apart from the targeted diester, major impurities include PEG, monoester, plant sterol, and succinic anhydride. To obtain a high-purity diester, effective separation and purification procedures are essential. Silica gel column chromatography is a commonly used lab separation method, but it is impractical for large-scale processing. This method relies on the differential adsorption abilities of substances on silica gel for separation. However, for large-scale samples, this method is not only time-consuming but may also fail to ensure the separation's purity and yield. To efficiently separate the diester, we adopted an organic phase/water phase extraction method. As PEG is water-soluble, adding saturated sodium chloride reduces PEG's solubility in the water phase, facilitating effective separation from the diester. Simultaneously, saturated sodium chloride aids in demulsification, ensuring a clearer separation between the two phases. In selecting the extraction solvent, we considered common solvents used in plant sterol crystallization processes, such as methanol, ethanol, isopropanol, acetone, and ethyl acetate. However, due to PEG's high solubility in methanol, ethanol, and isopropanol, these solvents are unsuitable for diester extraction. In preliminary experiments, we found that diester has good solubility in acetone. However, after extracting with acetone and a saturated sodium chloride aqueous solution, PEG's content in the acetone layer reached 40%. This indicated a significant amount of PEG was carried into the targeted product. Therefore, acetone was also excluded. Ultimately, we chose ethyl acetate with a saturated sodium chloride aqueous solution as the extraction agent for the diester. This method effectively removes PEG and other impurities, yielding a high-purity diester. Figure 1 displays the HPLC chromatogram of the unseparated and unpurified diester-1000 reactants. Figure 2 shows the preliminary separation and purification results for a 0.1g diester-1000 sample, indicating a noticeable improvement in diester purity. Figure 3 illustrates the HPLC chromatogram after the separation and purification of a diester-1000 sample, approximately 35g in quantity.
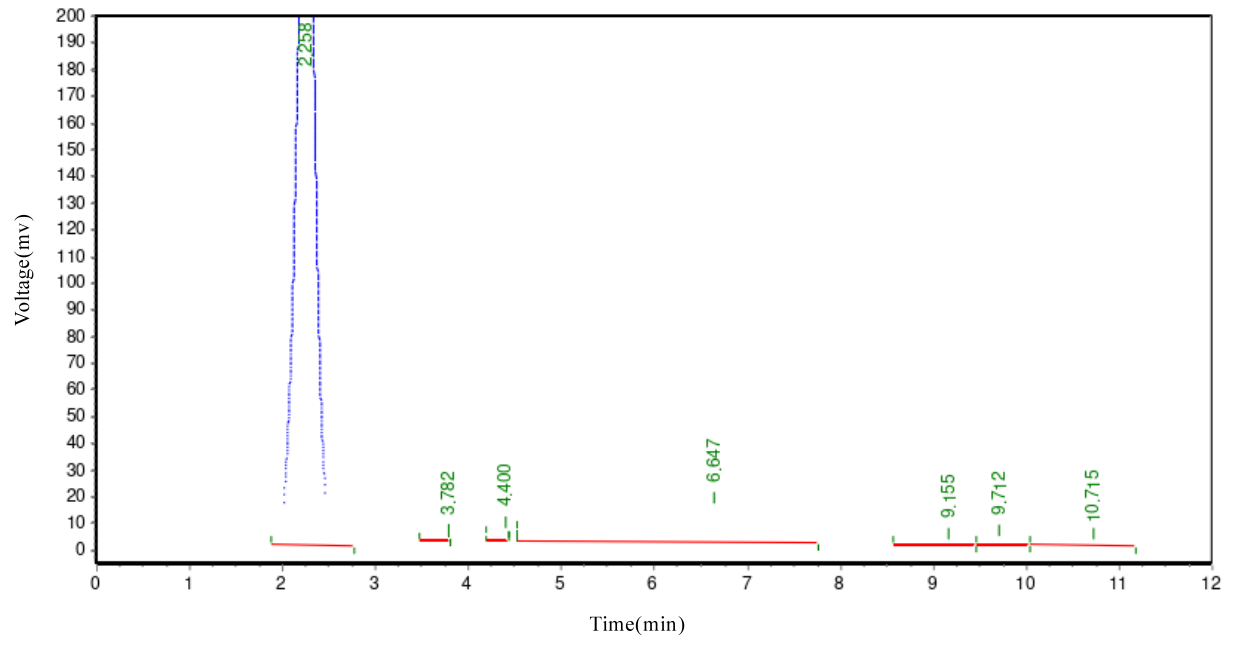
Figures 1. HPLC diagram of unisolated and purified diester-1000 reactant
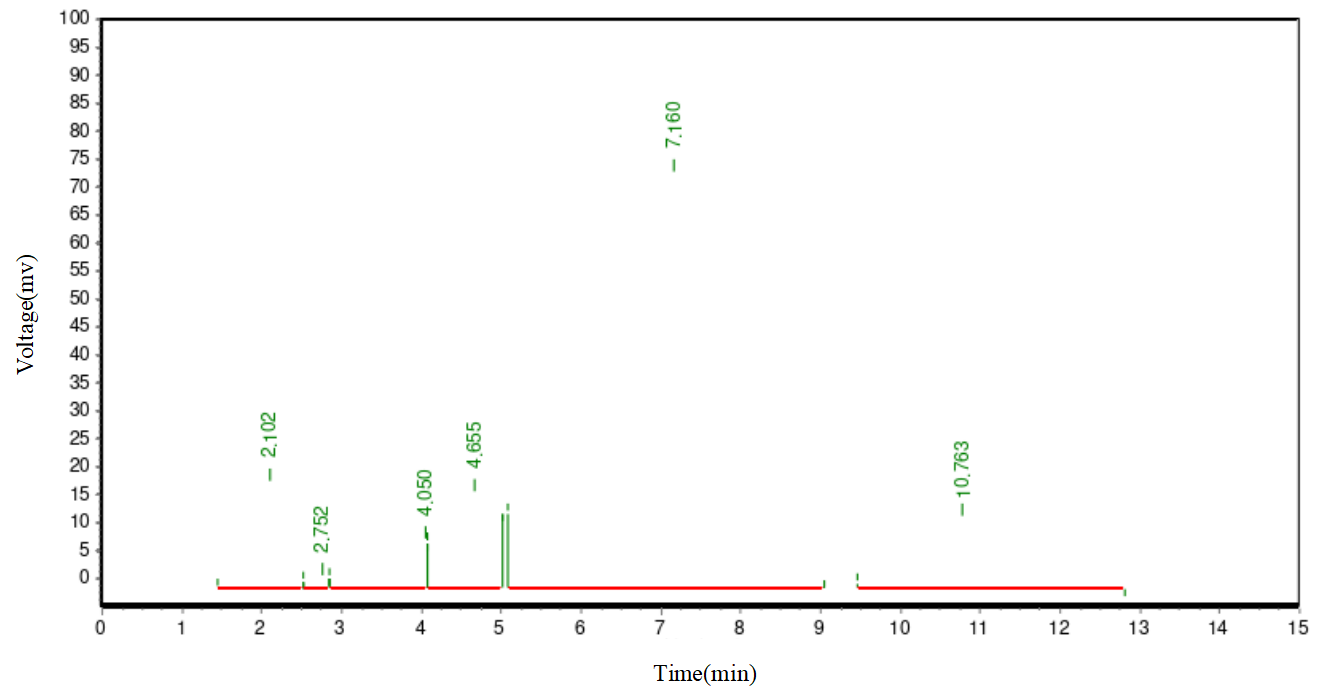
Figures 2. Pre-test results of separation and purification of 0.1g diester -1000 sample
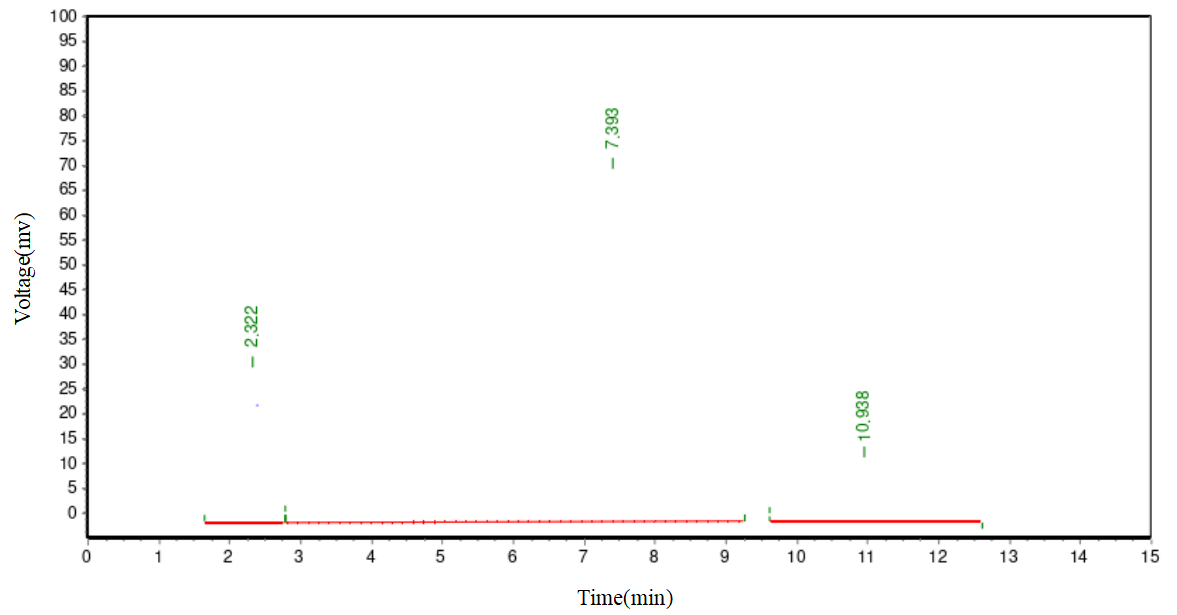
Figures 3. HPLC diagram of separation and purification of 35g diester -1000 sample
As depicted in Figures 1, 2, and 3, in the batch-produced diester-1000 samples, we observed a substantial presence of PEG1000, exceeding 70% by mass. To enhance purity, we employed ethyl acetate/saturated sodium chloride aqueous solution extraction. After an initial small-scale extraction, the diester mass fraction reached 89%, with the majority of PEG1000 removed. In large-scale processing, we adjusted the extraction agent ratio to 0.7:1 to meet demand. After batch extraction, the diester mass fraction stabilized between 85-88%, with PEG1000 content slightly exceeding 2-3%. For samples with diester mass fractions below 85%, increasing the extraction frequency improved purity. Similar methods were applied for purifying diester-600 and diester-2000, with purities exceeding 88%, suitable for subsequent research.
3.2. Discussing the Possibility of Water-Soluble Plant Sterol Derivatives Preparation Using Click Chemistry
Water-soluble plant sterol derivatives find crucial applications in various fields such as pharmaceutical development, food, and cosmetics industries. However, due to the chemical stability of plant sterols, their modification poses a challenge. Click chemistry provides a solution to this challenge. Specifically, reactions like CuAAC and SCoA efficiently and selectively bind plant sterols with various functional molecules, generating derivatives with specific biological activities. This opens up possibilities in areas like drug development, food additives, and cosmetics. Despite these advancements, certain challenges need addressing, including designing specific alkyne structures, optimizing reaction conditions, and improving product purity. In summary, despite the existing challenges, click chemistry offers a powerful tool for preparing water-soluble plant sterol derivatives, presenting vast application prospects.
4. Conclusion and Prospects
4.1. Conclusion
In the study of the preparation and purification of hydrophilic plant sterol derivatives, researchers successfully synthesized plant sterol succinate monoester and polyethylene glycol succinate diester through a series of chemical reactions. For the synthesis of the intermediate product monoester, researchers reacted dicarboxylic acid with plant sterol under specific conditions, obtaining crude product after a 2-hour reflux reaction. Subsequently, methods such as rotary evaporation and chromatographic separation were employed for the purification of the monoester, ultimately yielding a pure monoester product. Regarding the synthesis of the target product diester, researchers, in a solvent-free system, utilized a predetermined temperature and reaction time to catalyze PEG2000 with monoester through an ionic liquid. This process resulted in crude diester. Subsequently, diester separation and purification were performed using silica gel column chromatography, leading to a pure product. In thin-layer chromatography analysis, researchers conducted qualitative analysis through timed sampling, observing the presence of products in monoester and diester. High-performance liquid chromatography (HPLC) analysis provided quantitative measurements of the sample components. Additionally, infrared spectroscopy analysis further confirmed the product's structure. In the study of the product's water solubility, researchers found that methods such as heating, vortex oscillation, and ultrasonic treatment enabled complete dissolution of the diester in water, exhibiting excellent water solubility.
From the above research, the author concludes that, with optimized synthesis conditions and purification methods, hydrophilic plant sterol derivatives with excellent water solubility can be successfully prepared. This opens up new possibilities for the application of plant sterols in fields such as pharmaceuticals, food, and cosmetics.
4.2. Prospects
Firstly, despite successfully synthesizing plant sterol succinate monoester and polyethylene glycol succinate diester, reaction conditions and purification processes remain somewhat intricate and require further optimization. For instance, exploring simpler separation methods or more effective catalysts could enhance product yield and purity. Secondly, in the study of the product's water solubility, although methods like heating, vortex oscillation, and ultrasonic treatment were found effective, they may require considerable time and energy, potentially impractical for large-scale production. Therefore, further research is needed to enhance the solubility and stability of the product for better application in actual production. Lastly, attention should be paid to the limitations of the research. For example, the study of the biological activity of the product is not sufficiently in-depth, requiring further exploration of its prospects in pharmaceuticals, food, and cosmetics. Additionally, research and development of other types of plant sterol derivatives should be explored to expand their application scope in the biomedical field. In summary, for the preparation and purification of hydrophilic plant sterol derivatives, continuous efforts and exploration are needed to make greater contributions to related fields. At the same time, awareness of research limitations is crucial, prompting continuous improvement of experimental methods and approaches to propel the research into deeper development.
References
[1]. Nanotechnology - Nanodispersion; Research Conducted at Zhejiang University of Technology Has Updated Our Knowledge about Nanodispersion (Fabrication and Characterization of Water-soluble Phytosterol Ester Nanodispersion By Emulsification-evaporation Combined Ultrasonic Method) [J]. Chemicals & Chemistry, 2020.
[2]. Feng S, Wang Z, Zhao J, et al. Fabrication and characterization of water-soluble phytosterol ester nanodispersion by emulsification-evaporation combined ultrasonic method [J]. Journal of Food Engineering, 2020, 276, 109895-109895.
[3]. He W, Li L, Wang H, et al. Synthesis and cholesterol-reducing potential of water-soluble phytosterol derivative [J]. Journal of Functional Foods, 2019, 60, 103428-103428.
[4]. Yuyuan H, Chuanguo M, Xiaowei C, et al. Hydrophilic phytosterol derivatives: A short review on structural modifications, cholesterol-lowering activity and safety [J]. Grain & Oil Science and Technology, 2022, 5 (3).
[5]. Xue X, Mingxing R, WenSen H, et al. The preparation of phytosteryl succinyl sucrose esters and improvement of their water solubility and emulsifying properties. [J]. Food Chemistry, 2021, 373 (PB), 131501-131501.
[6]. Li H. Research on the Enzymatic Synthesis of Phospholipid Derivatives of Plant Sterols and Vitamin E [D]. Beijing University of Chemical Technology, 2023.
[7]. Guo Z. Construction of β-sitosterol Derivatives—Paclitaxel Nanoparticles and Their Antitumor Activity [D]. Harbin Institute of Technology, 2021.
[8]. Jing J. The Influence of Traditional Chinese Medicine Sterols and Their Molecular Analogs on Cholesterol Crystallization and Absorption [D]. Nanjing University of Chinese Medicine, 2019.
[9]. Wang H. Preparation and Lipid-lowering Efficacy of Hydrophilic Plant Sterol Derivatives [D]. Jiangsu University, 2018.
[10]. Wu S. Design, Synthesis, and Antioxidant Activity Evaluation of Novel Ester-type Catechins [D]. Zhejiang University, 2018.
[11]. Zheng W. Research on Water-soluble Modification of Plant Sterols [D]. Zhejiang Business Technology Institute, 2017.
[12]. Ren M. Preparation and Properties of Water-soluble Plant Sterol Succinyl Glucoside Esters [D]. Jiangnan University, 2015.
[13]. Zhang M. Preparation and Anti-tumor Efficacy of Mossitol Plant Sterol Esters [D]. Jiangnan University, 2015.
[14]. Liu P. Synthesis and Properties of Water-soluble Amino Acid Plant Sterol Esters [D]. Jiangnan University, 2014.
[15]. Sha O. Analysis and Testing of Plant Sterols and Their Ester Derivatives [D]. Yangzhou University, 2004.
Cite this article
Xiang,Q.;Mo,J.;Chen,B. (2024). Research on the preparation of water-soluble plant sterol derivatives. Theoretical and Natural Science,35,189-197.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Nanotechnology - Nanodispersion; Research Conducted at Zhejiang University of Technology Has Updated Our Knowledge about Nanodispersion (Fabrication and Characterization of Water-soluble Phytosterol Ester Nanodispersion By Emulsification-evaporation Combined Ultrasonic Method) [J]. Chemicals & Chemistry, 2020.
[2]. Feng S, Wang Z, Zhao J, et al. Fabrication and characterization of water-soluble phytosterol ester nanodispersion by emulsification-evaporation combined ultrasonic method [J]. Journal of Food Engineering, 2020, 276, 109895-109895.
[3]. He W, Li L, Wang H, et al. Synthesis and cholesterol-reducing potential of water-soluble phytosterol derivative [J]. Journal of Functional Foods, 2019, 60, 103428-103428.
[4]. Yuyuan H, Chuanguo M, Xiaowei C, et al. Hydrophilic phytosterol derivatives: A short review on structural modifications, cholesterol-lowering activity and safety [J]. Grain & Oil Science and Technology, 2022, 5 (3).
[5]. Xue X, Mingxing R, WenSen H, et al. The preparation of phytosteryl succinyl sucrose esters and improvement of their water solubility and emulsifying properties. [J]. Food Chemistry, 2021, 373 (PB), 131501-131501.
[6]. Li H. Research on the Enzymatic Synthesis of Phospholipid Derivatives of Plant Sterols and Vitamin E [D]. Beijing University of Chemical Technology, 2023.
[7]. Guo Z. Construction of β-sitosterol Derivatives—Paclitaxel Nanoparticles and Their Antitumor Activity [D]. Harbin Institute of Technology, 2021.
[8]. Jing J. The Influence of Traditional Chinese Medicine Sterols and Their Molecular Analogs on Cholesterol Crystallization and Absorption [D]. Nanjing University of Chinese Medicine, 2019.
[9]. Wang H. Preparation and Lipid-lowering Efficacy of Hydrophilic Plant Sterol Derivatives [D]. Jiangsu University, 2018.
[10]. Wu S. Design, Synthesis, and Antioxidant Activity Evaluation of Novel Ester-type Catechins [D]. Zhejiang University, 2018.
[11]. Zheng W. Research on Water-soluble Modification of Plant Sterols [D]. Zhejiang Business Technology Institute, 2017.
[12]. Ren M. Preparation and Properties of Water-soluble Plant Sterol Succinyl Glucoside Esters [D]. Jiangnan University, 2015.
[13]. Zhang M. Preparation and Anti-tumor Efficacy of Mossitol Plant Sterol Esters [D]. Jiangnan University, 2015.
[14]. Liu P. Synthesis and Properties of Water-soluble Amino Acid Plant Sterol Esters [D]. Jiangnan University, 2014.
[15]. Sha O. Analysis and Testing of Plant Sterols and Their Ester Derivatives [D]. Yangzhou University, 2004.









