1. Introduction
Alzheimer’s disease is a severe degenerative disease affecting majority of people over the age of 65. AD, the most prevalent kind of dementia, is characterized by cognitive impairment, changes of personality, and a decline of quality of life. As the world’s population ages, AD will certainly impose major social and economic burdens on the world.
Growing evidence has shown that the pathologies of AD are complex. Some features of AD in gray matter include excessive deposition of β-amyloid, neurofibrillary formatting, degeneration of Tau protein and neuroinflammation. Metabolic or mitochondria function are also abnormal, as shown in Figure 1. Furthermore, demyelination in white matter occurs in the early stage of AD. AD has a high heritability, there are some related gene like APP, PSEN1, APOE, which was verified to have a clear relationship with AD. Environment is also a factor of AD such as environmental pollution, medical development. Currently, the diagnosis of AD relies on some scales designed to assess the cognitive ability of patients. In terms of pathological evaluation of AD, the most commonly used medical procedures are brain imaging and lumbar puncture to identify the lesions in brain caused by AD. Compared to Imaging technology and lumbar puncture, the plasma-based detection is easy to use and less costly, and it is likely to become an alternative method for AD detection. In addition to blood markers like Aβ1-42, Aβ1-40, or phosphorylated Tau protein 181, some new cerebrospinal fluid biomarkers such as Neurogranin, SNAP25 have been added [1].
Pharmacotherapy is still the dominant treatment of AD. Currently approved drugs for AD in clinical use like cholinesterase inhibitors (ChEIs) and NMDA receptors antagonist(memantine) have beneficial effects on symptoms but do not effectively slow the progression of the disease. ChEIs are currently primary drugs for the treatment of mild to moderate AD, mainly including donepezil, rivastigmine, galantamine, and huperzine A. In recent years, a randomized double-blind trial in China found that donepezil was effective in the treatment of severe AD [2]. However, these drugs are suitable for the patients in early stage. After long-term use, some patients have great side effects, and the drug’s effective time is shortened. Although much efforts have been made to develop novel drugs targeting at neuroinflammation, mitochondrial function or metabolism other than β-amyloid and Tau protein, current clinical tests haven’t achieved promising results. Non-drug therapy can be adjunct to drug therapy. Besides, non-drug therapy is less invasive and harmful to patients compared to drug therapy. This paper introduces some new developing drugs for AD, targeting at different mechanisms.
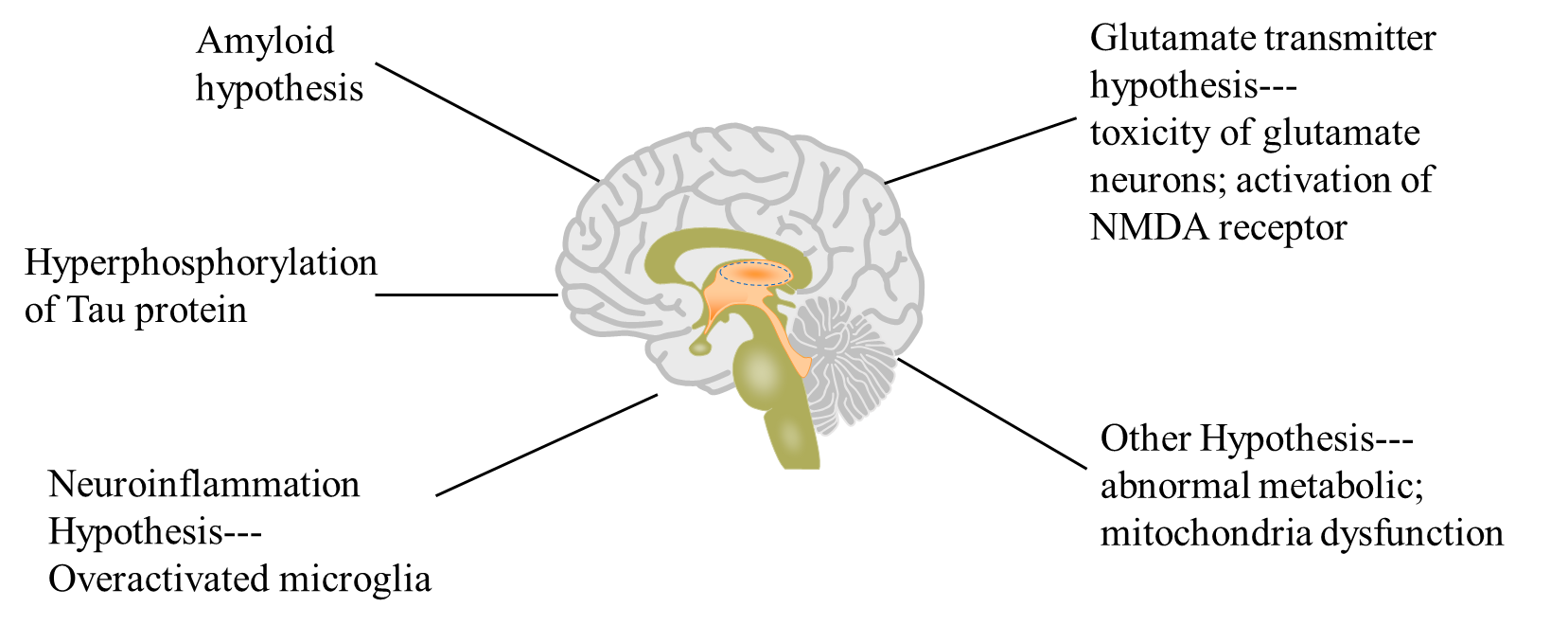
Figure 1. Different hypothesis of Alzheimer’s disease.
2. Non-drug therapy
2.1. Physical therapy
2.1.1. Muti-sensory gamma stimulation. Giving 40HZ light-sound stimulation to Hippocampus and prefrontal cortex has a profound impact on reducing the deposition of Aβ plaque and Tau protein. Long-term exposure to 40HZ light improves not only the function of synapsis and microglia acting through phagocytosis, but also alleviates immunological inflammation [3]. In mild and moderate patients, this innovative therapy has achieved Ⅱ-stage clinical findings. The results showed that after 6-months treatment, the sleep and cognition of AD patients improved noticeably, and the loss of brain volume decreased by 65 percent [4].
2.1.2. A deep brain stimulation. A deep brain stimulation (DBS) has been shown to help with Parkinson’s disease, memory and other conditions. DBS may work in memory circuits by reducing synaptic loss, boosting neurogenesis and improving glucose metabolism. DBS aims improve memory by coordinating activity across many brain regions. Furthermore, it has potential to lower Aβ plaque load DBS is a revolutionary treatment for AD that targets at entorhinal cortex and hippocampus directly [5].
2.2. Hyperbaric oxygen therapy
Patients who receive hyperbaric oxygen therapy (HBOT) breathe 100% oxygen in a controlled setting. Eliminating cerebral edema to reduce intracranial pressure and reducing oxygen deficiency to the brain are two probable methods of HBOT. Patients with AD improved in numerous areas after receiving HBOT treatment, including memory, blood flow in the brain, attention and their information processing ability [6]. This therapy is currently in preclinical stage.
2.3. Mesenchymal stem cell therapy
Lomecel-B, a novel mesenchymal stem cell treatment, promotes tissue healing while also regulating the immune system. This approach reduces brain inflammation and enhances the function of blood vessels. Anti-inflammatory molecules such as IL-4, IL-6, IL-10 were significantly increased in patients with different doses of Lpmecel-B in the phase Ⅰ of clinical trial as compared to placebo. Furthermore, the volume of left hippocampus was larger than that in both low-dose group and placebo. Patients given low-dose Lomecel-B saw an obvious improvement in their quality of life after 26-weeks of treatment [7].
2.4. Plasma exchange with albumin replacement
Plasma exchange with albumin replacement (PE) is a novel treatment for AD that has passed phase Ⅱb/Ⅲ trail. Plasma from AD patients contains Aβ as well as highly oxidized albumin, which impairs the albumin’s antioxidant activity. This therapy can improve the antioxidant activity of plasma. Some symptoms in patients with mild-AD such as processing capacity and speaking fluency got improved after PE treatment. The short-term memory in moderate-AD patients also got enhanced. Patients who had PE treatment had a higher quality of life in general [8].
2.5. Gene therapy
Microglia-specific microRNAs (miRNAs) contribute a lot in neuropathologies and therapy in AD. For example, MiRNA-200c, miR-206-3P, miR-30a-5p showed anti-AD properties [9,10]. However, because miRNAs are shared by hundreds of genes, some miRNAs gene or protein clusters may be better markers of AD. NHE6, a Na+-H+ exchanger, regulates endosome PH by exchanging acidic protons for sodium ions. When NHE6 gene was turned off in the mouse model, their deposition of Aβ was reduced. The PH-regulating protein can be produced with this therapy. As a result, intracellular environment becomes more acidic, and the accumulation of Aβ is prevented [11]. Apolipoprotein E4 (ApoE4) gene is a known risk factor for AD, which might be substituted with ApoE2 utilizing an adeno-associated virus (AAV) based gene therapy. ApoE2 plays a protective role in AD. ApoE4 homozygous persons are approximately 15 times more likely to develop AD than ApoE4 non-carriers [12]. The LEXEO firm developed an AAV-based gene therapy in which ApoE2 is delivered to central nervous system to replace ApoE4. This treatment lowered the level of Tau and phospho-Tau in patients’ brains.
3. New developing drug-therapy
3.1. Drugs regulating metabolism
3.1.1. GLP-1. AD called “Type 3 diabetes”, is thought to be associated with insulin resistance that is a major problem in type2 diabetes. Patients with type2 diabetes are more prone to develop AD. The incidence of dementia is reduced when they are treated with Recombinant Human Glucagon Like Peptide-1 (GLP-1) [13]. Many studies have shown that GLP-1 has neuroprotective effects in the transgenic mice model of AD. It can improve cognitive performance and oxidative stress by activating the PI3K/Akt pathway, which increases aerobic glycolysis and reduces oxidative phosphorylation [14]. Oral semaglutide which promotes insulin secretion and inhibits glucagon secretion has been approved in US, Europe and Japan. Its main function is to control blood glucose in patients with Type2 diabetes. Semaglutide is now undergoing clinical trials to determine its effectiveness on AD.
3.1.2. Targeting at mitochondrial function. Mitophagy plays a key role in inhibiting the accumulation of Aβ. UMI-77, a small molecule inhibitor of Myeloid cell leukemia-1(Mcl-1), was discovered to safely and effectively induce mitophagy by using a sensitive quantitative detection from the FDA-approved drug candidate. It doesn’t damage mitochondrial. It has the ability to degrade damaged mitochondria selectively. Mitophagy was successfully induced in mouse brain tissue after intraperitoneal injection of UMI-77 at a dose of 10mg/kg. The mechanism is that UMI-77 releases MCL-1 from Bax/Bak, which then combines with LC3A located on outer membrane of autophagy lysosome. The memory and cognitive abilities of AD mice improved after the treatment with UMI-77, and the area of Aβ plaques in the hippocampus was also reduced [15].
3.1.3. Targeting at gut microbial. Gut-Brain Axis is a potential pathway in AD. Sodium oligomannate (GV-971) is a marine algae-derived oral oligosaccharide first approved in November 2019 in China. It can help people with mild to moderate AD enhance their cognitive performance by regulating gut-microbiota-brain axis. It also inhibits aberrant increase of specific metabolites of intestinal flora. Furthermore, GV-971 has the ability to infiltrate the brain, and inhibits Aβ formation directly. GV-971 has been shown to be safe and tolerable in phage Ⅰ and Ⅱ studies. Objects with GV-971 900mg had improved cerebral glucose metabolism and dramatically enhanced memory in phage Ⅱ [16]. A 36-week study of GV-971 in phase Ⅲ clinical trial demonstrated obvious improvement of cognition in AD subjects(relative to placebo, p<0.0001) [17].
3.2. Targeting at Aβ
β-amyloid is a substance that can be cleared away from the brain in a healthy state. However, during the aging process of human brain, because of neuron’s intaking disorder, these amyloid plaques become insoluble amyloid fibrils. Deposition of insoluble amyloid fibrils leads to the death of nervous cells, resulting in impaired cognition. Because the cells in the brain generally don’t regenerate, excessive damage can be irreversible.
Donanemab is an antibody that targets Aβ epitope of N-terminal pyroglutamate, which reached a breakthrough in phase Ⅱ of clinical trial. Using an integrated Alzheimer’s disease scale(iADRS), researchers discovered that individuals who got donanemab medication for 76 weeks had a 32% lower in iADRS score than that in placebo group (p<0.05). It meant that patients who received domanemab had a lower rate of cognitive loss. Meanwhile, PET imaging technology revealed a rapid clearance of Aβ in patients’ brains after donanemab treatment, but there was no improvement of Tau position. Around 68% of patients had a PET-negative result after 18 months of treatment [18,19].
Lecanemab (BAN2401) is a humanized lgG1 monoclonal antibody that targets soluble aggregated Aβ (including oligomers and fibrils) and insoluble fibrils. It has completed phase Ⅱ of clinical trial. The highest dose about 10mg/kg of lecanemab was able to decrease Aβ in the patients’ brains. It also revealed a drop in Aβ levels that was time-dependent [20].
Aducanumab is a fully humanized IgG1 monoclonal antibody, it binds selectively with Aβ in patients’ brains, then it activates immune system to eliminate the deposition of Aβ. However, a study found that patients given a high dose of aducanumab experienced some negative effects. More studies are required to assess its effectiveness and safety [21].
Varoglutamstat is an oral small-molecule drug that inhibits pyroglutamate-A-beta (pGlu Aβ42), which is related to the formation of Aβ plaque. Tau pathology, neuroinflammation and synaptic function are all negatively affected by pGluAβ42. Varoglutamstat is currently in phase Ⅱ of clinical trials [22]. PBD-C06 is a humanized, deimmunized IgG1 antibody designed to bind to pGluAB42 in the brain and remove it. A research using mouse model showed that using a single treatment (Varoglutamstat or PBD-C06) reduced pGluAβ42 in brain of mice by 16-41%. Moreover, combining these two medications reduced pGluAβ42 by 45-65%, which was a considerable improvement [23].
3.3. Targeting at Tau protein
Protein Degradation Targeting Consortium (PROTAC) and Active Peptide Vaccine AADvac1 are two new therapies that target Tau protein and eliminate Tau aggregation.
PROTAC makes two kinds of protein close to each other, allowing for targeted protein degradation. It is composed of a ligand for binding target protein, a ligand for recruiting E3 ligase and a linker. PROTAC efficiently eliminated Tau aggregation in 3xTg-AD mouse model, improving cognition and synaptic functions [24].
AADvac1 is a Tau protein-targeted active peptide vaccination. A phase Ⅱ of clinical trial for this vaccine has been completed. It demonstrated that AADvac1 is relatively safe and tolerable. AADvac1 significantly reduced the accumulation of neurofilament light chain protein (NFL) by 58%, a key marker of neurodegenerative diseases. Furthermore, tau and phosphor-tau are reduced in cerebrospinal fluid (CSF) [25]. However, there was no statistically significant benefit from this vaccine in terms of cognition.
4. Others
4.1. Levetiracetam
About 10% to 20% of patients with AD have seizures, whereas an estimated 22-54% have asymptomatic epileptic activity. According to a clinical trial, low-dose levetiracetam improved spatial memory and executive function tasks in patients with AD. Levetiracetam (100 and 150 mg/kg) significantly attenuated learning impairments in rat model of AD [26]. However, there is no evidence that levetiracetam can slow the progression of AD, so more studies are needed [27].
4.2. Axitinib
Axitinib, an anticancer drug, is a small-molecule tyrosine kinase inhibitor that targets the vascular endothelial growth factor receptor. In the Tg2576 transgenic mouse, a typical pre-clinical model of AD, axitinib targets the pro-angiogenic pathway in AD, restoring the integrity of blood-brain barrier and reducing Aβ deposition [28]. As a result, rather than directly targeting Aβ, anticancer drugs that affect cerebral angiogenesis may be a viable therapy for AD.
4.3. Phosphodiesterase-5 inhibitors
Sildenafil is a selective phosphodiesterase-5 inhibitor, it has been approved to treat erectile dysfunction (ED) in males. Sildenafil may treat AD through a variety of potential mechanisms. The NO/cGMP pathway in the hippocampus, which is related closely to long-term potentiation (LTP) is disrupted in AD. The enzyme phosphodiesterase-5 which breaks down cGMP is elevated. PDE5 is inhibited by Sildenafil which raises cGMP levels [29]. In vitro experiments showed that Sildenafil increased neurite outgrowth and reduce aberrant phosphorylation of Tau protein. These findings show Sildenafil plays a potential role in treating AD.
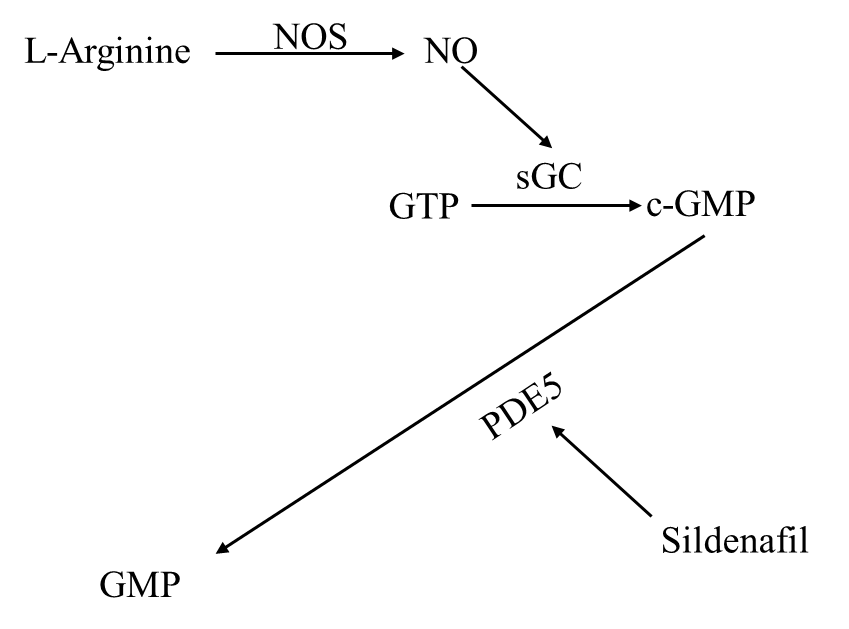
Figure 2. the flow chart from [29] shows NO/cGMP signal pathway.
4.4. Conventional Chinese herbal medicine
Huangqi sijunzi decoction may play a beneficial role in treating AD. The two most important components in Huangqi sijunzi decoction are Quercetin and Formononetin. The strongest inhibitor of acetylcholinesterase is Formononetin. In vitro experiments show that Formononetin has a neuroprotective effect and can protect brain cells form oxidative damage [30].
Table1. The potential drug for the treatment of AD.
Name | Characteristic | Effects/mechanism in AD |
Muti-sensory gamma stimulation | Physical therapy | Improves patients’ sleep and cognition; Decreases loss of brain volume |
Deep brain stimulation (DBS) | Improve memory; Reduce Aβ plaque load | |
Hyperbaric oxygen therapy | Non-drug therapy | Reduce intracranial pressure Improving oxygen deprivation to the brain |
Mesenchymal stem cell therapy | Reduces brain inflammation; Improves the function of blood vessels. | |
Gene therapy | Modifies some important genes that are risk of AD. | |
GLP-1 | Glucagon-like peptide-1 | Improves cognitive function and oxidative stress; PI3K/Akt pathway |
Sildenafil | Phosphodiesterase-5 inhibitors | Increase cGMP level by NO/cGMP pathway; increase neurite outgrowth; reduce aberrant phosphorylation of Tau protein. |
UMI-77 | Releases MCL-1 | Selectively degrade damaged mitochondria. |
GV-971 | Oral oligosaccharide, targeting at gut microbial | Suits mild or moderate AD improve cognitive function by regulating gut-microbiota-brain axis. |
Donanemab | An Aβ antibody targeting Aβ plaque | Clear the deposition of Aβ |
Lecanemab (BAN2401) | ||
Aducanumab | ||
Varoglutamstat | Targeting pGluAβ42 | Inhibiting pGluAβ42 |
PBD-C06 | A humanized, deimmunized IgG1 antibody, targeting pGluAβ42 | |
PROTAC | Targeting at Tau degradation | Clears Tau aggregation efficiently improves cognition and synaptic functions. |
AADvac1 | An active peptide vaccine targeting at Tau protein | Reduces NFL; reduces tau and phosphor-tau in CSF |
levetiracetam | Antiepileptic drug | Improves spatial memory and executive function |
Axitinib | Anti-cancer drug | Restores the integrity of blood-brain barrier; reduces Aβ deposition |
Table1. (continued).
Sildenafil | Phosphodiesterase-5 inhibitors | Increase cGMP level by NO/cGMP pathway; increase neurite outgrowth; reduce aberrant phosphorylation of Tau protein. |
Huangqi sijunzi decoction | Conventional Chinese herbal medicine | Inhibits acetylcholinesterase; alleviates oxidative damage of nerve cells. |
5. Conclusion
Alzheimer’s disease is the most common cause of dementia. According to the most recent estimates, the global incidence of dementia will quadruple by 2050. Some hypotheses are put forward that Aβ plaque maybe a consequence of AD pathology, other than a triggering factor. According to the current studies on the pathogenesis of AD, the pathways are very complicated. Some mechanisms are still being investigated or discovered, such as loss of mitochondrial dynamics, microglia-influencing hypothesis which opens up new avenues for therapeutic development. In addition, more developing drugs targets the supportive mechanisms of neurons like increasing energy metabolism in pathological conditions. Treatment for AD can only be symptomatic that can’t cure patients fundamentally until the pathogenesis of the disease is fully understood. In the future, there are some ways to treat AD: 1. Some deep study needed to learn the etiology and pathogenesis of the disease, new drugs should focus on specific molecular sites; 2. developing gene therapy to repair damaged nervous cells; 3. stem cells therapy has broad prospects in treating neurodegenerative diseases; 4. some drugs targeting at regulating gut microbial have a protective effect on AD.
References
[1]. S. Mazzucchi, G. Palermo, N. Campese, et al.The role of synaptic biomarkers in the spectrum of neurodegenerative diseases[J].Expert Review of Proteomics,2020,17:543-559.
[2]. J. Jia, C. Wei, L. Jia, et al.Efficacy and Safety of Donepezil in Chinese Patients with Severe Alzheimer's Disease: A Randomized Controlled Trial[J].Journal of Alzheimer's Disease : JAD,2017,56(4):1495-1504.
[3]. A. Martorell, A. Paulson, H. Suk, et al.Multi-sensory Gamma Stimulation Ameliorates Alzheimer's-Associated Pathology and Improves Cognition[J].Cell,2019,177(2):256-271.e22.
[4]. A. Cimenser, E. Hempel, T. Travers, et al.Sensory-Evoked 40-Hz Gamma Oscillation Improves Sleep and Daily Living Activities in Alzheimer's Disease Patients[J].Frontiers in Systems Neuroscience,2021,15:746859.
[5]. D. Yu, H. Yan, J. Zhou, et al.A circuit view of deep brain stimulation in Alzheimer's disease and the possible mechanisms[J].Molecular Neurodegeneration,2019,14(1):33.
[6]. R. Shapira, A. Gdalyahu, I. Gottfried, et al.Hyperbaric oxygen therapy alleviates vascular dysfunction and amyloid burden in an Alzheimer's disease mouse model and in elderly patients[J].Aging,2021,13(17):20935-20961.
[7]. M. Brody, M. Agronin, B. Herskowitz, et al.Results and insights from a phase I clinical trial of Lomecel-B for Alzheimer's disease[J].Alzheimer's & Dementia : the Journal of the Alzheimer's Association,2022,
[8]. M. Boada, O. López, J. Olazarán, et al.Neuropsychological, neuropsychiatric, and quality-of-life assessments in Alzheimer's disease patients treated with plasma exchange with albumin replacement from the randomized AMBAR study[J].Alzheimer's & Dementia : the Journal of the Alzheimer's Association,2021,
[9]. Y. Shao and T. Xu.A study on the neuroprotective effect of miR-206-3p on Alzheimer's disease mice by regulating brain-derived neurotrophic factor[J].Annals of Translational Medicine,2022,10(2):85.
[10]. T. Sun, K. Zhao, M. Liu, et al.miR-30a-5p induces Aβ production via inhibiting the nonamyloidogenic pathway in Alzheimer's disease[J].Pharmacological Research,2022,178:106153.
[11]. T. Pohlkamp, X. Xian, C. Wong, et al.NHE6 depletion corrects ApoE4-mediated synaptic impairments and reduces amyloid plaque load[J].eLife,2021,10
[12]. J. Rosenberg, M. Kaplitt, B. De, et al.AAVrh.10-Mediated APOE2 Central Nervous System Gene Therapy for APOE4-Associated Alzheimer's Disease[J].Human gene therapy. Clinical Development,2018,29(1):24-47.
[13]. C. Nørgaard, S. Friedrich, C. Hansen, et al.Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: Data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers[J]. Alzheimer's & Dementia (New York, N. Y.),2022,8(1):e12268.
[14]. J. Zheng, Y. Xie, L. Ren, et al.GLP-1 improves the supportive ability of astrocytes to neurons by promoting aerobic glycolysis in Alzheimer's disease[J].Molecular Metabolism,2021,47:101180.
[15]. X. Cen, Y. Chen, X. Xu, et al.Pharmacological targeting of MCL-1 promotes mitophagy and improves disease pathologies in an Alzheimer's disease mouse model[J].Nature Communications,2020,11(1):5731.
[16]. T. Wang, W. Kuang, W. Chen, et al.A phase II randomized trial of sodium oligomannate in Alzheimer's dementia[J].Alzheimer's Research & Therapy,2020,12(1):110.
[17]. S. Xiao, P. Chan, T. Wang, et al.A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer's dementia[J].Alzheimer's Research & Therapy,2021,13(1):62.
[18]. M. Mintun, A. Lo, C. Duggan Evans, et al.Donanemab in Early Alzheimer's Disease[J].The New England Journal of Medicine,2021,384(18):1691-1704.
[19]. S. Doggrell.Still grasping at straws: donanemab in Alzheimer's disease[J].Expert Opinion on Investigational Drugs,2021,30(8):797-801.
[20]. C. Swanson, Y. Zhang, S. Dhadda, et al.A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer's disease with lecanemab, an anti-Aβ protofibril antibody[J].Alzheimer's Research & Therapy,2021,13(1):80.
[21]. G. Alexander, S. Emerson and A. Kesselheim.Evaluation of Aducanumab for Alzheimer Disease: Scientific Evidence and Regulatory Review Involving Efficacy, Safety, and Futility[J].JAMA,2021,325(17):1717-1718.
[22]. E. Vijverberg, T. Axelsen, A. Bihlet, et al.Rationale and study design of a randomized, placebo-controlled, double-blind phase 2b trial to evaluate efficacy, safety, and tolerability of an oral glutaminyl cyclase inhibitor varoglutamstat (PQ912) in study participants with MCI and mild AD-VIVIAD[J].Alzheimer's Research & Therapy,2021,13(1):142.
[23]. T. Hoffmann, J. Rahfeld, M. Schenk, et al.Combination of the Glutaminyl Cyclase Inhibitor PQ912 (Varoglutamstat) and the Murine Monoclonal Antibody PBD-C06 (m6) Shows Additive Effects on Brain Aβ Pathology in Transgenic Mice[J].International Journal of Molecular Sciences,2021,22(21)
[24]. W. Wang, Q. Zhou, T. Jiang, et al.A novel small-molecule PROTAC selectively promotes tau clearance to improve cognitive functions in Alzheimer-like models[J].Theranostics,2021,11(11):5279-5295.
[25]. M. Vaz and S. Silvestre.Alzheimer's disease: Recent treatment strategies[J].European Journal of Pharmacology,2020,887:173554.
[26]. M. Alavi, S. Fanoudi, M. Hosseini and H. Sadeghnia.Beneficial effects of levetiracetam in streptozotocin-induced rat model of Alzheimer's disease[J].Metabolic Brain Disease,2022,37(3):689-700.
[27]. K. Vossel, K. Ranasinghe, A. Beagle, et al.Effect of Levetiracetam on Cognition in Patients With Alzheimer Disease With and Without Epileptiform Activity: A Randomized Clinical Trial[J].JAMA neurology,2021,78(11):1345-1354.
[28]. C. Singh, K. Choi, L. Munro, et al.Reversing pathology in a preclinical model of Alzheimer's disease by hacking cerebrovascular neoangiogenesis with advanced cancer therapeutics[J].EBioMedicine,2021,71:103503.
[29]. O. Sanders.Sildenafil for the Treatment of Alzheimer's Disease: A Systematic Review[J].Journal of Alzheimer's Disease Reports,2020,4(1):91-106.
[30]. W. Zhang, M. Lv, Y. Shi, et al.Network Pharmacology-Based Study of the Underlying Mechanisms of Huangqi Sijunzi Decoction for Alzheimer's Disease[J].Evidence-based Complementary and Alternative Medicine : eCAM,2021,2021:6480381.
Cite this article
You,H. (2023). New Treatment of Alzheimer’s Disease. Theoretical and Natural Science,3,44-52.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Biological Engineering and Medical Science (ICBioMed 2022), Part I
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. S. Mazzucchi, G. Palermo, N. Campese, et al.The role of synaptic biomarkers in the spectrum of neurodegenerative diseases[J].Expert Review of Proteomics,2020,17:543-559.
[2]. J. Jia, C. Wei, L. Jia, et al.Efficacy and Safety of Donepezil in Chinese Patients with Severe Alzheimer's Disease: A Randomized Controlled Trial[J].Journal of Alzheimer's Disease : JAD,2017,56(4):1495-1504.
[3]. A. Martorell, A. Paulson, H. Suk, et al.Multi-sensory Gamma Stimulation Ameliorates Alzheimer's-Associated Pathology and Improves Cognition[J].Cell,2019,177(2):256-271.e22.
[4]. A. Cimenser, E. Hempel, T. Travers, et al.Sensory-Evoked 40-Hz Gamma Oscillation Improves Sleep and Daily Living Activities in Alzheimer's Disease Patients[J].Frontiers in Systems Neuroscience,2021,15:746859.
[5]. D. Yu, H. Yan, J. Zhou, et al.A circuit view of deep brain stimulation in Alzheimer's disease and the possible mechanisms[J].Molecular Neurodegeneration,2019,14(1):33.
[6]. R. Shapira, A. Gdalyahu, I. Gottfried, et al.Hyperbaric oxygen therapy alleviates vascular dysfunction and amyloid burden in an Alzheimer's disease mouse model and in elderly patients[J].Aging,2021,13(17):20935-20961.
[7]. M. Brody, M. Agronin, B. Herskowitz, et al.Results and insights from a phase I clinical trial of Lomecel-B for Alzheimer's disease[J].Alzheimer's & Dementia : the Journal of the Alzheimer's Association,2022,
[8]. M. Boada, O. López, J. Olazarán, et al.Neuropsychological, neuropsychiatric, and quality-of-life assessments in Alzheimer's disease patients treated with plasma exchange with albumin replacement from the randomized AMBAR study[J].Alzheimer's & Dementia : the Journal of the Alzheimer's Association,2021,
[9]. Y. Shao and T. Xu.A study on the neuroprotective effect of miR-206-3p on Alzheimer's disease mice by regulating brain-derived neurotrophic factor[J].Annals of Translational Medicine,2022,10(2):85.
[10]. T. Sun, K. Zhao, M. Liu, et al.miR-30a-5p induces Aβ production via inhibiting the nonamyloidogenic pathway in Alzheimer's disease[J].Pharmacological Research,2022,178:106153.
[11]. T. Pohlkamp, X. Xian, C. Wong, et al.NHE6 depletion corrects ApoE4-mediated synaptic impairments and reduces amyloid plaque load[J].eLife,2021,10
[12]. J. Rosenberg, M. Kaplitt, B. De, et al.AAVrh.10-Mediated APOE2 Central Nervous System Gene Therapy for APOE4-Associated Alzheimer's Disease[J].Human gene therapy. Clinical Development,2018,29(1):24-47.
[13]. C. Nørgaard, S. Friedrich, C. Hansen, et al.Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: Data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers[J]. Alzheimer's & Dementia (New York, N. Y.),2022,8(1):e12268.
[14]. J. Zheng, Y. Xie, L. Ren, et al.GLP-1 improves the supportive ability of astrocytes to neurons by promoting aerobic glycolysis in Alzheimer's disease[J].Molecular Metabolism,2021,47:101180.
[15]. X. Cen, Y. Chen, X. Xu, et al.Pharmacological targeting of MCL-1 promotes mitophagy and improves disease pathologies in an Alzheimer's disease mouse model[J].Nature Communications,2020,11(1):5731.
[16]. T. Wang, W. Kuang, W. Chen, et al.A phase II randomized trial of sodium oligomannate in Alzheimer's dementia[J].Alzheimer's Research & Therapy,2020,12(1):110.
[17]. S. Xiao, P. Chan, T. Wang, et al.A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer's dementia[J].Alzheimer's Research & Therapy,2021,13(1):62.
[18]. M. Mintun, A. Lo, C. Duggan Evans, et al.Donanemab in Early Alzheimer's Disease[J].The New England Journal of Medicine,2021,384(18):1691-1704.
[19]. S. Doggrell.Still grasping at straws: donanemab in Alzheimer's disease[J].Expert Opinion on Investigational Drugs,2021,30(8):797-801.
[20]. C. Swanson, Y. Zhang, S. Dhadda, et al.A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer's disease with lecanemab, an anti-Aβ protofibril antibody[J].Alzheimer's Research & Therapy,2021,13(1):80.
[21]. G. Alexander, S. Emerson and A. Kesselheim.Evaluation of Aducanumab for Alzheimer Disease: Scientific Evidence and Regulatory Review Involving Efficacy, Safety, and Futility[J].JAMA,2021,325(17):1717-1718.
[22]. E. Vijverberg, T. Axelsen, A. Bihlet, et al.Rationale and study design of a randomized, placebo-controlled, double-blind phase 2b trial to evaluate efficacy, safety, and tolerability of an oral glutaminyl cyclase inhibitor varoglutamstat (PQ912) in study participants with MCI and mild AD-VIVIAD[J].Alzheimer's Research & Therapy,2021,13(1):142.
[23]. T. Hoffmann, J. Rahfeld, M. Schenk, et al.Combination of the Glutaminyl Cyclase Inhibitor PQ912 (Varoglutamstat) and the Murine Monoclonal Antibody PBD-C06 (m6) Shows Additive Effects on Brain Aβ Pathology in Transgenic Mice[J].International Journal of Molecular Sciences,2021,22(21)
[24]. W. Wang, Q. Zhou, T. Jiang, et al.A novel small-molecule PROTAC selectively promotes tau clearance to improve cognitive functions in Alzheimer-like models[J].Theranostics,2021,11(11):5279-5295.
[25]. M. Vaz and S. Silvestre.Alzheimer's disease: Recent treatment strategies[J].European Journal of Pharmacology,2020,887:173554.
[26]. M. Alavi, S. Fanoudi, M. Hosseini and H. Sadeghnia.Beneficial effects of levetiracetam in streptozotocin-induced rat model of Alzheimer's disease[J].Metabolic Brain Disease,2022,37(3):689-700.
[27]. K. Vossel, K. Ranasinghe, A. Beagle, et al.Effect of Levetiracetam on Cognition in Patients With Alzheimer Disease With and Without Epileptiform Activity: A Randomized Clinical Trial[J].JAMA neurology,2021,78(11):1345-1354.
[28]. C. Singh, K. Choi, L. Munro, et al.Reversing pathology in a preclinical model of Alzheimer's disease by hacking cerebrovascular neoangiogenesis with advanced cancer therapeutics[J].EBioMedicine,2021,71:103503.
[29]. O. Sanders.Sildenafil for the Treatment of Alzheimer's Disease: A Systematic Review[J].Journal of Alzheimer's Disease Reports,2020,4(1):91-106.
[30]. W. Zhang, M. Lv, Y. Shi, et al.Network Pharmacology-Based Study of the Underlying Mechanisms of Huangqi Sijunzi Decoction for Alzheimer's Disease[J].Evidence-based Complementary and Alternative Medicine : eCAM,2021,2021:6480381.









