1. Introduction
Adult Hippocampal Neurogenesis (AHN) is the process that involves the generation of new neurons from the division of stem and progenitor cells in the hippocampus of adult mammals [1]. Studies have suggested that the level of neurogenesis occurring in the hippocampus could be involved in the development of depression [2]. One reason for this may be due to the greater neuroplasticity and excitability shown by young neurons in comparison to mature neurons [3]. Our investigation examines this idea further by exploring the effect of altering the plasticity and excitability of young and mature neurons on depressive behaviour in mice. The outcome of our investigation may suggest the potential for analogous effects in humans and promote our understanding of how neurogenesis may be significant in human depression.
AHN occurs continuously in specific neurogenic regions located in the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the hippocampus throughout the lifespan of adult mammals [4], the SGZ being the area that our experiments will focus on. Adult neurogenesis generates granule neurons in the subgranular zone through the asymmetric division of radial glial stem cells [5], whose daughter cells then produce neuroblasts that move into the granule cell layer, where they undergo differentiation into granule neurons [6].
Adult neurogenesis has been shown to occur in many mammal species, including within the primate order, and is notably robust in rodents. Numerous studies have also presented findings supporting the occurrence of adult neurogenesis in the human hippocampus [1]. Although the precise role of adult neurogenesis remains unclear, past experiments that inhibited AHN led to behaviour impairments related to learning and memory, suggesting a role for newborn neurons in cognitive function [7]. Diminished neurogenesis has also been linked to the onset of anxiety and depression, although direct evidence is still needed to support this [2]. Several factors have been demonstrated to impact the rate of neurogenesis in the adult hippocampus. For example, stress often downregulates the process [4], whereas antidepressants such as fluoxetine [8] and stimulation of the amygdala can both upregulate the amount of neurogenesis taking place [9].
Since there is evidence that activating newborn adult neurons can achieve an antidepressant effect [8], our experiments aim to investigate the significance of neural plasticity and excitability in the apparent antidepressant effect of AHN by modifying these features of old and new neurons in mice. Young neurons generally have greater plasticity than old neurons, meaning they have a greater capacity to form Long Term Potentiation. This plasticity is influenced by the NMDA:AMPA receptor ratio, which is higher in young neurons. New neurons also have higher excitability – for example they are less constrained by GABAergic inhibition [3]; whereas old neurons have lower excitability – for example GIRK channels in mature dentate granule neurons lower their resting potential [10].
The prevailing assumption is that immature and plastic newborn neurons exert the most significant influence on behaviour [3]. One possible reason for this may be that new-born neurons, alongside astrocytes, can be incorporated into the pre-existing hippocampal neural circuitry. which has been shown to be necessary for recovery from stress and mood regulation [11].
In our investigation, we will reduce the plasticity of young neurons by reducing the number of NMDA receptors (thereby decreasing the NMDA:AMPA ratio). We will also increase the excitability of new and old neurons using Clozapine -N-Oxide (CNO), which is known to have this effect [8]. The consequence of these changes on depressive behaviour in mice will then be tested to shed further light on the significance of the plasticity and excitability of neurons in the hippocampus.
My hypothesis is that there will be a significant relationship between both plasticity and excitability on depressive behaviour in the mice. More specifically: reducing the plasticity of new neurons (and therefore making them behave more like old neurons) will increase depressive behaviour because mature neurons; increasing the excitability of new and old neurons will alleviate depressive behaviour; and increasing the excitability of new neurons will have a greater antidepressive effect than increasing the excitability of old neurons.
2. Methodology
2.1. Behavioural tests
Below are the behavioural tests that will be carried out to evaluate anxiety and depression-like behaviours of the mice:
2.2. Tail suspension test (TST)
Before the test, groups of mice are kept in a controlled environment with 12:12 light-dark cycles, along with access to food and water. In the test, the mouse’s tail will be attached to a clip at the top of a vertical structure, and the mouse will be suspended in a manner that prevents it from touching the floor or any other structure with its paws. The suspension typically proceeds for 6 minutes in a quiet and dimly lit room to minimise external disturbance. Each mouse will be tested individually in succession. The length of immobility time exhibited by the mouse will be recorded with a stopwatch. The immobility period is considered to represent a state of behavioural despair, and is therefore used as an indicator of depressive symptoms [8].
2.3. Open field test (OFT)
This test operates on rodents’ innate tendency to avoid bright areas and favour darkness. In the OFT, the mouse is transferred from its home cage to a novel and bright arena with enough space for free movement. The arena is divided into central and peripheral units, allowing for the recording of locomotion and rearing behaviour. Due to its photophobicity, the mouse would avoid the bright, open spaces, tending to remain in proximity to the walls. Exploratory or locomotive behaviour in relation to the distance from the walls will be assessed, as well as autonomic activities such as urination and defecation. An automated infrared beam array system is employed to measure locomotion, rearing, and the duration spent in distinct zones within the arena. However, it is worth nothing that this test is vulnerable to various internal and external factors [12].
2.4. Elevated-plus maze (EPM)
The EPM is constructed from black polypropylene and comprises two open arms and two closed arms that are raised 70 cm above the floor. In the center, there is a junction measuring 10 x 10 cm. Mice will be placed individually in the junction and allowed 5 minutes of free exploration. The total number of entries to different alleys will be counted, and the proportion of time spent in the open arms will serve as an indicator of anxiety. Mice with high anxiety would have fear-induced inhibition of open-alley exploration and therefore spend reduced time in the open arms of the maze [13].
2.5. Animals
All experiments will be performed accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, and all procedures are carried out in accordance with EU Directive 2010/63/EU and NIH guidelines on animal care and experimentation [8].
Two- months old naïve C57bl/6 male and female mice (200 – 250g) are housed three per cage and maintained under standard laboratory conditions (12 h light: 12 h dark cycles, 22˚C, relative humidity of 55%, ad libitum access to food and water) [11] for experiment 1 which is the knockout of NR1 gene. Groups of mice (n = 6 per group) are randomly assigned to 4 experimental groups: two of which will have their NR1 gene knocked out with CRISPR-Cas 9, and two control groups. For experiment 2, hM3Dq floxed mice are mated to mice containing tamoxifen-inducible Cre recombinase under the control of the Ascl1 promoter (Ascl1-CreERTM) to produce the transgenic progeny Ascl1- CreERTM;R26LSL−hM3Dq (+ hM3Dq) [8]. The progenies (n = 6 per group) are then randomly assigned to the following 4 experimental groups: +hM3Dq without NR1 gene + saline, +hM3Dq + saline, +hM3Dq without NR1 gene + CNO, +hM3Dq + CNO. The mice are genotyped with PCR using genomic DNA and primers, and the Cre and/or DREADD-negative littermates from heterozygote breeding are used as controls [8].
2.6. Experiment 1
To investigate whether the contrast in antidepressant effect of newborn adult neurons and mature neurons is caused by their difference in plasticity, the main factor affecting synaptic plasticity is considered to be the number of NMDA receptors present in the neurons. Previous research has indicated that augmenting the quantity of NMDA receptors may reduce the decline in synaptic plasticity [14], indicating that young neurons have more NMDA receptors and hence more plasticity compared to aged neurons. Therefore, by knocking out the gene that codes for the NMDA receptor on the newborn neurons, we can artificially induce mature neuron properties on the young ones in the hippocampus of the mice groups, then carry out behavioural tests to assess whether there is an increase or decrease in depressive behaviour and anxiety level.
The NMDA receptor complex consists of 4 subunits: two NR1, NR2 and NR3 [14]. By removing any one of these subunits, the functional receptor would not be produced, meaning the newborn neurons would have no NMDA receptors and thus behave like old neurons. Therefore to achieve this, we use CRISPR-Cas 9 to knockout the gene that encodes for the NR1 subunit by customising the guide RNA specific to the gene sequence, while the Cas-9 nuclease creates a double-stranded break and the DNA itself disrupts the gene of interest by non-homologous end joining [15].
To determine whether the knockout was successful, after CRISPR-Cas 9 has knocked out the gene we dissect the mouse to obtain the target neurons and lyse them, then Western blot analysis is performed. If the protein band for NMDA receptor isn’t present, then a successful knockout of the gene can be determined which will allow us to carry out the formal experiment on the 4 experimental mice groups. 2 groups will be injected with viral vectors with modified genes, and the 2 control groups will be injected with 0.9% saline. Behavioural tests are carried out 24 hours after injection during light hours.
2.7. Result prediction
The prediction is that the two experimental groups would show a greater extent of depressive behaviour compared to the two control groups.
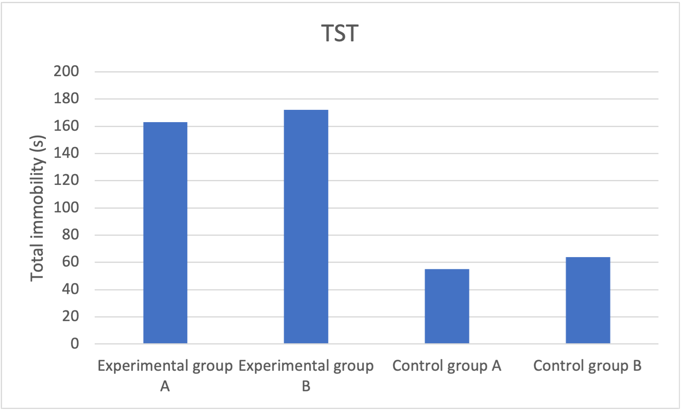
Figure 1a. Decreased neuroplasticity effect on tail suspension test predicted results
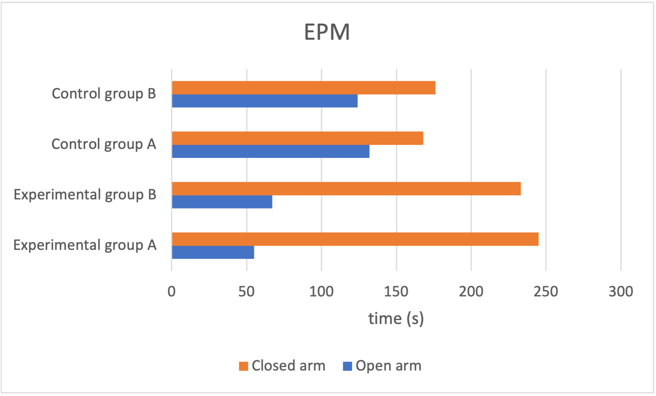
Figure 1b. Decreased neuroplasticity effect on elevated plus maze predicted results
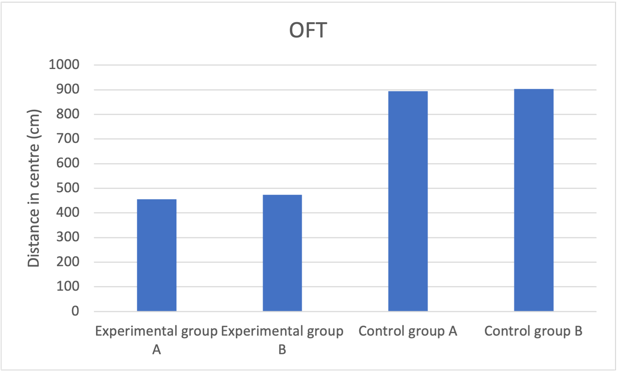
Figure 1c. Decreased neuroplasticity effect on open field test predicted results
Fig. 1 Reducing number of NMDA receptors in newborn neurons induces increased depressive behaviour and anxiety level. a Mice group with reduced neuroplasticity had longer freeze time, indicating increased anxiety compared to the control group. b Experimental group mice spent much longer in the closed arm with proportion to time spent to open arms compared to control groups, showing higher prevalence of depressive behaviour. c Experimental groups with reduced neuroplasticity exhibited shorter distance in centre, suggesting an inclination to stay closer to the walls, which shows heightened depressive behaviour.
Reduced number of NMDA receptors may result in heightened depressive behaviour due to less Long term potentiation (LTP) will be able to form and therefore the neurons would be less active. This would mean that decrease in neuroplasticity leads to an increase in anxiety and depression, implicating that any difference in antidepressant effect between newborn and mature neurons may also be influenced by their difference in plasticity. When all the new neurons have their NMDA receptor gene knocked out, all the neurons in the mouse’s hippocampus would be behaving like mature neurons, which is mimicking the phenomenon that happens when all animals age. Past studies found that neurogenesis declines with age in both humans and animals, meaning less and less newborn neurons will be generated as we age, leaving mainly mature neurons making up the neuronal population, while the prevalence of depression tends to increase with age [9]. This consolidates the idea that newborn neurons in the adult brain are necessary for regulating mood and are essential for the effectiveness of antidepressant treatments [9], and hence also the crucial role of adult neurogenesis in mood regulation.
2.8. Experiment 2
The aim of this experiment is to investigate whether activating neurons with reduced plasticity (i.e. old neurons) would also have an antidepressant effect like newborn neurons. We first cross the hM3Dq floxed mice with mice containing tamoxifen-inducible Cre recombinase to produce the transgenic progeny +hM3Dq mouse line, so upon injection of tamoxifen the Cre protein would remove unwanted genes and lead to the expression of the DREADD protein, which would activate neurons in the dentate gyrus when treated with Clozapine-N-oxide (CNO) [8].
4 experimental +hM4Dq mice groups (n = 6) will be used, 2 of which will have their NR1 gene knocked out with CRISPR-Cas 9, meaning they are artificially made-mature neurons. When the mice are 2 months old, inject 180 mg kg-1 tamoxifen intraperitoneally for 5 continuous days, while the control groups receive saline injection in the same time course. 2 hours before the behavioural tests, inject 2mg kg-1 CNO to the 2 experimental groups and 0.9% saline to the 2 control groups. CNO injection activates the DREADD tagged neurons and increases their excitability, which leads to increased firing of the neurons and therefore should decrease depression and anxiety in the behavioural tests, the results from which will be statistically analysed and compared.
Statistical analyses are done using SPSS software (SPSS, Chicago, IL, USA) [11]. Once the homogeneity of data is confirmed, statistical analyses are conducted. Behavioural results are assessed using one-way analysis of variance (ANOVA), with F-values and P-values derived from the between-group ANOVA analysis. Differences between groups are determined through Bonferroni’s post-hoc multiple comparison test, and the corresponding P-values are presented. In cases where appropriate, a t-test is utilized to evaluate differences between two groups. Statistical significance is considered when P<0.05 [11].
2.9. Result prediction
Both experimental groups that had CNO treatment would show lower levels of anxiety and depression compared to the control groups, and the mice group with a complete set of genes (with intact NMDA receptor gene) present greater antidepressant effects upon CNO treatment compared to the mice group with made-old neurons.
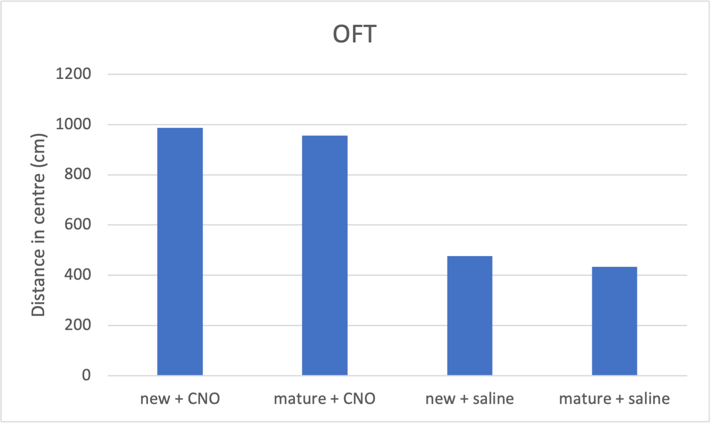
Figure 2a. Increasing neuron excitability on open field test predicted result
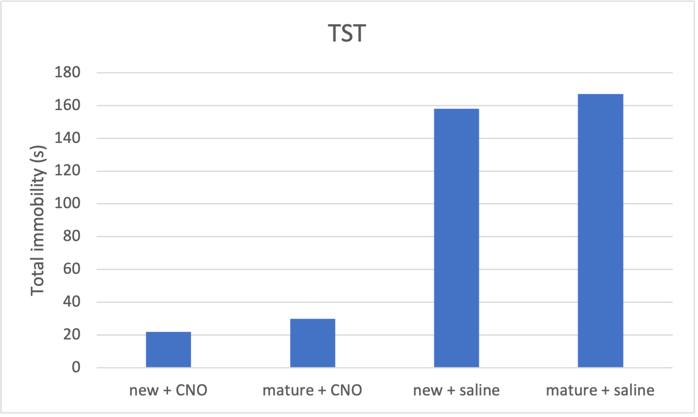
Figure 2b. Increasing neuron excitability on tail suspension test predicted result
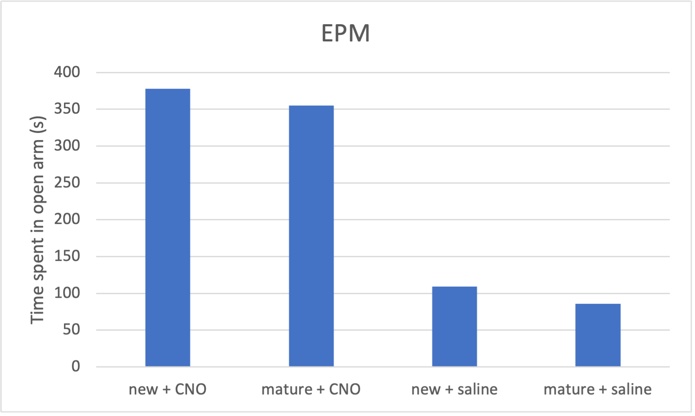
Figure 2c. Increasing neuron excitability on elevated plus maze predicted result
Fig. 2 CNO treatment induces an antidepressant effect, which is more prominent in newborn neurons compared to mature neurons. a Activating both mature and new neurons caused corresponding mice groups to spent longer distance in centre, indicating reduced depressive behaviour. Mice with activated newborn neurons show less anxiety compared to mature neurons. b Both mature and newborn neurons with increased excitability induced decrease in total immobility in mice, showing an antidepressant effect. Mice with activated newborn neurons exhibited even shorted immobility time than mature ones. c Increasing mature and newborn neuron excitability increase time spent in the open arms in elevated plus maze by mice groups, more so in the newborn neuron group.
Therefore I predict that activating mature neurons would also have an antidepressant effect, however not as robust as newborn neurons due to decreased plasticity and lower threshold for LTP. If activating mature neurons result in no positive effects in alleviating depressive behaviour, possible reasons may include a) Aged neurons may have experienced prolonged oxidative stress and inflammatory responses, leading to cellular damage and decreased function. b) The activity-enhancing methods used in the experiments may not be appropriate for aged neurons or may lead to abnormal activation of neural circuits causing the opposite effect. c) Older neurons having already integrated into the neural circuit may find it more difficult adapting to new environments or respond to stimuli, affecting their antidepressant effect when activated.
3. Limitations
The main limitation throughout the experiments is that the difference in number of NMDA receptors must not be the only characteristic that distinguishes between mature and newborn adult neurons. However it is not possible to artificially include all the factors that make a neuron old, due to the complexity of natural biological growth and other external influences such as inflammation or infections. Therefore we have included a supplementary experiment that can be carried out to overcome this limitation:
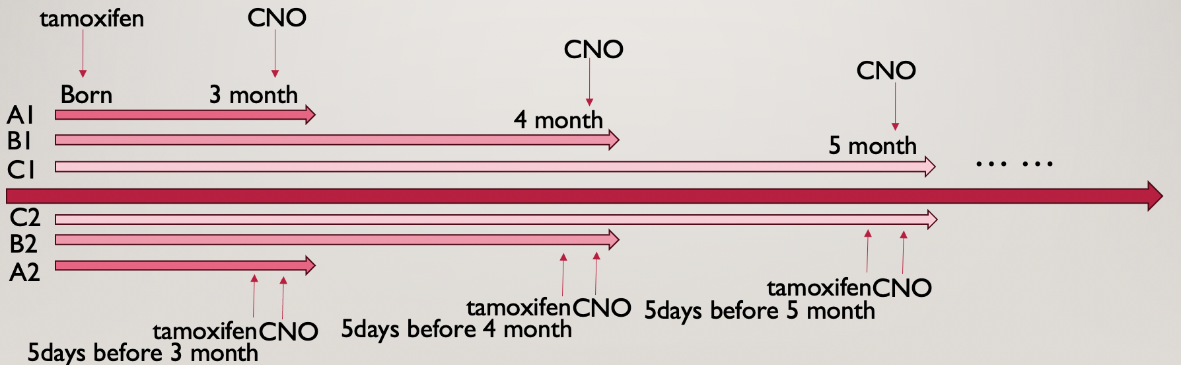
Figure 3. Alternative experiment timeline
As fig 3 shows, this experiment is carried out throughout the course of 6 months, starting from when the mice are 3 months old, inject tamoxifen and apply CNO treatment every month followed by the behavioural tests. This allows the neurons to age naturally and the antidepressant effects during different age stages can be assessed and compared. The groups can be compared horizontally, which is comparing the ability of neurons to regulate depression at different ages; or it can be compared vertically, which compares the ability of old vs. new neurons in alleviating depression and anxiety.
Additional limitations arise from variations in the levels of stress and fear induced by different behavioural tests. These variations can activate distinct subsystems of the neurogenic interactome, potentially introducing interference with the final results [9]. However, since all mice groups undergo the same experiments and tests, the slight impact that this has can be ignored. Furthermore, neuronal morphology is found to vary substantially, even within the same animal [3]. Therefore, the antidepressant effects of newborn and mature neurons may vary from mouse to mouse and might not be sufficient to draw a conclusion for all rodents or even humans.
4. Conclusion
To summarise, our first experiment tested the importance of neuroplasticity in inducing depressive behaviour by knocking out the NR1 gene that encodes for the NMDA receptor, which gave young neurons mature neuron properties, reducing their plasticity. The prediction is that decreased neuroplasticity would lead to increased depressive behaviour, which would highlight the importance of newborn adult hippocampal neurons in mood regulation.
Our second experiment used CNO to increase the excitability of both newborn and artificially made-mature neurons to evaluate which one would have a more significant antidepressant effect. Presumably the newborn neurons would have a greater effect in alleviating anxiety and depression since they have more impact on behaviour, according to Cole, Espinueva et al., 2020, and more plasticity.
Therefore, the distinctive features of newborn neurons and their clinical values can be further studied in application to future depression treatment and in academia.
References
[1]. Cope, E.C. and Gould, E. (2019) ‘Adult neurogenesis, glia, and the extracellular matrix’, Cell Stem Cell, 24(5), pp. 690–705. doi:10.1016/j.stem.2019.03.023.
[2]. Snyder, J. S., Soumier, A., Brewer, M., Pickel, J., & Cameron, H. A. (2011, August). ‘Adult hippocampal neurogenesis buffers stress responses and depressive behaviour’, Nature, 476(7361), pp. 458–461. doi:10.1038/nature10287.
[3]. Cole, J. D., Espinueva, D. F., Seib, D. R., Ash, A. M., Cooke, M. B., Cahill, S. P., O’Leary, T. P., Kwan, S. S., & Snyder, J. S. (2020, June 22). ‘Adult-born hippocampal neurons undergo extended development and are morphologically distinct from neonatally-born neurons’, The Journal of Neuroscience, 40(30), pp. 5740–5756. doi:10.1523/jneurosci.1665-19.2020.
[4]. Zhao, C., Deng, W. and Gage, F.H. (2008) ‘Mechanisms and functional implications of adult neurogenesis’, Cell, 132(4), pp. 645–660. doi:10.1016/j.cell.2008.01.033.
[5]. Seri B, García-Verdugo JM, McEwen BS, and Alvarez-Buylla A (2001). Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci 21, 7153–7160. [PubMed: 11549726]
[6]. Gonçalves JT, Schafer ST, and Gage FH (2016a). Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 167, 897–914. [PubMed: 27814520]
[7]. Cameron, H.A. and Glover, L.R. (2015) ‘Adult neurogenesis: Beyond learning and memory’, Annual Review of Psychology, 66(1), pp. 53–81. doi:10.1146/annurev-psych-010814-015006.
[8]. Tunc-Ozcan, E., Peng, C. Y., Zhu, Y., Dunlop, S., Contractor, A., & Kessler, J. A. (2019). Activating newborn neurons suppresses depression and anxiety-like behaviors’, Nature Communications, 10(1). doi:10.1038/s41467-019-11641-8.
[9]. Eisch, A.J. and Petrik, D. (2012) ‘Depression and hippocampal neurogenesis: A road to remission?’, Science, 338(6103), pp. 72–75. doi:10.1126/science.1222941.
[10]. Hansen, J. (2018, August 10). An ion channel differentiates newborn and mature neurons in the adult brain. ScienceDaily.
[11]. Mateus-Pinheiro, A., Pinto, L., Bessa, J., Morais, M., Alves, N. D., Monteiro, S., Patrício, P., Almeida, O. F. X., & Sousa, N. (2013). Sustained remission from depressive-like behavior depends on hippocampal neurogenesis. Translational Psychiatry, 3(1). doi:10.1038/tp.2012.141.
[12]. Fuchs, E. and Flügge, G. (2006) ‘Experimental animal models for the simulation of depression and anxiety’, Dialogues in Clinical Neuroscience, 8(3), pp. 323–333. doi:10.31887/dcns.2006.8.3/efuchs.
[13]. Bessa, J.M. (2009) ‘A trans-dimensional approach to the behavioral aspects of depression’, Frontiers in Behavioral Neuroscience, 3. doi:10.3389/neuro.08.001.2009.
[14]. Magnusson, K.R. (2012) ‘Aging of the NMDA receptor: From a mouse’s point of View’, Future Neurology, 7(5), pp. 627–637. doi:10.2217/fnl.12.54.
[15]. Khurana, A., Sayed, N., Singh, V., Khurana, I., Allawadhi, P., Rawat, P. S., Navik, U., Pasumarthi, S. K., Bharani, K. K., & Weiskirchen, R. (2022, September 21) ‘A comprehensive overview of CRISPR/Cas 9 technology and application thereof in Drug Discovery’, Journal of Cellular Biochemistry, 123(10), pp. 1674–1698. doi:10.1002/jcb.30329.
Cite this article
Lai,J. (2024). The importance of neuroplasticity and excitability of adult-born neurons in alleviating depression and anxiety-like behaviour in mice. Theoretical and Natural Science,46,1-8.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Cope, E.C. and Gould, E. (2019) ‘Adult neurogenesis, glia, and the extracellular matrix’, Cell Stem Cell, 24(5), pp. 690–705. doi:10.1016/j.stem.2019.03.023.
[2]. Snyder, J. S., Soumier, A., Brewer, M., Pickel, J., & Cameron, H. A. (2011, August). ‘Adult hippocampal neurogenesis buffers stress responses and depressive behaviour’, Nature, 476(7361), pp. 458–461. doi:10.1038/nature10287.
[3]. Cole, J. D., Espinueva, D. F., Seib, D. R., Ash, A. M., Cooke, M. B., Cahill, S. P., O’Leary, T. P., Kwan, S. S., & Snyder, J. S. (2020, June 22). ‘Adult-born hippocampal neurons undergo extended development and are morphologically distinct from neonatally-born neurons’, The Journal of Neuroscience, 40(30), pp. 5740–5756. doi:10.1523/jneurosci.1665-19.2020.
[4]. Zhao, C., Deng, W. and Gage, F.H. (2008) ‘Mechanisms and functional implications of adult neurogenesis’, Cell, 132(4), pp. 645–660. doi:10.1016/j.cell.2008.01.033.
[5]. Seri B, García-Verdugo JM, McEwen BS, and Alvarez-Buylla A (2001). Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci 21, 7153–7160. [PubMed: 11549726]
[6]. Gonçalves JT, Schafer ST, and Gage FH (2016a). Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 167, 897–914. [PubMed: 27814520]
[7]. Cameron, H.A. and Glover, L.R. (2015) ‘Adult neurogenesis: Beyond learning and memory’, Annual Review of Psychology, 66(1), pp. 53–81. doi:10.1146/annurev-psych-010814-015006.
[8]. Tunc-Ozcan, E., Peng, C. Y., Zhu, Y., Dunlop, S., Contractor, A., & Kessler, J. A. (2019). Activating newborn neurons suppresses depression and anxiety-like behaviors’, Nature Communications, 10(1). doi:10.1038/s41467-019-11641-8.
[9]. Eisch, A.J. and Petrik, D. (2012) ‘Depression and hippocampal neurogenesis: A road to remission?’, Science, 338(6103), pp. 72–75. doi:10.1126/science.1222941.
[10]. Hansen, J. (2018, August 10). An ion channel differentiates newborn and mature neurons in the adult brain. ScienceDaily.
[11]. Mateus-Pinheiro, A., Pinto, L., Bessa, J., Morais, M., Alves, N. D., Monteiro, S., Patrício, P., Almeida, O. F. X., & Sousa, N. (2013). Sustained remission from depressive-like behavior depends on hippocampal neurogenesis. Translational Psychiatry, 3(1). doi:10.1038/tp.2012.141.
[12]. Fuchs, E. and Flügge, G. (2006) ‘Experimental animal models for the simulation of depression and anxiety’, Dialogues in Clinical Neuroscience, 8(3), pp. 323–333. doi:10.31887/dcns.2006.8.3/efuchs.
[13]. Bessa, J.M. (2009) ‘A trans-dimensional approach to the behavioral aspects of depression’, Frontiers in Behavioral Neuroscience, 3. doi:10.3389/neuro.08.001.2009.
[14]. Magnusson, K.R. (2012) ‘Aging of the NMDA receptor: From a mouse’s point of View’, Future Neurology, 7(5), pp. 627–637. doi:10.2217/fnl.12.54.
[15]. Khurana, A., Sayed, N., Singh, V., Khurana, I., Allawadhi, P., Rawat, P. S., Navik, U., Pasumarthi, S. K., Bharani, K. K., & Weiskirchen, R. (2022, September 21) ‘A comprehensive overview of CRISPR/Cas 9 technology and application thereof in Drug Discovery’, Journal of Cellular Biochemistry, 123(10), pp. 1674–1698. doi:10.1002/jcb.30329.









