1. Introduction
Synaptic plasticity is a crucial aspect of the mammalian brain, allowing neural activity from experiences to modify circuit function and influence subsequent thoughts, feelings, and behavior. It involves the activity-dependent modification of synaptic transmission strength, enabling the brain to incorporate transient experiences into long-lasting memory traces, a process vital for memory formation.
There are many epidemiological studies on the relationship between degenerative diseases and metabolic diseases like obesity and diabetes. Animal models using high-fat diets, leptin deficiency, and genetic modifications have shown impaired synaptic function and accelerated cognitive decline associated with metabolic diseases. The mechanisms underlying the impaired synaptic function in metabolic diseases are complex. Increased apoptosis decreased levels of neurotrophic factors and reduced synaptic complexity in specific regions of the hippocampus are consistently observed in models of metabolic disease.
Hormones involved in metabolic diseases have been proven to influence synaptic function. Insulin resistance, often associated with metabolic diseases, may play a causal role in the impaired long-term potentiation (LTP). Insulin and insulin-like growth factors (IGF1 and IGF2) are crucial for hippocampal synaptic plasticity, adult neurogenesis, memory, and learning. Insulin and IGFs promote synaptic plasticity through various signaling pathways. Leptin, an adipokine involved in appetite regulation, also plays a role in protecting hippocampal neurons and stimulating dendrite growth.
As an upstream factor affecting metabolic diseases, diets can influence the synaptic plasticity of hypothalamic neuronal populations either through direct effect due to macronutrients or via peripheral appetite signals. Indeed, understanding the causal relationship between diet and synaptic function is essential, given the increasing prevalence of metabolic diseases and the potential improvements towards cognition and memory brought by healthy diets. Based on current research, the molecular mechanism of macronutrients in neurons are multifaceted. Thus, further research is needed to gain a deeper understanding of the mechanisms involved and explore interventions to synaptic dysfunction based on these mechanisms.
2. Previous Research
For long time, SFA has been believed as unhealthy and impedes cognition, yet recently increasing research papers point out that SFA benefit memory and learning under specific conditions. A previous research paper observed that, contrary to that worse episodic memory is associated with increasing amount of SFA among older people, young people perform better in the recollection test under high-level SFA [1]. SFA may thus contributes to brain function and development, while the excessive health risks caused by high-level SFA in older people outweigh its benefits. In particular, a diet high in SFA can increase the risk of cardiovascular disease and metabolic disorders, indirectly leading to inflammation [2]. Nevertheless, despite the health risks of SFA, it may protect brain against neural degeneration by reducing neuroinflammation under low-level intake. Lauric acid has recently been reported to have neuroprotection in neuroinflammatory degenerative diseases; caprylic acid can ameliorate rotenone induced inflammation; other SFA such as myristic acid and stearic acid also possess potential anti-inflammatory abilities [3]. In this way, SFA leads to better overall cognitive performance by reducing neuroinflammation.
According to previous research, we speculate that such anti-neuroinflammatory effect of SFA may be achieved through JAK2/STAT3 signaling pathway. Caprylic acid, a SFA found in palm and coconut oil, is involved in inhibiting inflammation through upregulation of the ABCA1/p-JAK2/p-STAT3 pathways [4]. Mice fed with caprylic acid-added diet has proven to have higher mRNA and protein expression levels of JAK2 and STAT3, with declined level of multiple inflammatory factors.
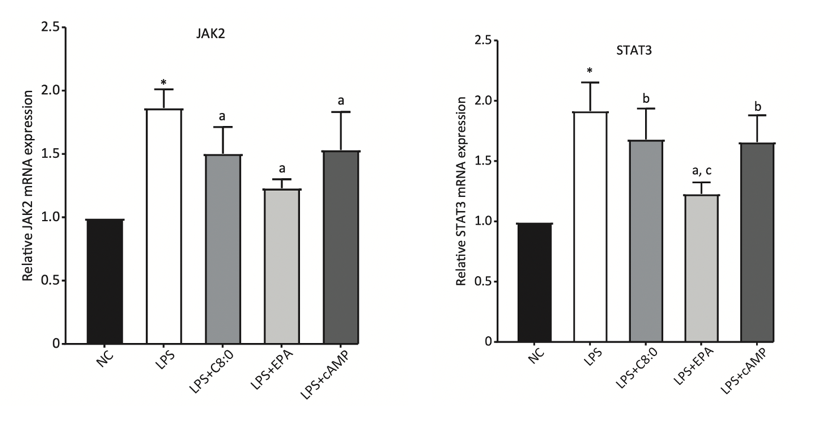
Figure 1. Relative JAK2 and STAT3 mRNA expression levels in C57BL/6 mice fed with different high-fat diets and normal diet (NC). C8:0 refers to 2% caprylic acid feed [5].
In fact, considering previous research on the modulation of synaptic plasticity through leptin signaling, there exist some similarities. Activation of leptin receptors causes following phosphorylation of JAK2 and dimerization along with translocation of STAT3, which phosphorylates NM2B, a subunit of NMDA receptor that is crucial to synaptic plasticity, and finally activates NMDA receptor, in turn promoting persistent changes in excitatory synaptic plasticity, usually in form of long-term potentiation (LTP). Within this procedure, JAK2/STAT3 cascade is a crucial component. Since caprylic acid can increase the production of signaling molecules within this cascade, it is reasonable to hypothesize that caprylic acid can enhance leptin signaling by increasing JAK2/STAT3 expression, leading to more NM2B phosphorylation and thus strengthening synaptic function.
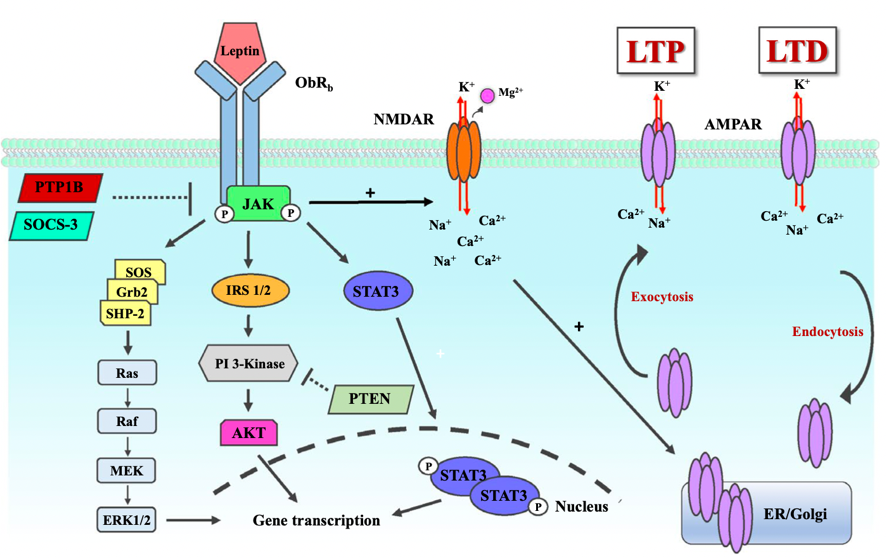
Figure 2. Leptin receptor signaling pathway [6].
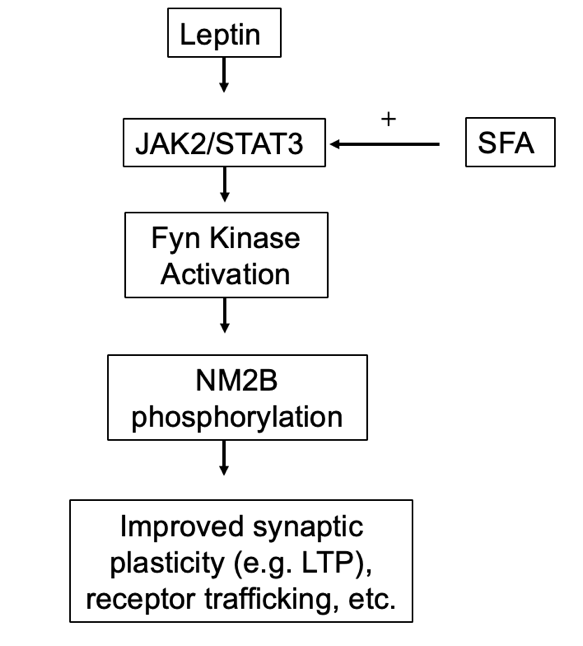
Figure 3. Speculated mechanism of SFA’s role in leptin regulation of synaptic function.
3. Methodology
To study the effect of caprylic acid on leptin/NR2B signaling pathway, experimental group and control group are first set. In the experimental group, C57BL/6 mice will be fed with 2% caprylic acid diet, having higher calories than the other group; in the control group, C57BL/6 mice will be fed with standard rodent chow. C57BL/6 mice from both groups will be fed with standard rodent chow for the first 8 weeks after birth and will be randomized into the experimental group and control group afterwards.
Then, mouse types in each group are determined. The widely used C57BL/6 C57BL/6 mice will be applied in this experiment. Male C57BL/6 mice are used because they have relative stable levels of hormones, which can affect the result, and they have lower amount of estrogen, which can directly influence the CRE protein.
The C57BL/6 mice will have three types: \( {LepR^{+}} \) (C57BL/6 mice with CRE-loxP system and no tamoxifen injection), \( {LepR^{-}}(C57BL/6 mice with CRE-loxP system and tamoxifen injection) \) , \( a \) nd WT (Wild -type C57BL/6 mice). The method of constructing CRE-loxP C57BL/6 mice models, as shown in figure 4, will be explained later. By comparing the performance of \( {LepR^{+}} \) and \( {LepR^{-}} \) C57BL/6 mice in experimental and control trials respectively, whether caprylic acid affects synaptic function through enhancing leptin, independently, or in both ways can be known. This will be explained more detailed in the Expected Outcomes. Also, the performance of WT C57BL/6 mice acted as a control group that tests the potential irrelevant variables, if any, brought by CRE-loxP gene editing beside the expression of leptin receptors; In theory, there shouldn’t be significant statistical difference between the performance of WT and \( {LepR^{+}} \) C57BL/6 mice (p<0.05). Sample size will be determined by power analysis to meet the 3R criteria; in previous research on the effect of caprylic acid on C57BL/6 mice brain, the sample sizes are mostly set between 5~10, so in this experiment, the sample size in each trial is set as 10 C57BL/6 mice. Consequently, there will be six trials and total of 60 C57BL/6 mice.
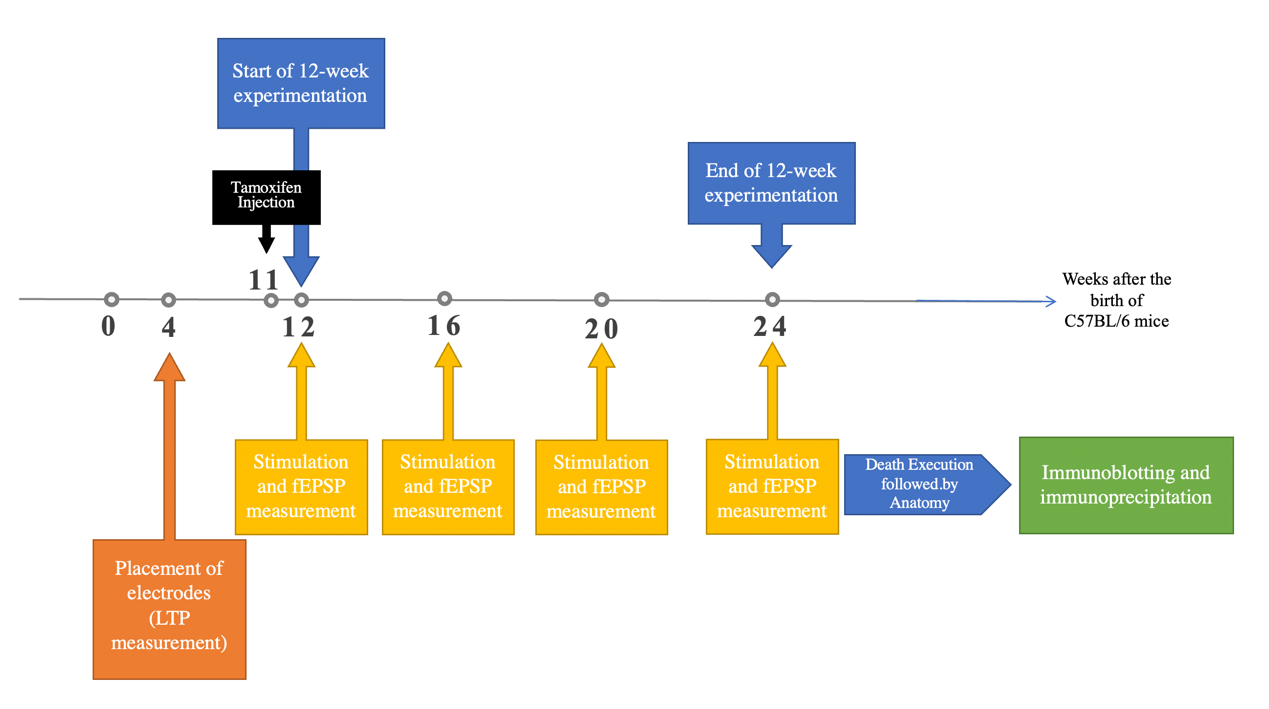
Figure 4. Timeline of the Experiment.
3.1. Transgene Construction and Transgenetic C57BL/6 mice Production
LepRb subtype of leptin receptors is chosen to be controlled through CRE-loxP system. The CRE-loxP system is a genetic tool that uses specific DNA sequences (loxP sites) and the enzyme CRE recombinase to perform DNA-editing. It allows for precise gene insertion, deletion, or rearrangement, enabling controlled and targeted genetic modifications in research. This is because LepRb [7] mRNA is primarily expressed in the dentate gyrus, which is spatially concentrated at the desired position. For promoter, Prox1 gene is chosen: it is known to be highly expressed in the dentate gyrus granule cells, which are a major neuronal population within the dentate gyrus region of the hippocampus. The Prox1 promoter has been widely used to drive gene expression specifically in dentate gyrus granule cells in C57BL/6 mice. Furthermore, with artificial injection of tamoxifen, the time of inhibition of LepRb can be decided precisely. Therefore, CRE-loxP system allows spatiotemporal operations on LepRb gene.
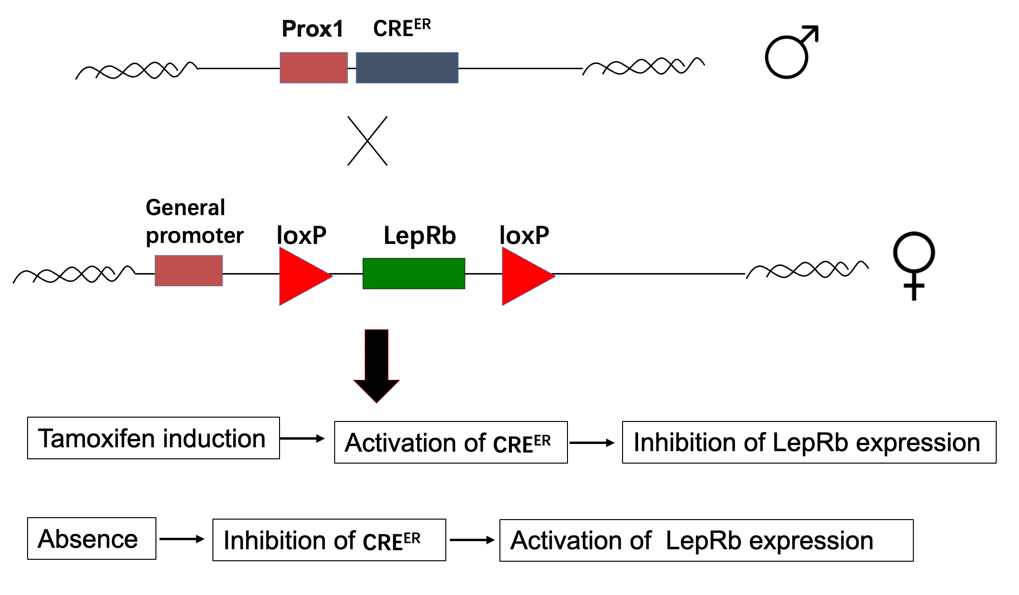
Figure 5. Simple illustration of the preparation of C57BL/6 mice with LepR-specific CRE-loxP system and the control of the LepR expression through tamoxifen.
Tamoxifen will be injected to induce inhibition of LepRb expression. Two methods of injection [8], subcutaneous injection and intraperitoneal injection, will be tested through pretrial. The one that causes less harm to C57BL/6 mice and help to achieve stable inhibition of LepRb expression will be used in the formal experimentation. Tamoxifen injection will be done one week ahead of the experiment, with injection of four consecutive days. Also, before the formal experimentation, patting and mock injection will be done to let C57BL/6 mice get used to surrounding environment and needles.
3.2. Leptin and serum lipid levels
The purpose of this measurement is to prove that the leptin and serum lipid levels of all C57BL/6 mice fell into a narrow range, so that self-produced leptin and serum lipid levels are standardized and thus won’t affect the experimentation.
The levels of serum lipid were determined using commercial kits. Blood serum of all C57BL/6 mice is collected first. Serum triglycerides (TG) (Wako,97Osaka, Japan), and HDL-C and LDL-C (Abcam, Cambridge, UK) were measured with commercial kits, and the ratio of HDL-C to LDL-C was subsequently calculated. To be more specific, TG will be measured through GPO-HMMPS, a method for triglyceride measurement. It involves the elimination of free glycerol and the conversion of triglycerides to dihydroxyacetone phosphate and hydrogen peroxide, which react with HMMPS and 4-aminoantipyrine to form a blue pigment for quantification. Also, the ratio of HDL-C to LDL-C will be determined through fluorometric detection method, which utilizes cholesterol oxidase to convert free cholesterol into a reactive compound that reacts with a probe to generate color and fluorescence.
Blood was collected at the end of study after 12 weeks and processed to measure serum leptin level using mouse leptin (no. EZML-82K, Millipore Sigma) ELISA kits, respectively, where leptin in the sample will bind to specific antibodies on a plate and thus enables fluorometric detection for analysis.
For procedural repetition, this measurement will be repeated for three times on each C57BL/6 mice brain slice.
3.3. fEPSP Measurements
The LTP of C57BL/6 mice is measured through fEPSP under live situation, by fixing electrodes in live C57BL/6 mice’s brains. By comparing the result between C57BL/6 mice fed with standard rodent chow and 2% caprylic acid, it can be determined whether caprylic acid diet can influence synaptic plasticity in the end.
Permanent monopolar recording electrode will be fixed for detecting responses of perforant path-dentate gyrus (DG) and hippocampal CA3. The placement of electrodes will be done to C57BL/6 mice with surgery under anesthetized condition between postnatal days 109 and 120. Electrodes will be placed both in the DG and CA3 region. Before the surgery, C57BL/6 mice will familiarize in the environment for at least one week. After the surgery, C57BL/6 mice will be given antibiotics and analgesics. Stimulation of the lateral and medial perforant paths produced monosynaptic responses in the DG and CA3, which were confirmed through decreased field excitatory postsynaptic potential (EPSP) slopes and paired pulse facilitation.
To measure responses, awake and behaving C57BL/6 mice will be subjected to constant current biphasic pulses. Input/output curves were collected to determine the current intensities that evoked field EPSPs at 50% of the maximal response. Response magnitudes were calculated by determining the slope of the field EPSP. C57BL/6 mice’s responses will be collected at 0, 4, 8, and 12-weeks after the beginning of the 12-week-experimentation. Each time, after 15 minutes of daily baseline responses, LTP will be induced by delivering five “theta burst” stimulation trains. The responses will be collected for an additional hour after the tetanus.
Data analysis involved normalizing and presenting the percent change in field EPSP slopes relative to baseline responses collected before tetanus. The magnitude of LTP was presented as the percent change in field EPSP slopes compared to baseline responses collected three days before tetanus.
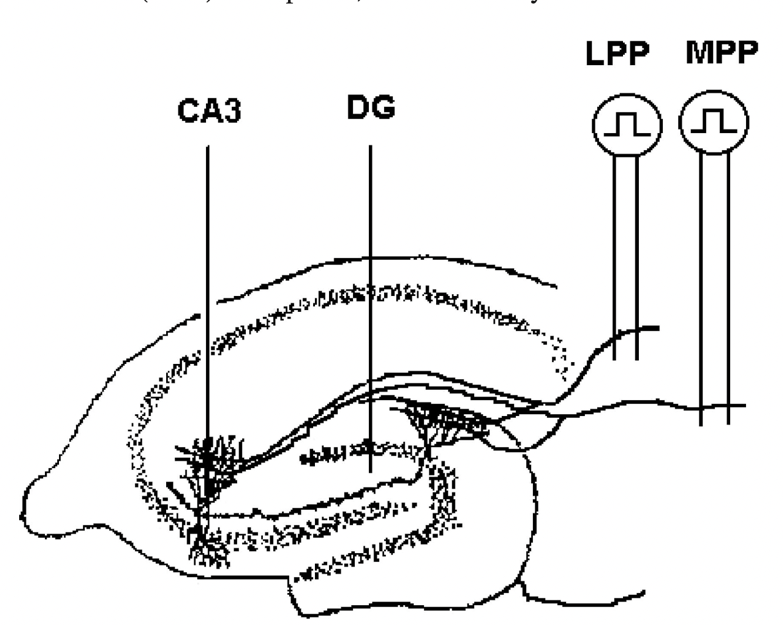
Figure 6. Electrode placement for perforant path for DG and CA3 responses [9].
3.4. Immunoblotting and Immunoprecipitation
Immunoblotting of LepRb, JAK2, and STAT3 are performed for quantifying the expression levels of these signaling molecules in all groups of C57BL/6 mice. Comparing the LepRb expression level between experimental groups and control groups can help to affirm the effectiveness of CRE-lop system in controlling the presence of LepRb ( \( {LepR^{-}} \) C57BL/6 mice should not have LepRb expression). Moreover, according to previous research, JAK2 and STAT3 level will increase in C57BL/6 mice fed with additional caprylic acid [10], so the immunoblotting of JAK2 and STAT3 will aid validation, reassuring caprylic acid’s role in enhancing leptin/NR2B signaling by increasing the amount of JAK2 and STAT3.
DG granular cell layer of all C57BL/6 mice will be collected and pre-treated. LepRb will be immunoprecipitated by Protein A/G agarose beads (Santa Cruz) pre-incubated with the correct amount of anti-LepRb antibody (1:500; no. ab5593, abcam), overnight at 4 °C [11]. In similar manners, immunoblotting of JAK2 and STAT3 will be done with their specific antibodies. The relative quantity of signaling molecules can bind to enzyme-linked secondary antibodies that can catalyze a colorimetric or chemiluminescent reaction, where the fluorescent signal can be quantified with specialized instruments.
For procedural repetition, this measurement will be repeated for three times on each C57BL/6 mice brain slice.
Immunoprecipitation will be done to p-JAK2, p-STAT3, and p-NM2B in DG granular cell layer with C57BL/6 mice brain slices. These assays are designed to test the influence of caprylic acid on specific signaling molecules participating in the leptin/NR2B pathway. With immunoprecipitation, the expression level of the above signaling molecules can be roughly compared between different trials; also, the interaction of these molecules can be studied by finding the overlapping fluorescent dots representing the presence of these molecules after staining. This uncovers the exact molecular mechanism of caprylic acid.
DG granular cell layer of all C57BL/6 mice will be collected. Proteins were separated by SDS–polyacrylamide gel electrophoresis and incubated with monoclonal antibodies to phosphe-JAK2 (1:1,000; no. 3771, Cell Signaling Technology), phospho-STAT3 (1:1,000; no. 9131, Cell Signaling Technology), and phospo-NR2B (1:1,000; no. 5355, Cell Signaling Technology).
For procedural repetition, immunoprecipitation experiment will be repeated for three times on each C57BL/6 mice brain slice.
4. Expected Results
Overall, it is expected that caprylic acid diet can strengthen synaptic plasticity through enhancing leptin/NR2B signaling. Specific expectations are discussed in the following passage: First, comparing the experiment group (caprylic acid diet) and control group, the experimental group should have higher JAK2, STAT3, p-JAK2, and p-STAT3 expression levels, as the leptin/NR2B signaling pathway is enhanced. Second, the experimental group may have greater changes in the fEPSP slope, bearing better LTP. Third, before the experiment, C57BL/6 mice from both groups should have similar serum lipid levels and leptin levels; it is assumed that these variables should be relatively constant in both groups.
5. Discussion
Admittedly, there are limitations in the methodology design, yet they are detailly addressed, as explained in the following passage:
First, the effective doses of tamoxifen injection may not correspond with previous experiment, as this is a novel CRE-loxP system. Therefore, pilot experiment will be done to determine the effective dose of tamoxifen injection. In particular, 0, 1, 10, 100, 200, 300 mg/kg/day will be tested on C57BL/6 mice for four consecutive days, and then the mice will be executed and tested the presence of LepRb with immunoprecipitation to see the effectiveness of tamoxifen in cutting off LepRb gene.
Second, another limitation is due to the self-produced leptin level in mice. Currently, this factor is assumed to be statistically insignificant (p<0.5) after controlling the mice’ diet and their subspecies. However, since the precise quantitative relationship between leptin and strength of leptin/NR2B signaling pathway is unknown, if the C57BL/6 mice is highly sensitive to leptin compared to caprylic acid, the influence of self-produced leptin level can be the main factor that affects the experiment result. Therefore, before the experiment, the fluctuation in self-produced leptin of all C57BL/6 mice will be assessed through T-test, and the fEPSP measurements may also be taken and analyzed through T-test. Also, if the fluctuation is too large, we may consider inhibiting the self-production of leptin in mice and artificially inject leptins regularly.
Third, the repetition in theta burst stimulation during fEPSP measurement can influence the experiment. Prolonged or excessive stimulation can potentially lead to tissue damage in the neural circuit under study. We will take precautions to ensure that the stimulation parameters used are within safe limits to minimize any potential harm.
Overall, the outcomes can contribute to the research problem by revealing the possible signaling mechanism of caprylic acid in enhancing leptin/NR2B. The experiment can reflect the interaction between caprylic acid and important signaling molecules in leptin/NR2B signaling pathway, such as JAK2 and STAT3. In this way, a detailed signaling mechanism of caprylic acid can be known.
6. Contribution To The Field Of Knowledge
Currently, the influence of macromolecules on brain function, in this case synaptic function and adult neurogenesis, lacks research. There can be contradictory relationship between these macromolecules and brain functions, especially for SFA, a formally believed totally unhealthy component that has been recently revealed to have some benefits in brain function. This research will continue this direction and reveal more possible influence of SFA on brain and health.
7. Conclusion
This proposal proposed a research on the effect of caprylic acid in the leptin/NM2B signaling pathway, based on previous results suggesting the common JAK2/STAT3 signaling existed in both caprylic acid’s signaling in anti-neuroinflammation and leptin/NM2B signaling. Hypothesizing that the existence of caprylic acid can increase the expression of JAK2/STAT3 signaling molecules, this study designed a detailed 12-week mice experiment, with general control group with standard rodent chow and experiment group with 2% caprylic acid, which focuses on the three following measurements: 1. fEPSP measurement will reveal the difference in LTP. 2. Immunoblotting regarding signaling molecules can reflect the difference in the expression of signaling molecules. 3. Immunoprecipitation suggests the regional expression pattern of these signaling molecules. Altogether, these potential results can give us a clue on the possible signaling mechanism of caprylic acid in enhancing leptin/NR2B. Therefore, this proposed research may offer a new perspective of the role of SFA in learning and cognition.
References
[1]. Cansino, S., Torres-Trejo, F., Estrada-Manilla, C., Flores-Mendoza, A., Ramírez-Pérez, G., & Ruiz-Velasco, S. (2021). Influence of dietary nutrient intake on episodic memory across the adult life span. Frontiers in Aging Neuroscience, 13. https://doi.org/10.3389/fnagi. 2021.724595
[2]. Micha, R., & Mozaffarian, D. (2010). Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids, 45(10), 893–905. https://doi.org/10.1007/s11745-010-3393-4
[3]. Huang, Q., Chen, C., Zhang, Z., & Xue, Q. (2023). Anti-inflammatory effects of myristic acid mediated by the NF-ΚB pathway in lipopolysaccharide-induced BV-2 microglial cells. Molecular Omics. https://doi.org/10.1039/d3mo00063j
[4]. Zhang, X., Zhang, P., Liu, Y., Liu, Z., Xu, Q., Zhang, Y., Liu, L., Yang, X., Li, L., & Xue, C. (2023). Effects of caprylic acid and eicosapentaenoic acid on lipids, inflammatory levels, and the JAK2/STAT3 pathway in ABCA1-deficient mice and ABCA1 knock-down raw264.7 cells. Nutrients, 15(5), 1296. https://doi.org/10.3390/nu15051296
[5]. Zhang, X., Zhang, P., Liu, Y., Liu, Z., Xu, Q., Zhang, Y., Liu, L., Yang, X., Li, L., & Xue, C. (2023). Effects of caprylic acid and eicosapentaenoic acid on lipids, inflammatory levels, and the JAK2/STAT3 pathway in ABCA1-deficient mice and ABCA1 knock-down raw264.7 cells. Nutrients, 15(5), 1296. https://doi.org/10.3390/nu15051296
[6]. McGregor, G., & Harvey, J. (2017). Leptin regulation of synaptic function at hippocampal TA-CA1 and SC-CA1 synapses: Implications for health and disease. Neurochemical Research, 44(3), 650–660. https://doi.org/10.1007/s11064-017-2362-1
[7]. Scott, M. M., Lachey, J. L., Sternson, S. M., Lee, C. E., Elias, C. F., Friedman, J. M., & Elmquist, J. K. (2009). Leptin targets in the Mouse Brain. The Journal of Comparative Neurology, 514(5), 518–532. https://doi.org/10.1002/cne.22025
[8]. Madisen, L., Mao, T., Koch, H., Zhuo, J., Berenyi, A., Fujisawa, S., Hsu, Y.-W. A., Garcia, A. J., Gu, X., Zanella, S., Kidney, J., Gu, H., Mao, Y., Hooks, B. M., Boyden, E. S., Buzsáki, G., Ramirez, J. M., Jones, A. R., Svoboda, K., … Zeng, H. (2012). A toolbox of cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nature Neuroscience, 15(5), 793–802. https://doi.org/10.1038/nn.3078
[9]. Barraza-Villarreal, A., Escamilla-Nuñez, C., Hernández-Cadena, L., Romieu, I., Ruiz-Velasco, S., & Sunyer, J. (2008). Reduction in measurement error: Barraza-Villarreal et al.. respond. Environmental Health Perspectives, 116(10). https://doi.org/10.1289/ehp.11804r
[10]. Cansız, D., Ünal, İ., Üstündağ, Ü. V., Alturfan, A. A., Altinoz, M. A., Elmacı, İ., & Emekli-Alturfan, E. (2021). Caprylic acid ameliorates rotenone induced inflammation and oxidative stress in the gut-brain axis in zebrafish. Molecular Biology Reports, 48(6), 5259–5273. https://doi.org/10.1007/s11033-021-06532-5
[11]. Liu, H., He, Y., Bai, J., Zhang, C., Zhang, F., Yang, Y., Luo, H., Yu, M., Liu, H., Tu, L., Zhang, N., Yin, N., Han, J., Yan, Z., Scarcelli, N. A., Conde, K. M., Wang, M., Bean, J. C., Potts, C. H., … Xu, Y. (2023). Hypothalamic GRB10 enhances leptin signalling and promotes weight loss. Nature
Cite this article
Ming,S. (2024). Caprylic acid promotes synaptic plasticity potentially through enhancing Leptin/NM2B signaling. Theoretical and Natural Science,46,30-38.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Cansino, S., Torres-Trejo, F., Estrada-Manilla, C., Flores-Mendoza, A., Ramírez-Pérez, G., & Ruiz-Velasco, S. (2021). Influence of dietary nutrient intake on episodic memory across the adult life span. Frontiers in Aging Neuroscience, 13. https://doi.org/10.3389/fnagi. 2021.724595
[2]. Micha, R., & Mozaffarian, D. (2010). Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids, 45(10), 893–905. https://doi.org/10.1007/s11745-010-3393-4
[3]. Huang, Q., Chen, C., Zhang, Z., & Xue, Q. (2023). Anti-inflammatory effects of myristic acid mediated by the NF-ΚB pathway in lipopolysaccharide-induced BV-2 microglial cells. Molecular Omics. https://doi.org/10.1039/d3mo00063j
[4]. Zhang, X., Zhang, P., Liu, Y., Liu, Z., Xu, Q., Zhang, Y., Liu, L., Yang, X., Li, L., & Xue, C. (2023). Effects of caprylic acid and eicosapentaenoic acid on lipids, inflammatory levels, and the JAK2/STAT3 pathway in ABCA1-deficient mice and ABCA1 knock-down raw264.7 cells. Nutrients, 15(5), 1296. https://doi.org/10.3390/nu15051296
[5]. Zhang, X., Zhang, P., Liu, Y., Liu, Z., Xu, Q., Zhang, Y., Liu, L., Yang, X., Li, L., & Xue, C. (2023). Effects of caprylic acid and eicosapentaenoic acid on lipids, inflammatory levels, and the JAK2/STAT3 pathway in ABCA1-deficient mice and ABCA1 knock-down raw264.7 cells. Nutrients, 15(5), 1296. https://doi.org/10.3390/nu15051296
[6]. McGregor, G., & Harvey, J. (2017). Leptin regulation of synaptic function at hippocampal TA-CA1 and SC-CA1 synapses: Implications for health and disease. Neurochemical Research, 44(3), 650–660. https://doi.org/10.1007/s11064-017-2362-1
[7]. Scott, M. M., Lachey, J. L., Sternson, S. M., Lee, C. E., Elias, C. F., Friedman, J. M., & Elmquist, J. K. (2009). Leptin targets in the Mouse Brain. The Journal of Comparative Neurology, 514(5), 518–532. https://doi.org/10.1002/cne.22025
[8]. Madisen, L., Mao, T., Koch, H., Zhuo, J., Berenyi, A., Fujisawa, S., Hsu, Y.-W. A., Garcia, A. J., Gu, X., Zanella, S., Kidney, J., Gu, H., Mao, Y., Hooks, B. M., Boyden, E. S., Buzsáki, G., Ramirez, J. M., Jones, A. R., Svoboda, K., … Zeng, H. (2012). A toolbox of cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nature Neuroscience, 15(5), 793–802. https://doi.org/10.1038/nn.3078
[9]. Barraza-Villarreal, A., Escamilla-Nuñez, C., Hernández-Cadena, L., Romieu, I., Ruiz-Velasco, S., & Sunyer, J. (2008). Reduction in measurement error: Barraza-Villarreal et al.. respond. Environmental Health Perspectives, 116(10). https://doi.org/10.1289/ehp.11804r
[10]. Cansız, D., Ünal, İ., Üstündağ, Ü. V., Alturfan, A. A., Altinoz, M. A., Elmacı, İ., & Emekli-Alturfan, E. (2021). Caprylic acid ameliorates rotenone induced inflammation and oxidative stress in the gut-brain axis in zebrafish. Molecular Biology Reports, 48(6), 5259–5273. https://doi.org/10.1007/s11033-021-06532-5
[11]. Liu, H., He, Y., Bai, J., Zhang, C., Zhang, F., Yang, Y., Luo, H., Yu, M., Liu, H., Tu, L., Zhang, N., Yin, N., Han, J., Yan, Z., Scarcelli, N. A., Conde, K. M., Wang, M., Bean, J. C., Potts, C. H., … Xu, Y. (2023). Hypothalamic GRB10 enhances leptin signalling and promotes weight loss. Nature









