1. Introduction
Bearing expectancy lowered by 5-20 years and a positive correlation with various fatal diseases such as cancer, obesity is indeed a chronic disease rather than a risk factor in the same sense as hypertension [1].
Other than environmental factors, including excess nutrition and little physical exercise, pathological dysregulation can also contribute to the accumulation of excess body weight as shown in Figure 1. For instance, Tourette syndrome is reported to have a strong positive correlation of 58%-77% with obesity. [1] Additionally, the human gut microbiome also modifies the energy harvested from ingested food [2]. Thus, these patients may gain more calories and weight due to the composition of the gut microbiome compared to normal people.
In both situations listed above, applying a healthier lifestyle can hardly have an impact on body weight because these patients abnormally gain more calories from ordinary food or have problems metabolizing excess fat. Additionally, incorporating more exercise and a healthier diet in life is a slow treatment process, with an annual weight loss of less than 4% [1]. Thus, a safe, long-term medication is required for people with a body mass index (BMI) larger than 30 kg/m-2, who cannot do excess exercise since they would develop musculoskeletal problems due to their own weight [3].
2. Obesity And Related Risks
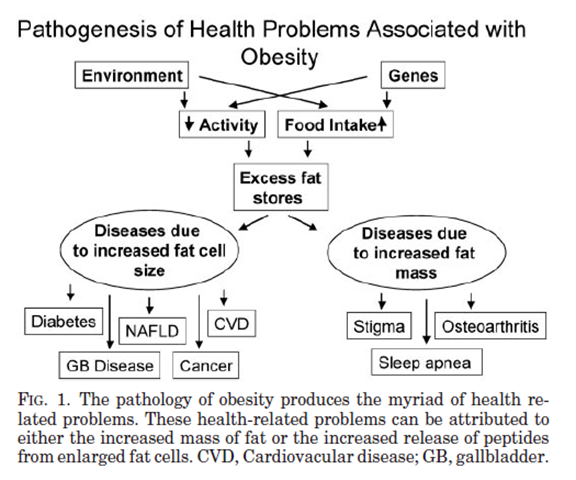
Figure 1. Pathology of Obesity [3]
Although obesity can be attributed to various factors, subsequent conditions are caused by either increased fat cell size or increased fat mass.
2.1. Diseases Due to Increased Fat Cell Mass
With an increased cell mass, coupled with volume, excess fat cells occupy the space that should be available for other tissues and organs to function normally. For instance, excess mass can cause a heavy burden on muscle and thus induce malfunction or irreversible damage to typical tissue.
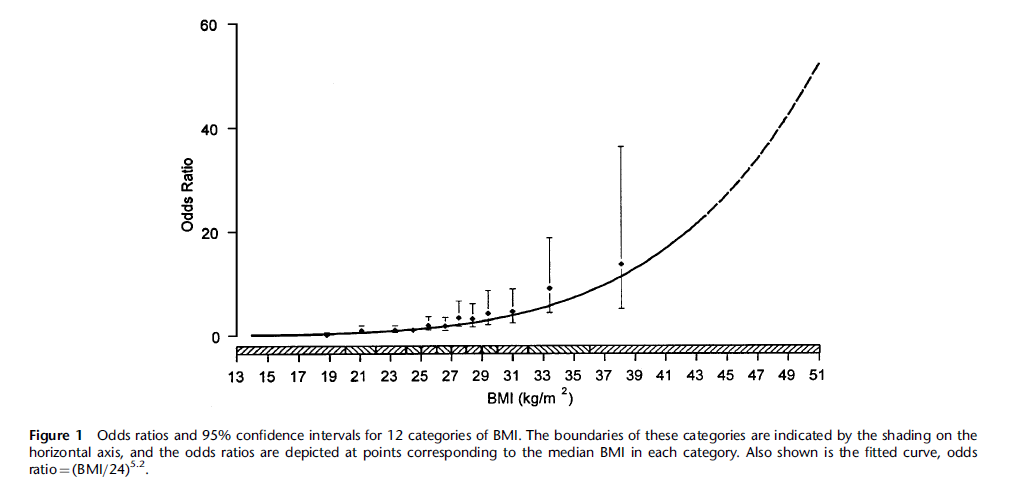
Figure 2. Knee Osteoarthritis Correlation with BMI [4]
For instance, knee osteoarthritis (OA), the breakdown of cartilage within the knee, is positively correlated with BMI as shown in Figure 2 [4]. With OA, the patient suffers pain, stiffness, swelling, and decreased flexibility in the knee. Such a condition further limits the patient’s ability to exercise enough to lose weight.
Additionally, excessive fat accumulated around the neck and upper airway may also trigger obstructive sleep apnea (OSA) [5]. With OSA, the patient will be continuously waked during sleep, which will trigger insomnia and hypersomnia during the daytime, which further influences the endocrine system.
2.2. Disease Due to Increased Fat Cell Size
Fat cells themselves can be viewed as a type of endocrine cell, especially with adipose tissue recognized as an endocrine organ responsible for secreting leptin [3]. The accumulation of visceral fat, fat around the abdominal cavity, triggers hyperinsulinemia, and thus insulin resistance [3]. Combining these two factors, obese patients have a high risk of developing type two diabetes (T2D).
Additionally, BMI has a positive correlation with triglycerides and a negative correlation with high-density lipoprotein (HDL) [3]. These two factors are both high-risk biomarkers for cardiovascular diseases (CVD) [3].
Bearing these risks, medication must be designed and assigned in consensus with providing risk-lowering effects and avoiding deteriorating existing problems, especially cardiac health.
3. Regulation Of Satiety
As a crucial part of life, the regulation of food intake is a complicated process that includes the cooperation of the nervous system, endocrine system, and digestive system as shown in Figure 3. Thus, anthropological control of satiety can either target the brain, the central nervous system, or peripheral organs, such as the pancreas.
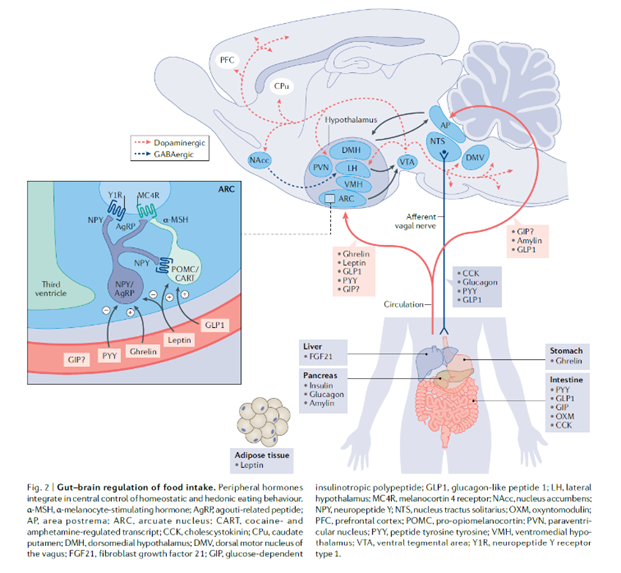
Figure 3. Regulation of Satiety [1]
In such a regulation process, many hormones are involved and some of them act on both brain and peripheral organs. For instance, glucagon-like peptide 1, GLP-1, can act on both intestine and brain and circulate through these two sites.
However, not all regulatory pathways can be chosen as a feasible target. For example, the medicating acting on the reward pathway, involving the ventral tegmental area, prefrontal cortex, and nucleus accumbens in the regulation of satiety, can have severe side effects, such as developing addiction, or even death after long-term dosage [1].
Additionally, due to an organism’s inherited instinct to preserve more energy from food intake, the human body has a series of mechanisms to prevent weight loss. For instance, natural satiety regulatory hormones usually have a half-life too short to be considered as a medication; the body automatically secretes some antagonists of AOMs in response to a decrease in some biomarkers, such as blood glucose level [1].
4. Historical Approach: Sympathicomimetic
Historically, eighteen medications were approved to treat obesity in the long term. These drugs can be divided into nine categories according to their mechanism. Now, only GLP1R agonists and pancreatic lipase inhibitors are still approved, with other seven types banned for having severe side effects [1]. Viewing from contemporary perspectives, some of the drugs are boldly approved as a treatment for obesity.
Sympathicomimetics are drugs that mimic endogenous catecholamines, neuron transmitters involved in the sympathetic nervous system. Catecholamines are the hormones made by adrenal glands and their derivatives, which include dopamine and epinephrine. Due to Sympathicomimetic drugs’ activity in the reward pathway, there is a high risk for abusiveness and addiction, such as in the case of methamphetamine, which was approved in 1947-1979 for the treatment of obesity [1].
5. Contemporary Approach
5.1. Pancreatic Lipase Inhibitor: Orlistat
5.1.1. Chemical Properties & Pharmacodynamics
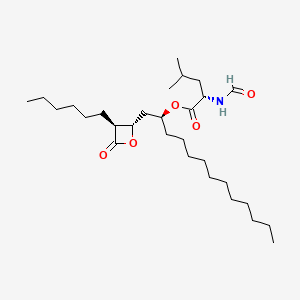
Figure 4. Structure of Orlistat [6]
Orlistat, with the structure shown in Figure 4, is a hydrophobic compound that is insoluble in water but soluble in various organic solvents, including methanol, ethanol, and chloroform [7]. Due to this lipophilic nature, orlistat is minimally absorbed into circulation and has a relatively short half-life of 14-19 hours [8]. Thus, only 1% of the Orlistat taken orally would be found in urine with 96% excreted in feces [8]. With a melting point between 40 and 50 degrees Celsius, Orlistat should not be exposed to high temperatures for a long time.
5.1.2. Mechanism. Acting on pancreatic lipase, Orlistat is a hydrogenated derivative of lipstatin, a natural lipase inhibitor produced by Streptomyces toxytricini [9], that binds covalently to the active site of pancreatic lipase stably [8]. Binding to that site, the complex causes a change in the structure of the pancreatic lipase that alters the function of this enzyme. Specifically, this binding leads to the acylation of a hydroxyl group on the serine residue, forming a lid-like structure in the active site [8]. With this modification, the lipase loses its function to hydrolyze fat into fatty acids and monoglycerides [8]. Thus, the excess fat ingested will be directly passed into feces and will not be absorbed. Hence, the patient can limit their fat consumption and thus lose weight.
5.1.3. Efficacy. Two doses are tested on slim people to ensure the safety of the drug, which is to decrease lipid absorption by no more than 35% [8]. For low dosage, 80mg three times per day (t.i.d), fat absorption is decreased by 20%. For high dosages up to 400 mg t.i.d, reduction in lipid absorption also maintains at 35% as shown in Figure 5 [8].
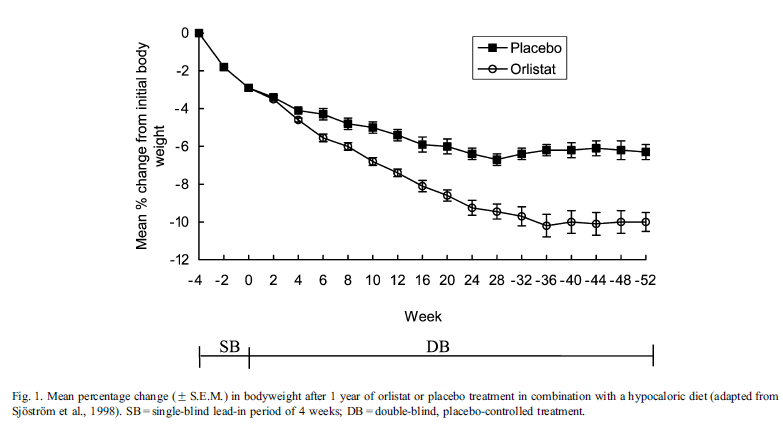
Figure 5. Efficacy of Orlistat [8]
Although the percentage absorption remains in a safe range, the net amount of fat unabsorbed increases with increased fat intake. This indicates that the treatment of Orlistat must be coupled with a diet with a relatively low lipid proportion, or severe gastrointestinal conditions may happen due to the fat that enters the large intestine maintained in a liquid state, which would also affect the state of the feces and cause diarrhea [8]. With a low-calorie diet and regular exercise, orlistat decreases weight by 0.5 kg per week [8], or around 5% of the body weight relative to placebo after a 6-month treatment [10]. Such a low-calorie diet should be well-balanced, mild, and contain approximately 30% of calories from fat [10].
Apart from the weight-losing effect, Orlistat also contributes to beneficial changes in several cardiovascular risk factors, including dyslipidemia, insulin and glucose metabolism, and blood pressure [10].
5.1.4. Manufacture
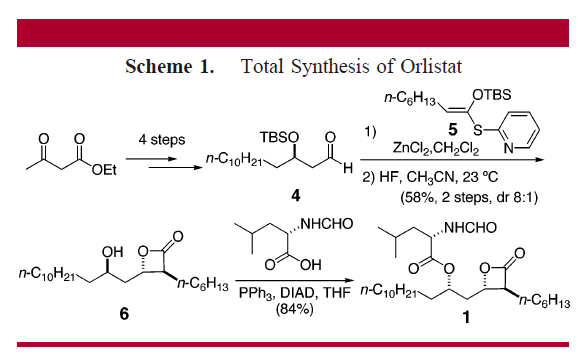
Figure 6. Total Synthesis of Orlistat [11]
Since Orlistat is a relatively small molecule, its manufacture is often total synthesis based on the synthesis strategy of some other chemicals, such as panclicin D, another natural derivative of Orlistat with 2-fold higher potency inhibiting pancreatic lipase shown in Figure 6 [11]. The applied strategy involves the tandem Mukaiyama aldol-lactonization (TMAL) process as a key step, which delivers trans-beta-lactones with high diastereoselectivity, allowing facile modification on the alpha-side chain [11]. Other versatile strategies include cuprate addition and olefin metathesis, which contribute to the modification of the delta-side chain [11].
5.2. GLP1R Agonists: Semaglutide
5.2.1. Chemical Properties of Semaglutide
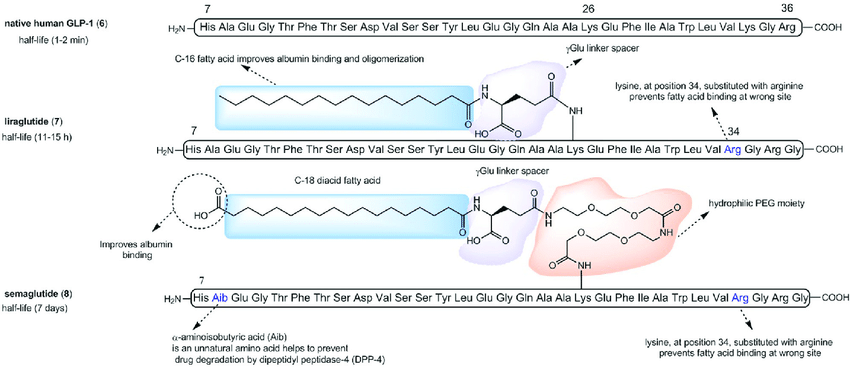
Figure 7. Structure of GLP-1 Ras [12]
Being a next-generation GLP-1 RA, Semaglutide follows the designing criteria of keeping a structure similar to native GLP-1 and not introducing unnecessary changes in amino acids to avoid immunogenicity responses as shown in Figure 7 [13]. This design is also based on previous GLP-1 RAs that had been used in markets with completed tests, such as liraglutide, which has only one amino acid substitution and a less modified side chain [13]. Eventually, Semaglutide only has two amino acids substitution at position 8 and position 34, and acylation of the lysine at position 26 (Lys26) with a linker containing a glutamic acid moiety and a C-18 fatty diacid side chain [13]. At position 8, alanine is changed to alpha-aminosobutyric acid, which provides relative resistance to DDP-4-induced degradation, which is not present in the previous generation liraglutide, which only has a half-life of 11-15 hours compared to Semaglutide’s 183 hours [13]. At position 34, lysine is changed into arginine, which also presents in the liraglutide as the only amino acid substitution [13]. This substitution avoids the intramolecular binding of the C-18 fatty diacid tail to this position and thus restricts the binding site to the only other remaining lysine [13]. The linker allows Semaglutide to bind to albumin, with a se, which allows Semaglutide to stay in the plasma with a 70%-80% portion of the total dosage, and free to be released to cross the blood-brain barrier to act on the hypothalamus with the function influenced at a minimum portion [13].
5.2.2. Mechanism. Predominantly, AOMs targets mainly either peripheral or central pathway, not both pathways with equal significance [1]. This is also true in the case of GLP-1 RAs, although GLP-1 is a hormone involved in both pathways. In peripheral pathways, in which native GLP-1 is normally involved, Semaglutide promotes insulin secretion in beta-pancreatic cells and suppresses glucagon production in alpha-pancreatic cells glucose-dependently [4]. This is how Semaglutide decreases plasma glucose and acts as a drug treating T2D. Additionally, Semaglutide acts on the inhibitory feedback between nutrients and the small intestine to prolong gastro emptying, which also contributes partly to the weight-lowering effect [14].
Despite various effects on the peripheral organs, Semaglutide reduces weight mainly because it activates GLP-1 receptors in the human brain increases satiety, and regulates food reward [15]. Eating ad libitum, patients are observed with a lowered total energy intake attributable to suppression of appetite, rather than nausea or food aversion [14]. In rodents, these effects are reached by activating proopiomelanocortin and cocaine- and amphetamine-regulated transcript neurons within the arcuate nucleus of the hypothalamus, but not via any peripheral action [14]. Despite the side effects of Semaglutide including nausea and vomiting, these side effects only contribute to 0.07-0.5 kg of weight loss [14].
5.2.3. Efficacy. Being the latest medication approved to treat obesity in the long term, when paired with a diet of lower calory and regular exercise, 2.4mg Semaglutide issued to be injected subcutaneously weekly has the greatest weight loss percent of nearly 12% in a 6-month trial in both humans and rodents among medications [1]. The only treatment with a greater effect is bariatric surgery, which removes part of the stomach permanently by surgery [1]. Additionally, Semaglutide is the only medication that led to a weight loss larger than 10% when minus the weight loss of placebo after a 68-week treatment [1]. Moreover, Semaglutide also has a weight-lowering effect when the patient eats ad libitum since it causes a 24% reduction in the patient’s energy intake every day [15].
5.2.4. Manufacture. As a polypeptide with a branched side chain with fatty acid modification, the synthesis of Semaglutide is easily influenced by favored peptide folding and aggregation [16]. The latest synthesis strategy is to start with the linear precursor peptide in genetically modified host cells and then add other modifications in vitro as shown in Figure 8 [16]. Due to the similarity of the structures of Liraglutide and Semaglutide, their synthesis is often researched together and in a similar manner.
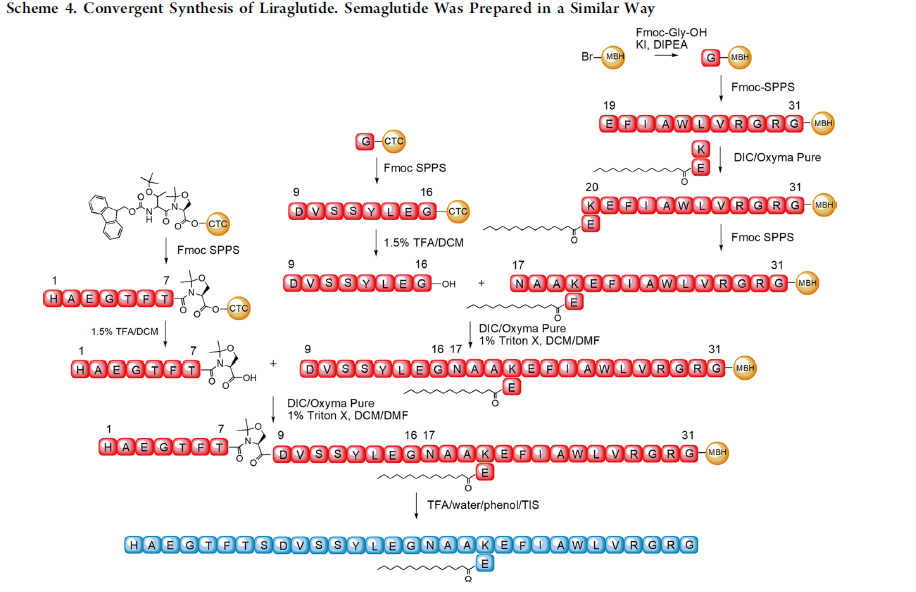
Figure 8. Synthesis of GLP-1 Ras [16]
In the synthesis of Liraglutide, to properly prepare the palmitoylated intermediate, which is the intermediate of the fatty diacid chain that is to be attached to the side chain, the use of Copper (II) lysinate, a Liraglutide Intermediate, is involved [16]. This Copper (II) complex contains trifunctional amino acids, such as Lys, Asp, or Glu [16]. Together, they offer temporary protection for the selective introduction of protective groups into the side chain [16]. A similar strategy can also be applied to Semaglutide, with the copper (II) lysinate substituted with a complex of Copper (II) and an intermediate of Semaglutide [16].
In industrialized factories, this method is often applied for its efficiency and relatively low cost. Due to the low toxicity of copper (II) salts, the reagents involved have a safe, relatively simple storage and disposal process. (Guryanov et al.)
Additionally, to enhance the coupling efficiency, a pseudoproline moiety is introduced into the structure, which prevents peptide aggregation during fragment condensation. This pseudoproline cannot be epimerized upon carboxylic group activation due to the absence of an amide proton, which allows it to avoid aggregation in conditions such as the racemization of the intermediate 2,4-diakyl-5(4H)-oxazolone. Thus, this approach is a potent strategy in the large-scale synthesis of GLP-1 RAs, such as Semaglutide [16].
5.3. Comparison of Orlistat and Semaglutide
Dividing Orlistat and Semaglutide into two categories based on their acting site, each of them has the best efficacy within each group of approved weight-losing medication. Especially, Orlistat is the only peripheral inhibitor that is still approved for treating weight loss. However, Semaglutide (2.4 mg) has a nearly doubled weight loss percentage after a six-month treatment period [1]. Moreover, Orlistat must be coupled with a low-calorie diet whereas the patient can eat ad libitum with Semaglutide with only a lower weight loss but no significant side effects. Overall, Orlistat has more reports of more severe gastrointestinal effects, which are sometimes associated with consuming too much lipid. Semaglutide is reported to cause vomiting and nausea, as well as gastrointestinal effects, including both constipation and diarrhea [1]. It is also notable that Semaglutide is responsible for inducing thyroid cancer in rodents. Nevertheless, such effects are yet to be studied in humans. Thus, patients who are going to take Semaglutide should take a thyroid examination in advance to eliminate high-risk factors [15].
The two drugs are also administered differently. Semaglutide has a half-life of nearly one week, thus only required to be injected subcutaneously once per week. Orally taken Semaglutide is the only GLP-1 RA that can be taken orally. However, this kind of medication is still under trial and development and has not been approved yet. Orlistat, on the other hand, should be taken orally three times per day [13].
As a drug developed nearly 15 years ago, Orlistat has a relatively more stable supply. On the contrary, Semaglutide was launched in 2019, which faces severe supply problems coupled with the global pandemic and demand from patients with T2D. Such problems still occur even in 2023, as Diabetes UK reported a shortage of Semaglutide due to the off-label prescription of treating obesity with Semaglutide [17].
6. Conclusion
With the success of Semaglutide, more GLP-1 RAs are undergoing development and even clinical trials. For instance, Tirzepatide, a dual GIP and GLP-1 RA, underwent its SURPASS clinical trials in 2020 for treating T2D [16]. In phase 1 and phase 2 trials, tripeptide has already demonstrated dose-dependent weight loss of up to 11.3 kg in patients with T2D. [18] Overall, though analogs of Orlistat or other peripheral-acting medications report no significant development, GLP-1 RAs, medications acting on incretin hormones in CNS, have a promising future and are still being heavily researched.
References
[1]. Müller, Timo D., et al. “Anti-obesity Drug Discovery: Advances and Challenges - Nature Reviews Drug Discovery.” Nature, 23 Nov. 2021, https://doi.org/10.1038/s41573-021-00337-8.
[2]. Tsai, Franklin, and Walter J. Coyle. “The Microbiome and Obesity: Is Obesity Linked to Our Gut Flora? - Current Gastroenterology Reports.” SpringerLink, 19 July 2009, https://doi.org/10.1007/s11894-009-0045-z.
[3]. Bray, George A. “Medical Consequences of Obesity.” OUP Academic, 1 June 2004, https://doi.org/10.1210/jc.2004-0535.
[4]. Coggon, D., et al. “Knee Osteoarthritis and Obesity - International Journal of Obesity.” Nature, 7 May 2001, https://doi.org/10.1038/sj.ijo.0801585.
[5]. “Sleep Apnea - Symptoms and Causes.” Mayo Clinic, 6 Apr. 2023, www.mayoclinic.org/diseases-conditions/sleep-apnea/symptoms-causes/syc-20377631.
[6]. https://pubchem.ncbi.nlm.nih.gov/compound/Orlistat#section=Related-Records
[7]. PubChem. “Orlistat.” Orlistat | C29H53NO5 | CID 3034010 - PubChem, pubchem.ncbi.nlm.nih.gov/compound/3034010.
[8]. Al-Omar, Mohamed A., et al. “Safety and mechanism of action of orlistat (tetrahydrolipstatin) as the first local antiobesity drug.” Journal of Applied Sciences Research 2.4 (2006): 205-208.
[9]. Guerciolini, R., 1997. Mode of action of orlistat. Int. J. Obes. Relat. Metab. Disord. 21 (suppl.3), S12-S23
[10]. Ballinger, Anne, and Steven R. Peikin. “Orlistat: Its Current Status as an Anti-obesity Drug.” European Journal of Pharmacology, vol. 109–117, no. 2–3, 1 Apr. 2002, https://doi.org/10.1016/s0014-2999(02)01422-x.
[11]. Ma, Gil, et al. “Total synthesis and comparative analysis of orlistat, valilactone, and a transposed orlistat derivative: Inhibitors of fatty acid synthase.” Organic Letters 8.20 (2006): 4497-4500.
[12]. https://www.researchgate.net/figure/Chemical-structures-of-native-human-GLP-1-6-liraglutide-7-and-semaglutide-8_fig8_348287453
[13]. Christou, Georgios A., et al. “Semaglutide as a promising antiobesity drug.” Obesity Reviews 20.6 (2019): 805-815.
[14]. Marathe, Chinmay S., et al. “Relationships Between Gastric Emptying, Postprandial Glycemia, and Incretin Hormones.” American Diabetes Association, 1 May 2013, https://doi.org/10.2337/dc12-1609.
[15]. Blundell, John, et al. “Effects of once‐weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity.” Diabetes, Obesity and Metabolism 19.9 (2017): 1242-1251.
[16]. Guryanov, Ivan, et al. “Copper (II) lysinate and pseudoproline assistance in the convergent synthesis of the GLP-1 receptor agonists liraglutide and semaglutide.” Organic Process Research & Development 25.7 (2021): 1598-1611.
[17]. Guryanov, Ivan, et al. “Copper (II) lysinate and pseudoproline assistance in the convergent synthesis of the GLP-1 receptor agonists liraglutide and semaglutide.” Organic Process Research & Development 25.7 (2021): 1598-1611.
[18]. Min, Thinzar, and Stephen C. Bain. “The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type2 Diabetes: The SURPASS Clinical Trials - Diabetes Therapy.” SpringerLink, 15 Dec. 2020, https://doi.org/10.1007/s13300-020-00981-0.
Cite this article
Shi,J. (2024). Review of current anti-obesity medication. Theoretical and Natural Science,44,245-253.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Müller, Timo D., et al. “Anti-obesity Drug Discovery: Advances and Challenges - Nature Reviews Drug Discovery.” Nature, 23 Nov. 2021, https://doi.org/10.1038/s41573-021-00337-8.
[2]. Tsai, Franklin, and Walter J. Coyle. “The Microbiome and Obesity: Is Obesity Linked to Our Gut Flora? - Current Gastroenterology Reports.” SpringerLink, 19 July 2009, https://doi.org/10.1007/s11894-009-0045-z.
[3]. Bray, George A. “Medical Consequences of Obesity.” OUP Academic, 1 June 2004, https://doi.org/10.1210/jc.2004-0535.
[4]. Coggon, D., et al. “Knee Osteoarthritis and Obesity - International Journal of Obesity.” Nature, 7 May 2001, https://doi.org/10.1038/sj.ijo.0801585.
[5]. “Sleep Apnea - Symptoms and Causes.” Mayo Clinic, 6 Apr. 2023, www.mayoclinic.org/diseases-conditions/sleep-apnea/symptoms-causes/syc-20377631.
[6]. https://pubchem.ncbi.nlm.nih.gov/compound/Orlistat#section=Related-Records
[7]. PubChem. “Orlistat.” Orlistat | C29H53NO5 | CID 3034010 - PubChem, pubchem.ncbi.nlm.nih.gov/compound/3034010.
[8]. Al-Omar, Mohamed A., et al. “Safety and mechanism of action of orlistat (tetrahydrolipstatin) as the first local antiobesity drug.” Journal of Applied Sciences Research 2.4 (2006): 205-208.
[9]. Guerciolini, R., 1997. Mode of action of orlistat. Int. J. Obes. Relat. Metab. Disord. 21 (suppl.3), S12-S23
[10]. Ballinger, Anne, and Steven R. Peikin. “Orlistat: Its Current Status as an Anti-obesity Drug.” European Journal of Pharmacology, vol. 109–117, no. 2–3, 1 Apr. 2002, https://doi.org/10.1016/s0014-2999(02)01422-x.
[11]. Ma, Gil, et al. “Total synthesis and comparative analysis of orlistat, valilactone, and a transposed orlistat derivative: Inhibitors of fatty acid synthase.” Organic Letters 8.20 (2006): 4497-4500.
[12]. https://www.researchgate.net/figure/Chemical-structures-of-native-human-GLP-1-6-liraglutide-7-and-semaglutide-8_fig8_348287453
[13]. Christou, Georgios A., et al. “Semaglutide as a promising antiobesity drug.” Obesity Reviews 20.6 (2019): 805-815.
[14]. Marathe, Chinmay S., et al. “Relationships Between Gastric Emptying, Postprandial Glycemia, and Incretin Hormones.” American Diabetes Association, 1 May 2013, https://doi.org/10.2337/dc12-1609.
[15]. Blundell, John, et al. “Effects of once‐weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity.” Diabetes, Obesity and Metabolism 19.9 (2017): 1242-1251.
[16]. Guryanov, Ivan, et al. “Copper (II) lysinate and pseudoproline assistance in the convergent synthesis of the GLP-1 receptor agonists liraglutide and semaglutide.” Organic Process Research & Development 25.7 (2021): 1598-1611.
[17]. Guryanov, Ivan, et al. “Copper (II) lysinate and pseudoproline assistance in the convergent synthesis of the GLP-1 receptor agonists liraglutide and semaglutide.” Organic Process Research & Development 25.7 (2021): 1598-1611.
[18]. Min, Thinzar, and Stephen C. Bain. “The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type2 Diabetes: The SURPASS Clinical Trials - Diabetes Therapy.” SpringerLink, 15 Dec. 2020, https://doi.org/10.1007/s13300-020-00981-0.









