1. Introduction
1.1. History of Lung Cancer
Over 150 years ago, lung cancer was still classified as an uncommon disease. Out of all the cancer autopsies in 1878, lung cancer only made up 1% [1]. By 1918, the percentage had risen to almost 10% and was higher than 14% in 1927. Therefore, as the world develops and industrializes, the rate of lung cancer is also on the rise. The most common type of lung cancer is squamous cell carcinoma, and the relationship between cancer rates and smoking habits was initially identified in 1961 and proven right in the last 20 years [2].
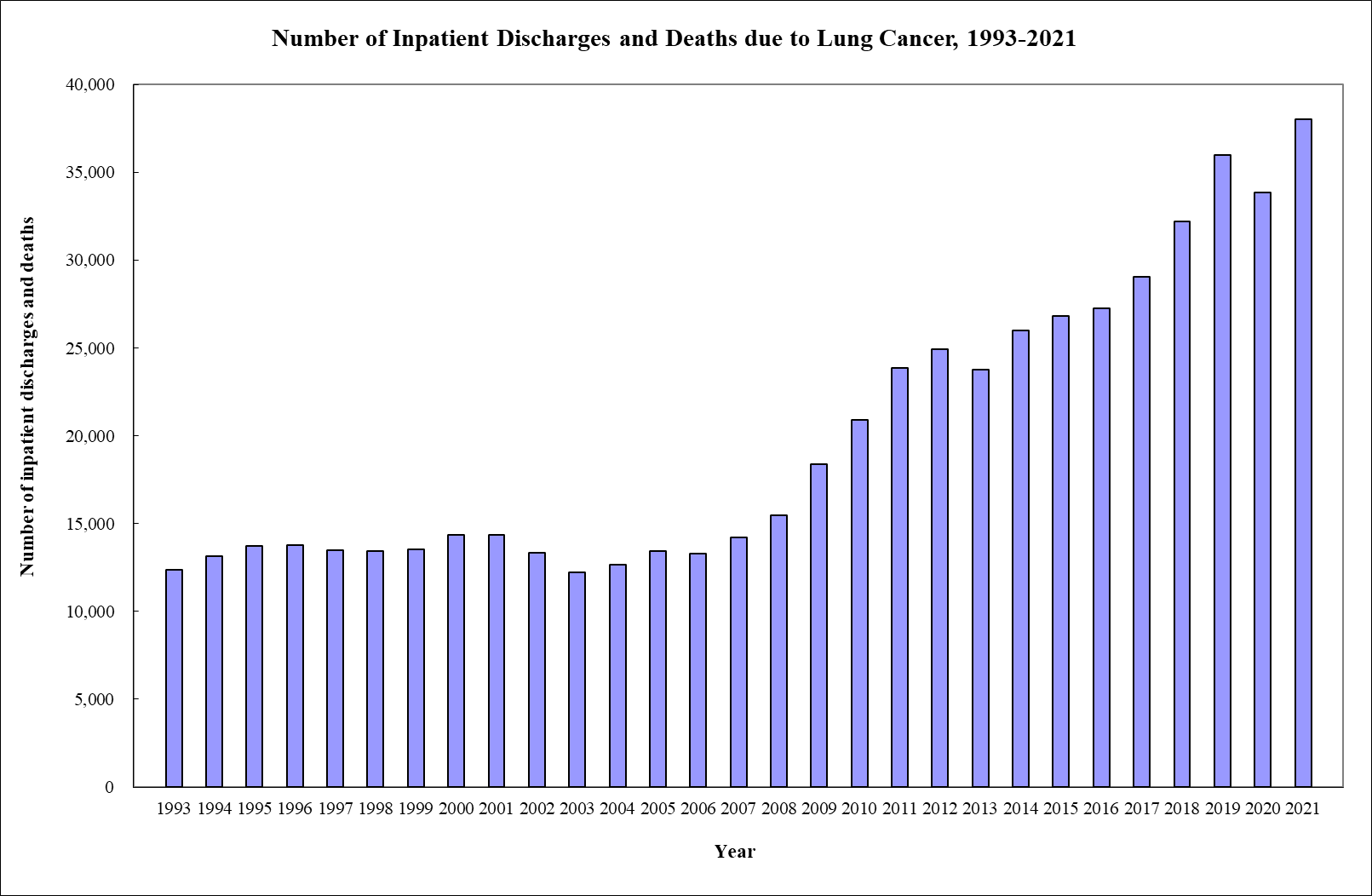
Figure 1. The rate of discharges and deaths due to lung cancer in Hong Kong from 1993 to 2021 [3].
1.2. Classification of Lung Cancer
As we all know, cancer arises when damaged cells in our body divide uncontrollably, and mutated cells spread to the rest of the organ by a process known as metastasis. Lung cancer contributes to the largest number of cancer-related deaths worldwide. The two major groups are non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Although both categories of lung cancer result from the spread of cancer cells in tissues, SCLC is much less common (20%) and often starts to develop from the trachea, which goes deeper down into the lungs and then into the finer structures of the respiratory system, while NSCLC starts in the center of the lung [4]. SCLC spreads much quicker than NSCLC and can even infect other parts of the body. In comparison, the symptoms of NSCLC are generally more gradual, leading to a later diagnosis of the disease most of the time.
1.3. TP53 mutations due to smoking
The TP53 gene is a type of tumor suppressor gene that contains information on the synthesis of p53, which is a tumor suppressor protein. A cell can mutate somatically through genetic fusion. This is when chromosomes rearrange and fuse to create a damaged gene sequence. This mutated version of the p53 protein forms an oncogene that has the potential to cause cancer. Normally, when the DNA sequence becomes damaged, the protein will be able to differentiate them. Hence, the cell will self-destruct before the damaged DNA can be replicated, preventing cells from proliferating uncontrollably. As time passes, the number of mutations can accumulate to cause the cancer cells to be more aggressive, and up to 60 mutations can be found in most cancer cells [5].
Tobacco is one of the main ingredients in a cigarette, and in its vapor phase, it contains thousands of particulate phase compounds. At least 60 are categorized as carcinogens [6]. The chronic exposure of the epithelium of the lungs to these compounds leads to covalent interactions forming between the chemical compound and the DNA of the epithelial cells. In this instance, carcinogens such as polycyclic aromatic hydrocarbons (PAHs) and the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), increase the likelihood of lung tumor formation, are the main causes of the transversions of G to T nucleotides [7]. The Cancer Genome Atlas and several other institutions had been carrying out experiments and collecting data to prove the relationship between tobacco exposure and diseases such as squamous cell carcinoma or lung adenocarcinoma affecting the head and neck.
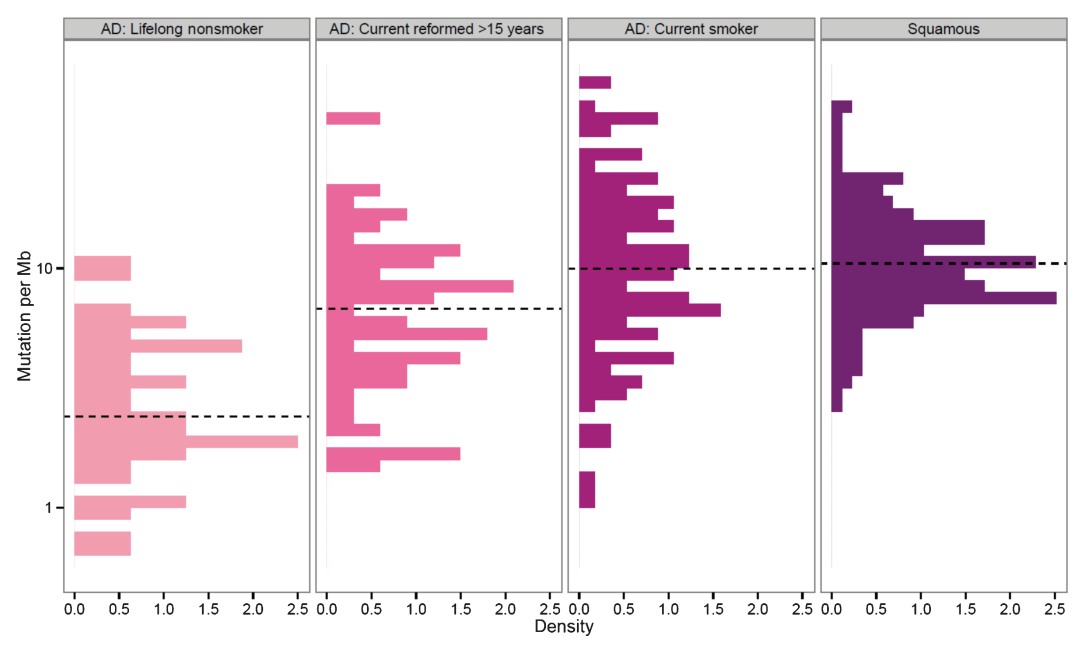
Figure 2. The genome-wide mutation densities of squamous cell carcinoma and lung adenocarcinoma (AD). 96% of the patients with squamous cell carcinoma are smokers, hence the groups when not categorized.
Analyzing Figure 2, we observe that mutation densities in patients who are current or former smokers are much higher than those in non-smokers when compared to their respective diseases. For lung adenocarcinoma, the medium number of mutations per mutational burden (Mb) rises from non-smokers to reformed smokers to current smokers, which have about 10 mutations per Mb. This data also applies to squamous cell carcinoma, where it also has a medium of about 10.
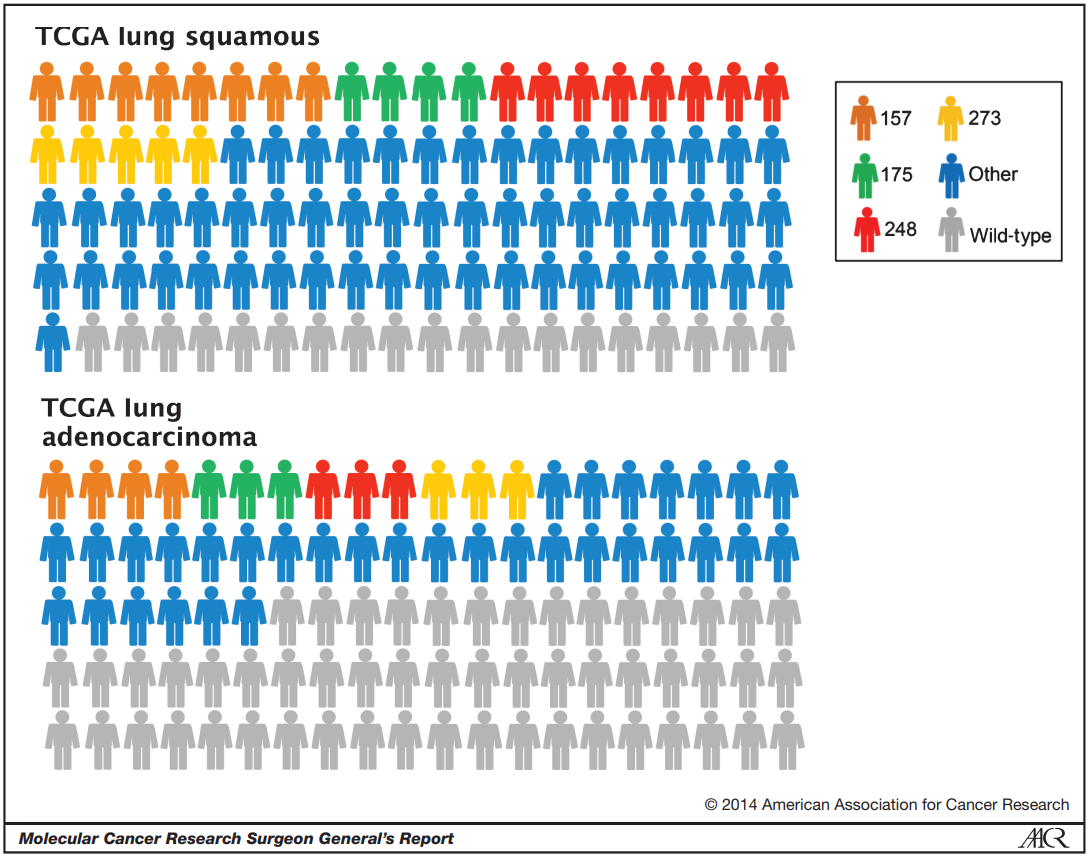
Figure 3. The kind of mutation in each type of lung cancer. Colors all represent p53 mutations, while gray represents wild-type p53. The colors orange, green, red, and yellow represent various hotspot areas around the amino acid. The blue represents other parts of the cell.
From Figure 3, we can observe that lung cancer types, both lung squamous and lung adenocarcinoma, have very high levels of p53 mutation rates in hotspot regions. The mutation rate is about 80% in squamous cells and 50% in lung adecarcinoma.
In a nutshell, we can observe that as the years of smoking get longer, the rate of mutations increases, of which p53 mutations make up a high percentage.
1.4. Hereditary mutations, EGFR gene T790M mutations
In recent research, it has been discovered that lung cancer can be hereditary, although in extremely rare circumstances. This is caused by the germline mutation of the EGFR gene on chromosome 7, which codes for the production of epidermal growth factor receptors, which contribute to cell growth. EFGR proteins are found throughout the cell surface membrane, and different ends of the protein stay on different sides of the cell. The receptor binds to ligands in the outside environment so that signals can be transmitted. Once the binding to a ligand finishes, the EGFR protein can dimerize to form a receptor complex and increase the rate of cell proliferation.
This mutation is known as T790M, and lung cancer patients very frequently have this mutation in their normal cells too. A study has been done to prove that this gene mutation can increase the risk of lung cancer in non-smokers. They sampled a Japanese family of 14 individuals over 5 generations and combined the results with another study of 15 individuals to test their hypothesis.
The T790M mutation was found in 1% of NSCLC patients and less than 1 in 7,500 healthy individuals. The germline T790M mutation was inheritance-dominant and favored females. The mutation led to lung adenocarcinoma in most scenarios, and 73% of the mutation carriers end up developing another EGFR gene mutation. The ratio of smokers to non-smokers with T790M diagnosed with cancer is 0.31. Therefore, they concluded that the germline T790 mutation ultimately increases the chances of lung cancer in non-smokers by approximately 31%, although data proves the opposite for heavy smokers [8].
2. Cancer therapy
There are different categories in the treatment of cancer. There are ‘local’ treatments, such as surgery and radiation therapy, that are commonly used to treat tumors or specific areas of the body. Another category would be drug treatments that include chemotherapy, immunotherapy, or targeted therapy. Drug treatments are often known as ‘systemic’ therapy because they can have side effects on the entire body. They are administered intravenously most of the time, but they can also be taken orally. The oldest type of cancer treatment would be surgical therapy. This therapy was known as pneumonectomies, and it has been implemented since the 1930s. With the development of technology, radical radiotherapy came into existence and the use of megavoltage linear accelerators was implemented in the 1950s. The earliest form of chemotherapy for NSCLC would be during the 1940s, treating with nitrogen mustards. Different treatments have their own pros and cons, and to maximize efficiency, combinations of treatments are prescribed to patients nowadays. In this review, the focus will be on drug treatments for cancer.
2.1. Carboplatin
2.1.1. Origins of Carboplatin. Carboplatin, also known as Paraplatin, is a chemotherapy drug grouped as a DNA alkylating agent together with cisplatin and oxaliplatin. It was developed in the 1980s and authorized by the FDA in 1989. Nowadays, it is widely used in cancer treatment, such as lung, ovarian, or neck, due to its minimal side effects compared to cisplatin, which is its parent compound.
2.1.2. Chemical Structure
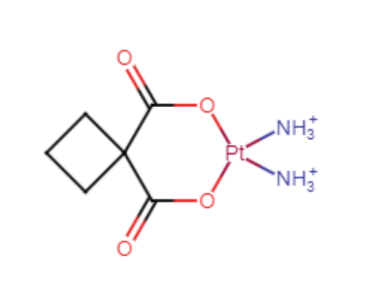
Figure 3. The structural formula of Carboplatin.
The chemical formula of Carboplatin is C6H12N2O4Pt. It consists of a bidentate dicarboxylate moiety as its leaving group. The most essential aspect of the molecule, however, would be the Pt atom in the ring. It is also bonded to two amino groups of nitrogen atoms and is hypervalent. This Pt atom helps this atom function as a drug in cancer by forming covalent bonds with DNA, which will be addressed further in this review.
2.1.3. Properties
Table 1. Properties of Carboplatin [9]
Properties | DATA |
Appearance | White solid powder |
Solubility | Water soluble |
Melting Point | 228-230°C |
Stability | Stable but reactive (incompatible) with strong oxidizing agents |
2.1.4. Pharmacodynamics and Pharmacology. In essence, Carboplatin inhibits or slows down the rate of cancer cell growth by binding to cell DNA. There are two main steps in the mechanism of Carboplatin: Aquation and Activation.
For the carboplatin molecule to function, it must first enter the cell through the selectively permeable cell surface membrane. They enter the cell through a high-affinity copper uptake protein known as CTR1 [10]. Cancer cells frequently overexpress the CTR1 protein, and this increases the absorption of platinum compounds, in this example, carboplatin. During Aquation, Carboplatin hydrolyzes at the 1,1-cyclobutane dicarboxylate, and the terminals become charged positively. This permits the various nucleophilic reactions with DNA, RNA, or proteins through the covalent bonding of carboplatin to the N7 site of the A and G base nucleotides. Then, the activation of carboplatin happens.

Figure 4. The hydrolysis of Carboplatin in the cell.
The reactive platinum complexes can cause cross-linkages that are difficult to repair within the DNA strands and hence damage the genetic code. The DNA strand will not be able to unzip during transcription, and this leads to a cytotoxic effect where DNA replication is halted. This is accomplished by increasing errors in the code, stopping cells in the G2 growth phase of the cell cycle, and, most importantly, triggering apoptosis in some cases [11].

Figure 5. The inter- and intrastrand cross-linking of DNA [12]
2.1.5. Modes of Administration. The amount of Carboplatin prescribed is adjusted by measuring the number of platelets and neutrophils in the body. Generally, it would be prescribed intravenously at approximately 300 mH/m2 IV once every 4 weeks for 6 cycles [13]. Needles should not be made of aluminum because they can react with carboplatin and form an inactive precipitate.
2.1.6. Side Effects. Carboplatin kills a large number of blood cells within the bone marrow, which increases the risk of infection and bleeding. Some common side effects are nausea, vomiting, diarrhea, sore throats, and hair loss. The more severe side effects would be such things as fainting, changes in vision, or ringing in the ears [14].
Carboplatin also leads to long-term side effects, even after the chemotherapy treatment. This, therefore hearing problems due to damage to the inner ear, heart problems, and cognitive problems that lead to brain fogginess.
2.1.7. Limitations. There are many challenges in the administration of carboplatin. These include hypersensitivity, renal toxicity, and ototoxicity, of which hypersensitivity is the largest aspect.
Hypersensitivity refers to an inappropriate immune response in the body when an antigen or allergen is introduced. They are a particular concern because of their high rates and unpredictability. They appear in approximately 15-20% of women, and the symptoms vary according to other agents that carboplatin is used in combination with. These symptoms include blood pressure changes, emesis, chest tightness, etc. Moreover, hypersensitivity reactions can occur throughout the whole diagnosis or even after its completion.
Another major factor is the toxicity of carboplatin to the body. The toxic effects of Carboplatin, which is a platinum compound, can accumulate in the body and affect treatments and interventions for relapses of cancer [15].
2.1.8. Synthesis. As mentioned before, cisplatin is the parent compound of carboplatin. This hence includes the biosynthesis of carboplatin, which is made from cisplatin.
The most common route for the synthesis of cisplatin would be the Dhara method. Firstly, [PtCl4]2- in aqueous form is changed to [PtI4]2- by the reaction with excess KI. The intermediate is then reacted with ammonia to form cis-[Pt(NH3)2I2], which is a yellow precipitate. To remove the iodide ligands, AgNO3 is added, which leads to the precipitation of AgI, which will then be filtered away. This leaves behind cis-[Pt(OH2)2(NH3)2]2+, and excess KCl is added to form the final yellow precipitate cisplatin, with the formula cis-[PtCl2(NH3)3] [16]. The yield of this method is very high, up to 80% [17].
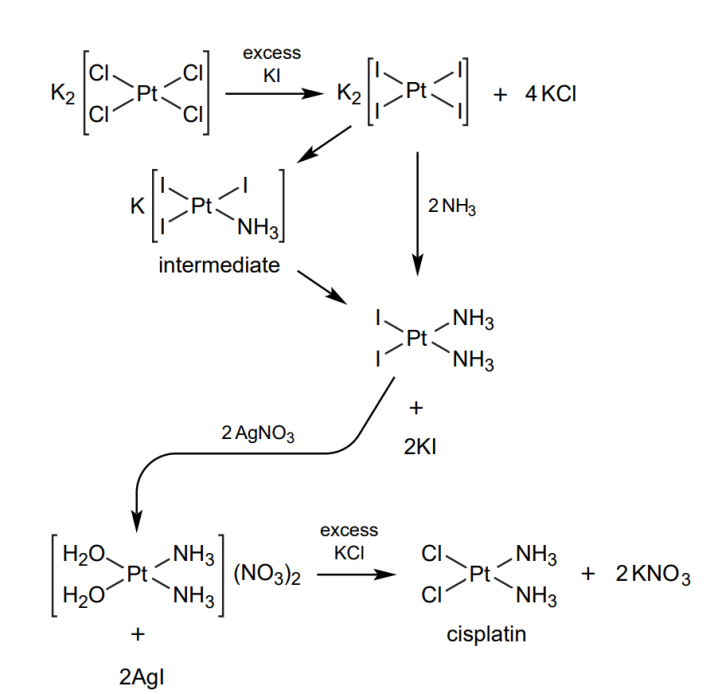
Equation 1. The reaction scheme for the synthesis of Cisplatin.
To synthesize carboplatin from cisplatin, the Cl-leaving groups need to be replaced with the nucleophile. The fact that carboplatin is soluble in water restricts some reaction schemes. In this reaction, an Ag(I) salt with the cyclobutane-1,1-dicarboxylic acid fragment reacts directly with cisplatin. This produces carboplatin and AgCl as by-products, which can be filtered away. The remaining filtrate can be evaporated to give pure carboplatin [18,19].
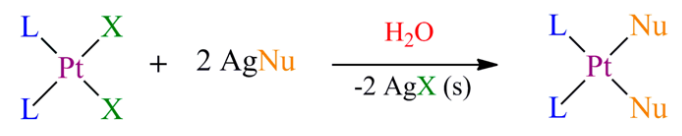
Equation 2. The reaction scheme for the synthesis of carboplatin from cisplatin. [20]
2.1.9. Pharmaeconomics. The cost of 5 ml of carboplatin solution with a concentration of 10 mg/ml is around $13.79 [21]. Recently, however, there has been a shortage of Carboplatin due to the coronavirus that has disrupted the manufacturing of this compound, and 93% of centers are going through a shortage of Carboplatin. [22] This might lead to an increase in the price of carboplatin.
2.2. Afatinib
2.2.1. Origins of Afatinib. Afatinib, also known as Gilotrif, is a targeted therapy drug and is categorized as a tyrosine kinase inhibitor. It was developed recently by Boehring Ingelheim Pharmaceuticals Inc. and approved by the FDA on July 12 of 2013 [23]. Currently, Afatinib is being used in the therapy of general NSCLC that involves resistance to platinum-based or non-resistant EGFR mutations.
2.2.2. Chemical Structure
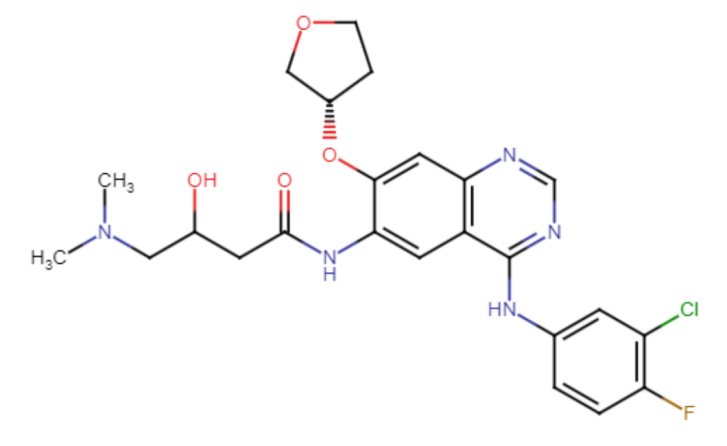
Figure 6. The chemical structure of Afatinib
Afatinib is a complex compound and contains quinazoline, furans, organofluorine, enamide, aromatic ether, tertiary amine, monochlorobenzene, and a secondary carboxamide group. Its IUPAC naming is N-[4-[(3-Chloro-4-fluoro phenyl) amino] -7-[[(3S)-tetra hydro-3-furanyl]oxy]-6-quinazolinyl]-4(dimethylamino)-2-butenamide.
2.2.3. Properties
Table 2. Properties of Afatinib [24]
Properties | DATA |
Appearance | Pale yellow powder |
Solubility | DMSO, or ethanol-soluble |
Melting Point | 102°C |
Stability | 1 year after the date of purchase, stable. A maximum of two months can be spent storing solutions in DMSO at -20°C. |
2.2.4. Pharmacodynamics and Pharmacology. Receptor tyrosine kinases are a category of tyrosine kinases, and they are in charge of mediating communication between cells and carrying out biological functions such as cell growth, motility, and metabolism. These receptors are found on the cell membrane, and they contain a ligand-binding pocket and an intracellular catalytic domain. When an extracellular growth factor ligand is formed, dimerization happens in the receptor, and dimers are produced. These dimers activate the receptors’ tyrosine kinase activity, and intracellular signals are given out by the process of phosphorylation.
Among lung adenocarcinoma patients, the most frequent EGFR mutations are the deletions in exon 19 as well as the substitutions in exon 21. As shown in the diagram below, the conformation of the EGFR is changed by the exon 19 deletion and L858R. The deletion of EGFR exon 19 increases kinase activity and brings oncogenic properties to EGFR. The L858R mutation stabilizes EGFR in its active conformation and increases its sensitivity to tyrosine kinase inhibitors.
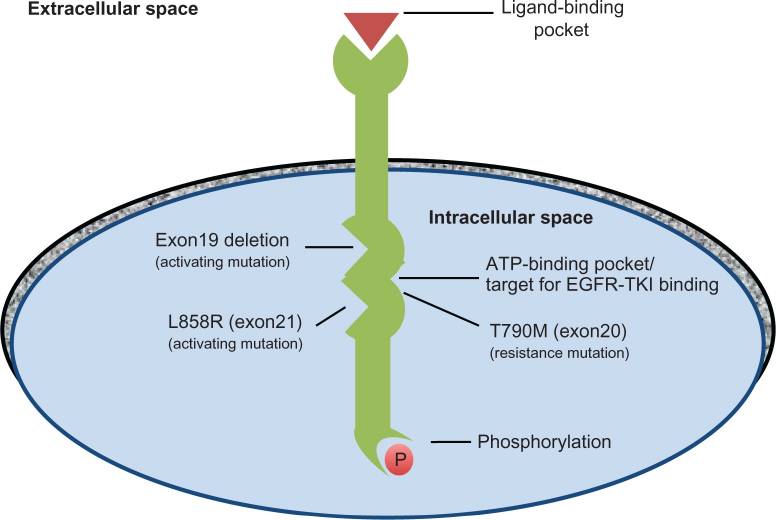
Figure 7. The structure of EGFR.
The main mechanism by which Afatinib functions is by covalently bonding to receptors (EGFR, HER2, HER4) at the ATP-binding site and inhibiting them irreversibly, thus reducing their ability to carry out tyrosine kinase activity as well as phosphorylation. Consequently, it blocks the signaling of all ErbB family members.
The reaction process is called a Michael addition. At the 6th position, the dimethyamino crotonamide group acts as a Michael acceptor and ultimately forms covalent bonds to the EGFR cysteine 797 at the sulfur atom.
Figure 7 shows the thioester bond formed between Afatinib and cysteine 797 [25].

Figure 8. The thioester bond formed between Afatinib and cysteine 797
There are also dipole-dipole interactions, such as hydrogen bonding between the 1st nitrogen of the quinazoline scaffold of Afatinib and the amino backbone of Met793 [26].
Both of these types of covalent bonding can be observed in Figure 7 below.
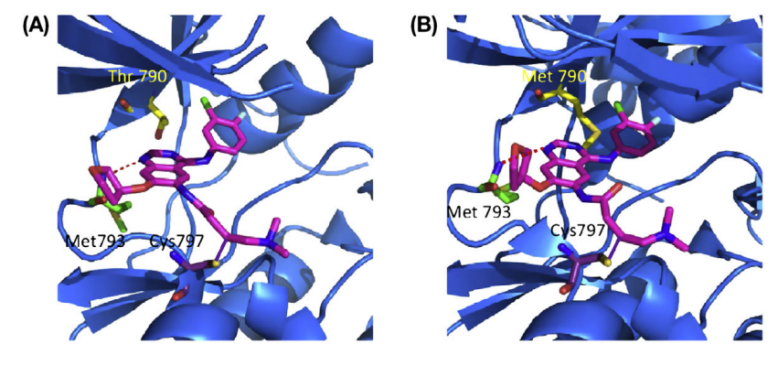
Figure 9. A crystallography that shows the bonding of Afatinib to wild-type EFGR (A) and a mutated T790M-EFGR (B).
This blockage of signals from all these growth receptors ultimately leads to a halt in the proliferation of cancer cells. In other studies, it was found that afatinib causes cell apoptosis in NSCLC cells that do not have the EGFR mutation.
2.2.5. Modes of Administration. For NSCLC, Afatinib is usually made into a tablet (20, 30, or 40 mg) and administered orally. It is advised to be consumed on an empty stomach once every day, and this includes at least 2 hours after eating and 1 hour before eating a meal. The dosing is adjusted with changes in creatinine clearance [27].
2.2.6. Side Effects. Some common side effects are dry skin, loss of appetite, acne, and nose bleeds. Severe side effects might be breathing difficulty, dehydration, sensitivity to light, or blurred vision [28].
2.2.7. Limitations. The T790M mutation gives cancer cells resistance, especially to first-generation EFGR Tyrosine Kinase Inhibitors, for instance, Gefitinib. As Afatinib is a second-generation Tyrosine Kinase Inhibitor, it is affected less by T790M as it is equally potent for both mutated and wild-type EGFR. Hence, it has a larger target and causes more side effects for patients. With the development of third-generation Tyrosine Kinase Inhibitors such as Rociletinib, which target specifically EGFR with the T790M mutation, this decreases their toxicity and is more efficient than the aforementioned.
2.2.8. Synthesis
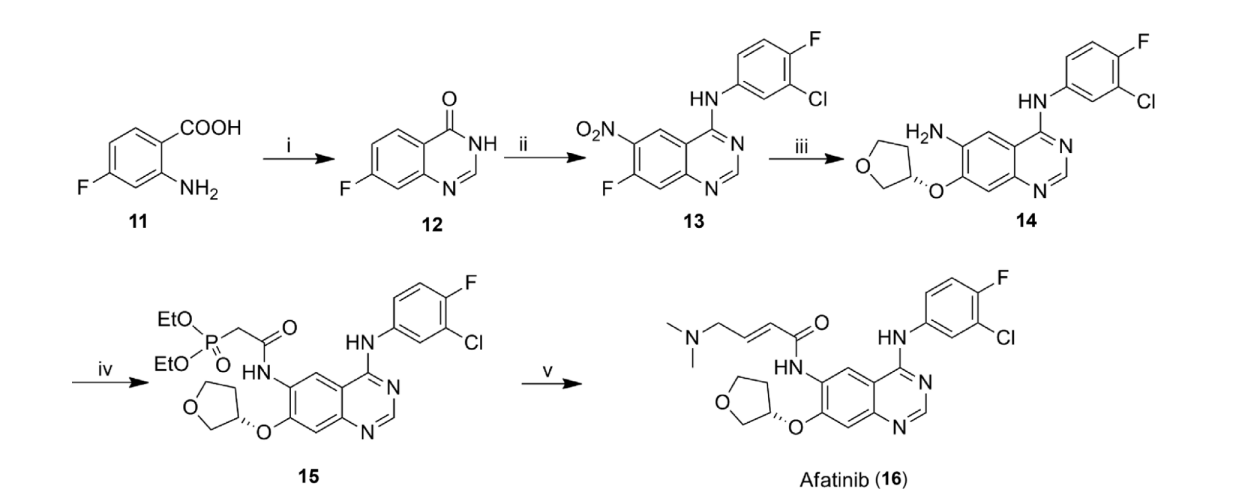
Equation 3. The full synthesis reactions to yield Afatinib.
The first step (i) includes 2-amino-4-fluorobenzoic acid (11) reacting with both 2-methoxy ethanol and formamidine acetate under reflux to form 7-fluoroquinolone acid (12).
The nitro group would be added at position 6 during the second step, and the processes of chlorination and condensation are carried out at position 4 with 3-chloro-4-fluoroaniline to yield compound (13). This process requires an acid HNO3/H2SO4 catalyst and a temperature of 100°C. Chlorination requires refluxing SOCl2 and CH3CN. Condensation requires 3-chloro-4-fluoroaniline.
To yield compound 14, a nucleophilic substitution reaction happens at the 7-fluoro group with (S)-(+)-3-hydroxy tetrahydrofuran, and a reduction of the 6-nitro group is followed. The conditions required are (S)-(+)-3-hydroxy-tetrahydrofuran in the substitution and H2, Pd/C, AcOH, and EtOH in the reduction.
The fourth reaction requires the presence of 1,1-carbonyldiimidazole (CDI), and compound 14 is reacted with diethylphosphonoacetic to form the peptide bond, producing compound 15. This reaction also requires a temperature of 40°C and tetrahydrofuran.
Lastly, compound 14 undergoes a Wittig-Horner homologation with 2-(dimethyl amino)-acetaldehyde in the conditions of LiCl, KOH, THF, and 5°C to finally yield Afatinib.
This reaction scheme only yields afatinib at 70%, but there is also another synthesis route known as the Wittig-Horner-Emmons reaction, which gives a 90% yield [29].
2.2.9. Pharmaeconomics. The cost of afatinib is very high. For a supply of 30 oral tablets, each of 20mg, the price is around $11713. This gives about $390 per tablet. Due to the daily administration, this leads to a very high cost of treatment in the long run [30].
3. Conclusion
Table 3. Summarized differences between Carboplatin and Afatinib
Summarized differences | Carboplatin | Afatinib |
Chemical Structure | Contains platinum group | Only consists of non-metal elements |
Mechanism | Cross-links to cell DNA | Blocking signals to growth receptors |
Cost of treatment | Lower | Very High |
Side effects | Very severe | Less severe as it is a targeted drug |
Limitations | Problems with the body’s immune system as well as the toxic build-up in body, which can cause relapse | Specific mutations in the cell DNA can lead to resistance to drug |
There are many different types of drugs used in the treatment of NSCLC. Carboplatin and Afatinib are just two of many. Carboplatin is a widely used platinum-based compound due to its reduced side effects compared to Cisplatin, and it acts as an alkylating agent that damages the DNA of cancer cells, thus preventing DNA replication. On the other hand, Afatinib is a targeted therapy drug (TKI), and it inhibits receptors such as EGFR to prevent cell growth and division. Although the targets of these two drugs are different, they both have the same function of preventing the proliferation of cancer cells and triggering cell apoptosis. It is very difficult to distinguish which is the more effective treatment, as both have their own pros and cons. One might say that Afatinib is superior because it produces fewer side effects, but on the other hand, the type of cancer mutation has a significant impact on its efficiency. Afatinib treatment is also much more costly than Carboplatin.
As time passes, the development and understanding of cancerous tumors advance. What will be the new first-line treatments for cancer in 10 years’ time? Perhaps it might be personalized medicine, as we are able to look deeper into the genetic code of human cellular DNA and develop drugs that can be specially tailored for patients, or it might be the rise of stem cell treatment, by growing a whole new tissue or organ for the patient.
References
[1]. Witsch, H. (2001, November 1). Short History of Lung Cancer. Academic.oup.com. https://academic.oup.com/toxsci/article/64/1/4/1637703
[2]. Document.write(title_ar[p,Lang]);. healthyhk.gov.hk. (2023, January 10). https://www.healthyhk.gov.hk/phisweb/en/chart_detail/55/
[3]. Burns, D. (2014, July). Standardized incidence of lung cancer by gender and histology (age ... Changing Rates of Adenocarcinoma of the Lung. https://www.researchgate.net/figure/ Standardized-incidence-of-lung-cancer-by-gender-and-histology-age-adjusted-to-2000-US_fig1_264054903
[4]. Ciupka, N. W. B. (2023, February 9). SCLC vs. NSCLC: What’s the difference?: NFCR Lung Cancer. NFCR. https://www.nfcr.org/blog/small-cell-lung-cancer-vs-non-small-cell-lung-cancer-whats-the-difference/
[5]. Nature Publishing Group. (n.d.). Cell Division and Cancer. Nature news. https://www.nature.com/scitable/topicpage/cell-division-and-cancer-14046590/#:~:text=Cells%20become%20cancerous%20after%20mutations,possess%2060%20or%20more%20mutations
[6]. Gibbons, D. L., Byers, L. A., & Kurie, J. M. (2014). Smoking, p53 mutation, and lung cancer. Molecular cancer research : MCR, 12(1), 3–13. https://doi.org/10.1158/1541-7786.MCR-13-0539
[7]. Suntharalingam, K., A. Duarte, A., J. Mann, D., & Ramon Vilar. (2013, February 19). A platinum complex that binds non-covalently to DNA and induces cell death via a different mechanism than cisplatin. Metallomics. https://pubs.rsc.org/en/content/ articlehtml/2013/mt/c3mt20252f
[8]. Gazdar, A., Robinson, L., Oliver, D., Xing, C., Travis, W. D., Soh, J., Toyooka, S., Watumull, L., Xie, Y., Kernstine, K., & Schiller, J. H. (2014). Hereditary lung cancer syndrome targets never smokers with germline EGFR gene T790M mutations. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer, 9(4), 456–463. https://doi.org/10.1097/JTO.0000000000000130
[9]. Carboplatin: 41575-94-4. ChemicalBook. (n.d.). https://www.chemicalbook.com/ ChemicalProductProperty_EN_CB6702418.htm
[10]. Uniprot website fallback message. UniProt. (n.d.). https://www.uniprot.org/uniprotkb/ P49573/entry
[11]. Sousa, G. F. de, Wlodarczyk, S. R., & Monteiro, G. (2014). Carboplatin: Molecular mechanisms of action associated with Chemoresistance. Brazilian Journal of Pharmaceutical Sciences. https://www.scielo.br/j/bjps/a/9F6tZpxsm7spMKjGn6Z3kvr/
[12]. Stefanou, D. T., Souliotis, V. L., Zakopoulou, R., Liontos, M., & Bamias, A. (2021, December 31). DNA damage repair: Predictor of platinum efficacy in ovarian cancer?. MDPI. https://www.mdpi.com/2227-9059/10/1/82
[13]. Vandergriendt, C. (2021, April 21). Long-term side effects of chemotherapy. Healthline. https://www.healthline.com/health/cancer/long-term-side-effects-of-chemotherapy#cognitive-difficulties
[14]. U.S. National Library of Medicine. (2021, September 15). Carboplatin Injection: Medlineplus Drug Information. MedlinePlus. https://medlineplus.gov/druginfo/meds/a695017.html
[15]. Fotopoulou, C. (2014, December). Limitations to the use of carboplatin-based therapy in Advanced ovarian cancer. EJC supplements : EJC : official journal of EORTC, European Organization for Research and Treatment of Cancer ... [et al.]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4683379/#:~:text=There%20appear%20to%20be%20three,%2C%20renal%20toxicity%2C%20and%20ototoxicity
[16]. Alderden, R. A., Hall, M. D., & Hambley, T. W. (2006). The Discovery and Development of Cisplatin. Products of Chemistry. https://home.iitk.ac.in/~sprath/Lecture_1.pdf
[17]. F.D.Rochon. (1985, October 1). Iodo-bridged complexes of platinum(I1) and synthesis of cis mixed-amine platinum(I1) compounds . https://cdnsciencepub.com/doi/pdf/10.1139/v86-312
[18]. Vardanyan, R. S., & Hruby, V. J. (2006). Synthesis of essential drugs. Elsevier.
[19]. Wilson, J. J., & Lippard, S. J. (2014). Synthetic methods for the preparation of platinum anticancer complexes. Chemical reviews, 114(8), 4470–4495. https://doi.org/10.1021/cr4004314
[20]. Wilson, J. J., & Lippard, S. J. (2014, April 23). Synthetic methods for the preparation of platinum anticancer complexes. Chemical reviews. https://www.ncbi.nlm.nih.gov/pmc/ articles/PMC3999256/
[21]. Carboplatin prices, coupons, Copay & Patient Assistance. Drugs.com. (n.d.). https://www.drugs.com/price-guide/carboplatin
[22]. Michael Ganio, P. (2023, July 27). Pharmacy expert details causes of carboplatin/cisplatin shortage in the U.S. Cancer Network. https://www.cancernetwork.com/view/pharmacy-expert-details-causes-of-carboplatin-cisplatin-shortage-in-the-u-s-
[23]. Gilotrif (afatinib) FDA approval history. Drugs.com. (n.d.-b). https://www.drugs.com/history/gilotrif.html#:~:text=Company%3A%20Boehringer%20Ingelheim%20Pharmaceuticals%2C%20Inc
[24]. Afatinib. Afatinib CAS#: 850140-72-6. (n.d.). https://www.chemicalbook.com/ ProductChemicalPropertiesCB62507657_EN.htm
[25]. Wurglics, S.-Z. (n.d.). Neue Arzneimittel Frühjahr 2013.
[26]. Hossam, M., Lasheen, D. S., & Abouzid, K. A. (2016). Covalent EGFR Inhibitors: Binding Mechanisms, Synthetic Approaches, and Clinical Profiles. Archiv der Pharmazie, 349(8), 573–593. https://doi.org/10.1002/ardp.201600063
[27]. Moosavi, L., & Polineni, R. (2022). Afatinib. NIH.
[28]. U.S. National Library of Medicine. (2017, February 15). Afatinib: Medlineplus Drug Information. MedlinePlus. https://medlineplus.gov/druginfo/meds/a613044.html
[29]. V. Prakash Reddy, V. P. ReddyV. P. R., & are, A. therapeutics for cancer. (2015, February 20). Organofluorine compounds as anticancer agents. Organofluorine Compounds in Biology and Medicine. https://www.sciencedirect.com/science/article/abs/pii/ B9780444537485000095
[30]. Gilotrif prices, coupons, Copay & Patient Assistance. Drugs.com. (n.d.-c). https://www.drugs.com/price-guide/gilotrif#:~:text=Gilotrif%20Prices%2C%20Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
Cite this article
Yeung,K.P.J. (2024). Comparison between Carboplatin and Afatinib in the drug treatment of Non-Small Cell Lung Cancer. Theoretical and Natural Science,44,45-56.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Witsch, H. (2001, November 1). Short History of Lung Cancer. Academic.oup.com. https://academic.oup.com/toxsci/article/64/1/4/1637703
[2]. Document.write(title_ar[p,Lang]);. healthyhk.gov.hk. (2023, January 10). https://www.healthyhk.gov.hk/phisweb/en/chart_detail/55/
[3]. Burns, D. (2014, July). Standardized incidence of lung cancer by gender and histology (age ... Changing Rates of Adenocarcinoma of the Lung. https://www.researchgate.net/figure/ Standardized-incidence-of-lung-cancer-by-gender-and-histology-age-adjusted-to-2000-US_fig1_264054903
[4]. Ciupka, N. W. B. (2023, February 9). SCLC vs. NSCLC: What’s the difference?: NFCR Lung Cancer. NFCR. https://www.nfcr.org/blog/small-cell-lung-cancer-vs-non-small-cell-lung-cancer-whats-the-difference/
[5]. Nature Publishing Group. (n.d.). Cell Division and Cancer. Nature news. https://www.nature.com/scitable/topicpage/cell-division-and-cancer-14046590/#:~:text=Cells%20become%20cancerous%20after%20mutations,possess%2060%20or%20more%20mutations
[6]. Gibbons, D. L., Byers, L. A., & Kurie, J. M. (2014). Smoking, p53 mutation, and lung cancer. Molecular cancer research : MCR, 12(1), 3–13. https://doi.org/10.1158/1541-7786.MCR-13-0539
[7]. Suntharalingam, K., A. Duarte, A., J. Mann, D., & Ramon Vilar. (2013, February 19). A platinum complex that binds non-covalently to DNA and induces cell death via a different mechanism than cisplatin. Metallomics. https://pubs.rsc.org/en/content/ articlehtml/2013/mt/c3mt20252f
[8]. Gazdar, A., Robinson, L., Oliver, D., Xing, C., Travis, W. D., Soh, J., Toyooka, S., Watumull, L., Xie, Y., Kernstine, K., & Schiller, J. H. (2014). Hereditary lung cancer syndrome targets never smokers with germline EGFR gene T790M mutations. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer, 9(4), 456–463. https://doi.org/10.1097/JTO.0000000000000130
[9]. Carboplatin: 41575-94-4. ChemicalBook. (n.d.). https://www.chemicalbook.com/ ChemicalProductProperty_EN_CB6702418.htm
[10]. Uniprot website fallback message. UniProt. (n.d.). https://www.uniprot.org/uniprotkb/ P49573/entry
[11]. Sousa, G. F. de, Wlodarczyk, S. R., & Monteiro, G. (2014). Carboplatin: Molecular mechanisms of action associated with Chemoresistance. Brazilian Journal of Pharmaceutical Sciences. https://www.scielo.br/j/bjps/a/9F6tZpxsm7spMKjGn6Z3kvr/
[12]. Stefanou, D. T., Souliotis, V. L., Zakopoulou, R., Liontos, M., & Bamias, A. (2021, December 31). DNA damage repair: Predictor of platinum efficacy in ovarian cancer?. MDPI. https://www.mdpi.com/2227-9059/10/1/82
[13]. Vandergriendt, C. (2021, April 21). Long-term side effects of chemotherapy. Healthline. https://www.healthline.com/health/cancer/long-term-side-effects-of-chemotherapy#cognitive-difficulties
[14]. U.S. National Library of Medicine. (2021, September 15). Carboplatin Injection: Medlineplus Drug Information. MedlinePlus. https://medlineplus.gov/druginfo/meds/a695017.html
[15]. Fotopoulou, C. (2014, December). Limitations to the use of carboplatin-based therapy in Advanced ovarian cancer. EJC supplements : EJC : official journal of EORTC, European Organization for Research and Treatment of Cancer ... [et al.]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4683379/#:~:text=There%20appear%20to%20be%20three,%2C%20renal%20toxicity%2C%20and%20ototoxicity
[16]. Alderden, R. A., Hall, M. D., & Hambley, T. W. (2006). The Discovery and Development of Cisplatin. Products of Chemistry. https://home.iitk.ac.in/~sprath/Lecture_1.pdf
[17]. F.D.Rochon. (1985, October 1). Iodo-bridged complexes of platinum(I1) and synthesis of cis mixed-amine platinum(I1) compounds . https://cdnsciencepub.com/doi/pdf/10.1139/v86-312
[18]. Vardanyan, R. S., & Hruby, V. J. (2006). Synthesis of essential drugs. Elsevier.
[19]. Wilson, J. J., & Lippard, S. J. (2014). Synthetic methods for the preparation of platinum anticancer complexes. Chemical reviews, 114(8), 4470–4495. https://doi.org/10.1021/cr4004314
[20]. Wilson, J. J., & Lippard, S. J. (2014, April 23). Synthetic methods for the preparation of platinum anticancer complexes. Chemical reviews. https://www.ncbi.nlm.nih.gov/pmc/ articles/PMC3999256/
[21]. Carboplatin prices, coupons, Copay & Patient Assistance. Drugs.com. (n.d.). https://www.drugs.com/price-guide/carboplatin
[22]. Michael Ganio, P. (2023, July 27). Pharmacy expert details causes of carboplatin/cisplatin shortage in the U.S. Cancer Network. https://www.cancernetwork.com/view/pharmacy-expert-details-causes-of-carboplatin-cisplatin-shortage-in-the-u-s-
[23]. Gilotrif (afatinib) FDA approval history. Drugs.com. (n.d.-b). https://www.drugs.com/history/gilotrif.html#:~:text=Company%3A%20Boehringer%20Ingelheim%20Pharmaceuticals%2C%20Inc
[24]. Afatinib. Afatinib CAS#: 850140-72-6. (n.d.). https://www.chemicalbook.com/ ProductChemicalPropertiesCB62507657_EN.htm
[25]. Wurglics, S.-Z. (n.d.). Neue Arzneimittel Frühjahr 2013.
[26]. Hossam, M., Lasheen, D. S., & Abouzid, K. A. (2016). Covalent EGFR Inhibitors: Binding Mechanisms, Synthetic Approaches, and Clinical Profiles. Archiv der Pharmazie, 349(8), 573–593. https://doi.org/10.1002/ardp.201600063
[27]. Moosavi, L., & Polineni, R. (2022). Afatinib. NIH.
[28]. U.S. National Library of Medicine. (2017, February 15). Afatinib: Medlineplus Drug Information. MedlinePlus. https://medlineplus.gov/druginfo/meds/a613044.html
[29]. V. Prakash Reddy, V. P. ReddyV. P. R., & are, A. therapeutics for cancer. (2015, February 20). Organofluorine compounds as anticancer agents. Organofluorine Compounds in Biology and Medicine. https://www.sciencedirect.com/science/article/abs/pii/ B9780444537485000095
[30]. Gilotrif prices, coupons, Copay & Patient Assistance. Drugs.com. (n.d.-c). https://www.drugs.com/price-guide/gilotrif#:~:text=Gilotrif%20Prices%2C%20Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit









