1. Introduction
Zika virus (ZIKV) is a positive-strand RNA flavivirus mainly transmitted through mosquitoes that inflicts damage upon human organs, notably affecting the nervous, cardiovascular, and reproductive systems [1]. Despite the widespread of ZIKV infection and its severeness, there are currently no appropriate drugs or preventive vaccines to cure the disease.
In recent years, however, a breakthrough in the scientific exploration of indigenous Chinese plants has uncovered a species possessing anti-Zika properties, including compounds called Wulfenioidin [2]. This plant, previously utilized for alleviating symptoms like coughing, edema, and arthritis, concealed an undiscovered facet of its capabilities [2].
The roots of Wulfenioidin plant were extracted for analysis. The structures and absolute configurations were characterized by spectroscopic methods, single crystal X-ray diffraction, and electronic circular dichroism analyses. According to the research conducted by Wen-Chao Tu, Yong-Xiang Huang and others, Wulfenioidin F was isolated as a colourless, oily substance and its formula is C21H28O3 [3]. The IR spectrum exhibited typical absorptions of a hydroxyl and a benzene ring. As the five double bonds accounted for five indices of hydrogen deficiency, Wulfenioidin F was tricyclic.
Upon further scrutiny by scientists, Wulfenioidin F underwent extensive biological testing. Abundant data, including EC50 values, demonstrated the compound’s proficiency in inhibiting viral replication, thereby inhibiting them from replication and protecting the host from further defection. In conclusion, Wulfenioidin F is qualified as one of the candidates for future research as antiviral drugs against ZIKV infection.
2. Synthesis Pathway Description
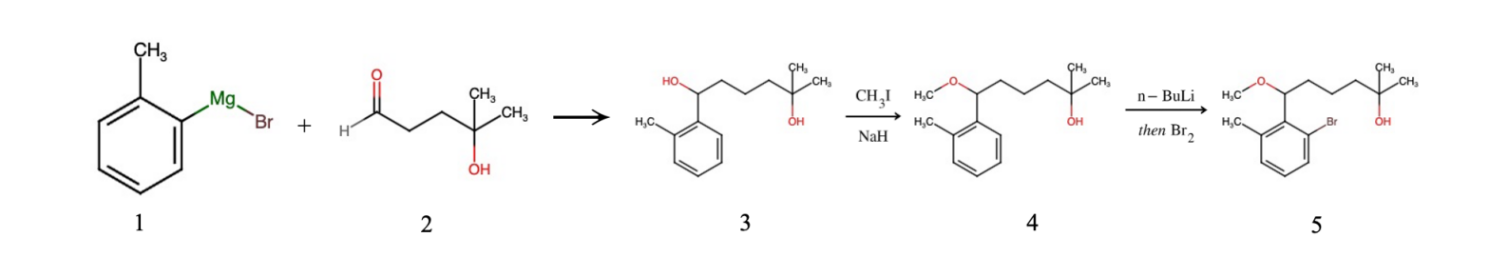
Figure 1. Preparation of 6-(2-bromo-6-methylphenyl)-6-methoxy-2-methylhexan-2-ol
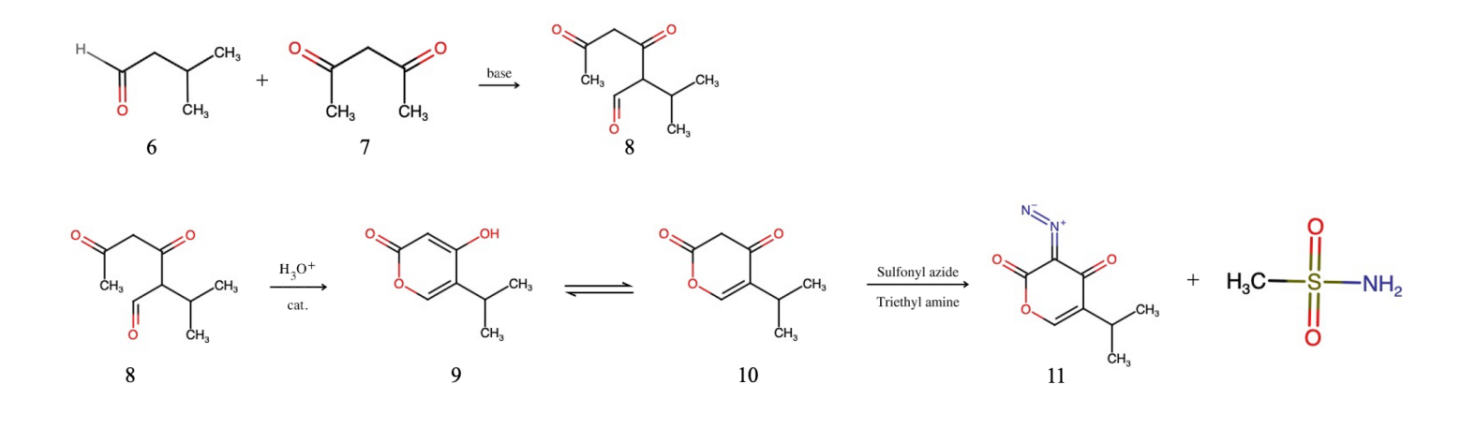
Figure 2. Preparation of 5-(propan-2-yl)-3-(I^5-diazynylidene)-3,4-dihydro-2H-pyran-2,4-dione
To synthesize the desired compound, we begin with two raw materials to prepare: bromo(2-methylphenyl) magnesium and 5-hydroxy-5-methylhexan-2-one. Figure 1 shows the process of synthesizing 6-(2-bromo-6-methylphenyl)-6-methoxy-2-methylhexan-2-ol. Initially, they undergo a Grignard reaction, leading to product 2. Subsequently, upon the addition of NaH and CH3I, an ether is formed, leading to product 3. Since the bond linking the benzene ring and the carbon chain can rotate freely, through rotation, product 4 will react with the strong base n-BuLi, resulting in an intermediate reactive substance, from which one hydrogen is removed. Finally, the addition of Br2 leads to an addition of Br functional group on the benzene ring, which serves as a “handle” for the ultimate connection of two subparts. This process yields our first target molecule, product 5.
The second stage of the whole synthetic pathway is the preparation of 5-(propan-2-yl)-3-(I^5-diazynylidene)-3,4-dihydro-2H-pyran-2,4-dione, as shown in figure 2. To start with, use isovaleraldehyde and acetylacetone as starting material. Both compounds are commonly used and can be bought at a relatively low price. First, add excessive base into the mixture, and both compounds will form enolates due to the deprotonation of carbonyls. The acetylacetone enolate is more stable because it can resonate and form a symmetrical structure. Thus, the isovaleraldehyde enolate, being more reactive, will add on to the carbon linked to the ketone functional group of the acetylacetone, forming product 8. Afterwards, add acid and catalyst into the mixture. The oxygen on the ketone functional group in product 8 is partially negative. Hence, they combine with the excessive protons in the environment, and the bonds rearrange to form product 9. Product 9 then resonates in the form of structure 10, which is the needed molecule for diazo transfer to occur. Add sulfonyl azide and triethyl amine, serving as reagents for diazo transfer reaction, into the mixture. Compound 10 reacts with the two reagents, forming compound 11 and byproduct methane sulfonamide.
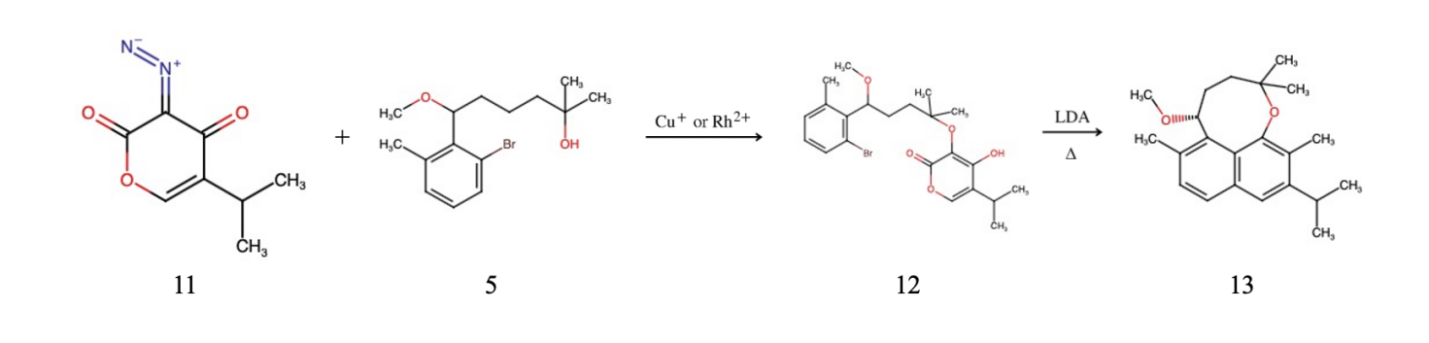
Figure 3. Scheme 2. Combination of two subparts and advancement to Wulfenioidin F
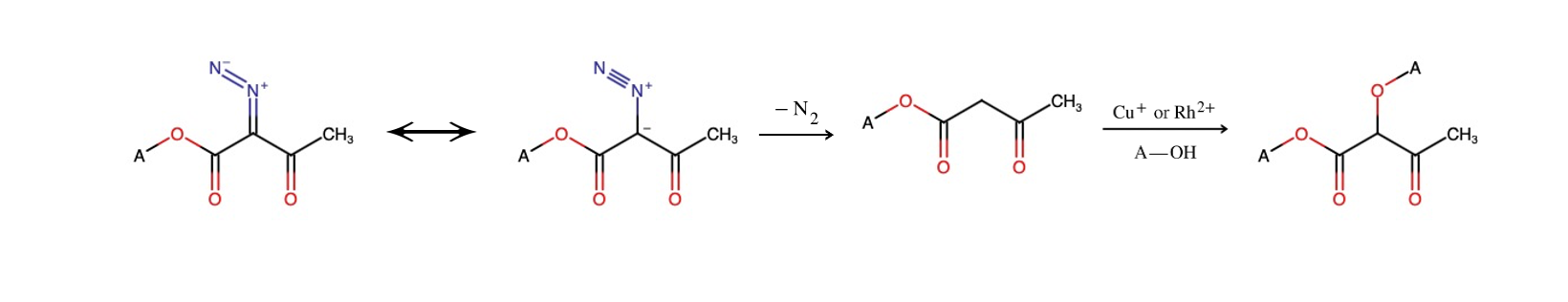
Figure 4. Schematic view of the reaction mechanism for Diazo carbonyl and alcohol
The ultimate step entails the amalgamation of the two entities previously synthesized. Nevertheless, prior to initiating this connection, it is necessary to ascertain whether there will be a reaction in the wrong orientation, leading to the production of unwanted byproducts. To prevent this from happening, we must first form the eight-carbon ring through the linkage of two subparts, which ends up in the formation of ether. Referring to Figure 3 above, which offers an outline of how to combine the subparts, the N2 exhibits a strong stability under natural conditions, thus the bond connecting N and C isn’t stable and can easily break. This results in the formation of carbene, which easily reacts with alcohol under the presence of catalysts such as Cu+ or Ru2+, as the catalyst can stabilize the carbene and facilitate the interaction of the two molecules, resulting in the addition of an alcohol to the central carbon. This catalytic process contributes to the production of ether. Figure 4. indicates an effective schematic pathway for Diazo transfer.
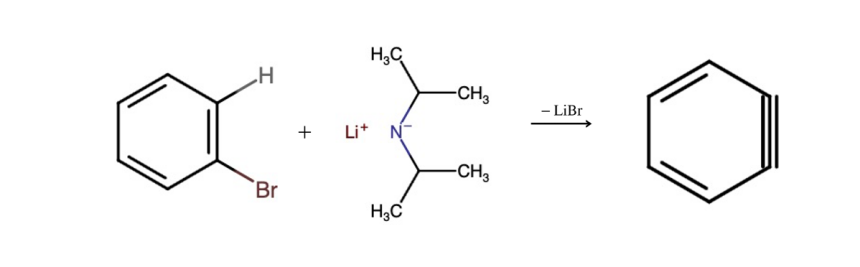
Figure 5. Schematic view of the reaction mechanism for Benzyne
Then, we need to synthesize benzyne as it serves as an intermediate compound that allows the Diels-Alders reaction to happen, which is crucial for the entire process. Referring to Figure 5, which provides a method of synthesizing benzyne that will be utilized to further process, we use a benzene ring with a Bromine functional group to react with strong base, in this case, LDA. Because the Nitrogen from the strong base has an affinity for hydrogen, it forms a bond with the hydrogen. The bond between the hydrogen and the benzene breaks, forming a new bond on the benzene, and the Bromine leaves the benzene ring. According to the Pka data [4], it is evident that the hydrogen-nitrogen bond is low in acidity, indicating that it is stable. Consequently, this reaction yields benzyne along with two by-products. Thus, this is why LDA is used in the final step of scheme 2.
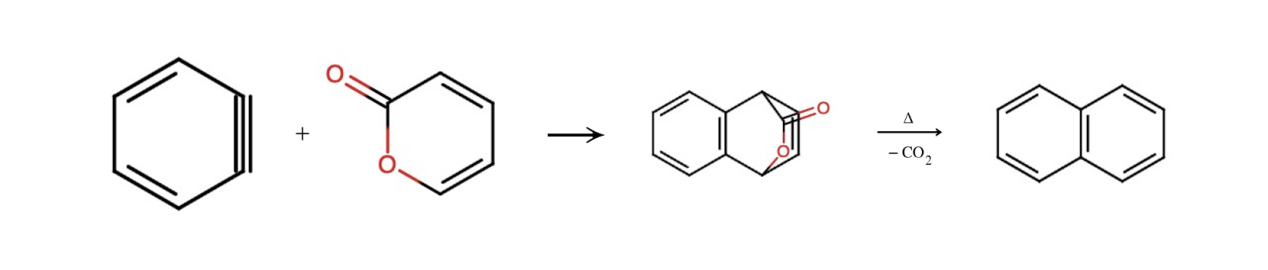
Figure 6. Schematic view of Diels-Alder reaction between benzyne and pyrone that forms a naphthalene
Finally, we establish a connection between benzyne (an intermediate form of product 5 in Scheme 2, with the benzene converted into benzyne) and pyrone (product 11 in Scheme 2) using the Diels-Alder reaction. During this reaction, the benzyne and the pyrone are connected, and part of the pyrone is folded upwards, creating an angle. Under a heating condition, the CO2 component dissociates, breaking the bonds and yielding the object, naphthalene. Thus, this is why heating is required in the final step of scheme 2. This leads us to the final product, Wulfenioidin F. Figure 6 refers to the connection of lower two parts, which is the merge of naphthalene [5].
3. Conclusion
This paper presents a synthesis pathway that leads to the production of Wulfenioidin F, which can be adopted as a drug defending against ZIKA virus. This work segregates the intended substance to two subparts and offers details of synthesis procedure. Also, the order of combining two subparts mentioned in the final step is emphasized. The whole work enables researchers to approach the final compound, Wulfenioidin F, thus helping them to produce it more efficiently.
References
[1]. Calvet, G.A., Kara, E.O., Bôtto-Menezes, C.H.A. et al. (2023) Detection and persistence of Zika virus in body fluids and associated factors: a prospective cohort study. Sci Rep 13, 21557.
[2]. Zhu, Y., Wei, H. (2020) The Annals of National Medicine in Yunnan. The Nationalities Publishing House of Yunnan, 2010: 239−240.
[3]. Tu, W. (2023) Wulfenioidins D−N, Structurally Diverse Diterpenoids with Anti-Zika Virus Activity Isolated from Orthosiphon wulfenioides. J. Nat. Prod, 86: 2348−2359.
[4]. 2005. Bordwell. (2005) pKa table. http://www.chem.wisc.edu/areas/reich/pkatable/index.htm
[5]. Hafeman, N. (2020) The Total Synthesis of (−)-Scabrolide A. J. Am. Chem. Soc. 142, 19: 8585–8590.
Cite this article
Sun,R.;Wang,Y. (2024). The total synthesis of Wulfenioidin F. Theoretical and Natural Science,44,123-126.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Calvet, G.A., Kara, E.O., Bôtto-Menezes, C.H.A. et al. (2023) Detection and persistence of Zika virus in body fluids and associated factors: a prospective cohort study. Sci Rep 13, 21557.
[2]. Zhu, Y., Wei, H. (2020) The Annals of National Medicine in Yunnan. The Nationalities Publishing House of Yunnan, 2010: 239−240.
[3]. Tu, W. (2023) Wulfenioidins D−N, Structurally Diverse Diterpenoids with Anti-Zika Virus Activity Isolated from Orthosiphon wulfenioides. J. Nat. Prod, 86: 2348−2359.
[4]. 2005. Bordwell. (2005) pKa table. http://www.chem.wisc.edu/areas/reich/pkatable/index.htm
[5]. Hafeman, N. (2020) The Total Synthesis of (−)-Scabrolide A. J. Am. Chem. Soc. 142, 19: 8585–8590.









