1. Introduction
Alzheimer's disease (AD) is a widely recognized progressive neurological disorder marked by memory loss and cognitive decline, culminating in the eventual loss of autonomy for affected individuals [1,2]. The neuropathological signature of AD centers around the buildup of Amyloid Beta (Aβ) protein, which contributes to neuronal demise, synaptic deterioration, and ultimately, neurodegeneration, leading to compromised brain function [3].
One significant genetic risk factor associated with late-onset sporadic Alzheimer's disease is the APOE ε4 allele. The e4 variant of the APOE gene has been extensively linked to an elevated risk of developing the disease. Importantly, individuals inheriting one or two copies of the APOE e4 allele exhibit reduced efficiency in clearing amyloid plaques compared to other alleles, contributing to their increased susceptibility to the disease [4,5]. Herpes Simplex Virus 1 (HSV-1) is a common virus primarily transmitted through oral contact, often causing infections in and around the mouth, commonly known as cold sores [6,7]. Recent evidence has suggested a potential association between HSV-1 and Alzheimer's disease pathogenesis. Studies have shown that HSV-1 infected cells display an accumulation of excess Aβ2, which could potentially contribute to an accelerated progression of AD [8,9].
Emerging research has pointed towards a possible interplay between the APOE4 allele and HSV-1 reactivation in Alzheimer's disease patients [10]. This intriguing connection raises the hypothesis that APOE4 may not only influence Aβ aggregation but also play a role in the reactivation of latent HSV-1, exacerbating Aβ accumulation and hastening the progression of AD.
Understanding the complex relationship between APOE4, HSV-1, and Aβ accumulation is of paramount importance for unraveling the underlying mechanisms of Alzheimer's disease. Therefore, this research proposal aims to investigate the impact of APOE4 on HSV-1 reactivation and its potential role in accelerating Aβ accumulation in AD. Through a multidimensional approach encompassing in vivo and in vitro experiments, this study seeks to shed light on this intricate interplay and contribute to the broader understanding of AD pathogenesis. Ultimately, the findings from this research may pave the way for novel therapeutic interventions and personalized prevention strategies for Alzheimer's disease.
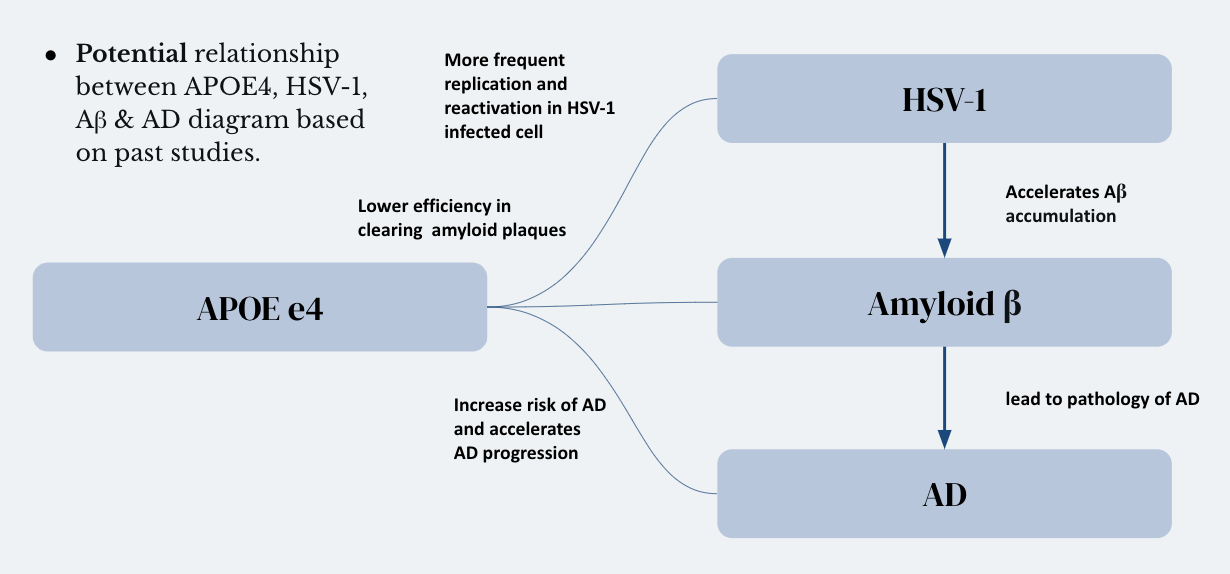
Figure 1. Potential relationship between APOE4, HSV-1, Aβ & AD diagram based on past studies.
2. Method
By addressing these critical research questions, we aim to contribute significant insights to the field of Alzheimer's disease and pave the way for the development of targeted interventions that may lead to improved patient outcomes.
In our research proposal, we have meticulously selected the IC-0001 human neuronal cell line as the primary cell line for our experiments. This cell line exhibits a unique ability to differentiate into human neurons from embryonic neural stem cells, making it an optimal candidate for injection into mice embryos in subsequent studies. This approach aims to minimize any discrepancies in the effects of Herpes Simplex Virus 1 (HSV-1) between human and mice subjects.
For our investigations, we will utilize the 5xFAD mice model, a well-established and widely employed model in Alzheimer's disease (AD) research. This model effectively replicates several AD-related phenotypes, notably manifesting an aggressive and progressive amyloid plaque pathology. Additionally, we intend to introduce a mouse model carrying the human APOE4 gene, which will be crossbred with the 5xFAD mice, ultimately generating the 5xFAD & APOE4 mice model.
It is important to emphasize that we have taken rigorous measures to control for potential confounding variables within our experimental groups. Specifically, we have ensured consistency in genetic background, age, gender, diet, and health status, thereby facilitating more precise and reliable comparisons between the various experimental conditions.
In future experiments, we will conduct Cervical Dislocation following the guidelines outlined by the National Research Council (NRC) to ensure a humane procedure that minimizes the mice's pain during the experiments.
By employing these carefully chosen cell lines and animal models, while simultaneously implementing robust controls, our research endeavors to shed light on the intricate interplay between HSV-1, Aβ accumulation, and APOE4, thereby providing valuable insights into the pathogenesis of Alzheimer's disease.
3. Experiment
3.1. Experiment 1
In the first experiment, our primary objective is to investigate whether the presence of HSV-1 in wild-type mice leads to an accumulation of Aβ42, thereby accelerating the progression of Alzheimer's disease (AD). This investigation will involve two experimental groups: the HSV-1 injected group and the non-injected group. For the study, we will utilize wild-type mice, all aged 3 months at the beginning of the experiment, which is equivalent to approximately 20-30 years in human age. The total number of mice involved in the experiment will be 30, with 15 in each group (experimental and control). The duration of this experiment is expected to be about 4 months.
To elucidate the effect of HSV-1 on amyloid accumulation, we will employ two complementary approaches. Firstly, we will conduct in vivo functional Magnetic Resonance Imaging (fMRI) three times a month to observe amyloid beta accumulation in the brain. This non-invasive imaging technique will allow us to monitor changes in functional connectivity and brain structure associated with Aβ42 accumulation in response to HSV-1 infection. Secondly, we will perform in vitro western blot analysis to investigate the expression levels of amyloid beta protein in each group. This quantitative analysis will enable us to compare the levels of Aβ42 between the HSV-1 infected and non-infected wild-type mice.
A threshold image analysis will be applied to the fMRI data to quantitatively measure Aβ accumulation in specific brain regions. Statistical analysis, such as t-tests, will be employed to compare the mean levels of Aβ42 accumulation between the experimental and control groups. These analyses will help us assess the correlation between HSV-1 infectivity and Aβ42 oligomers accumulation in wild-type mice.
Based on existing research and information, we hypothesize that the results of our study will reveal a higher level of Aβ42 oligomers accumulation in the brains of HSV-1 infected wild-type mice compared to the non-infected wild-type mice. This observation would suggest that HSV-1 infection leads to the accumulation of Aβ42 in wild-type mice, providing valuable insights into the potential link between viral infections and Alzheimer's disease progression.
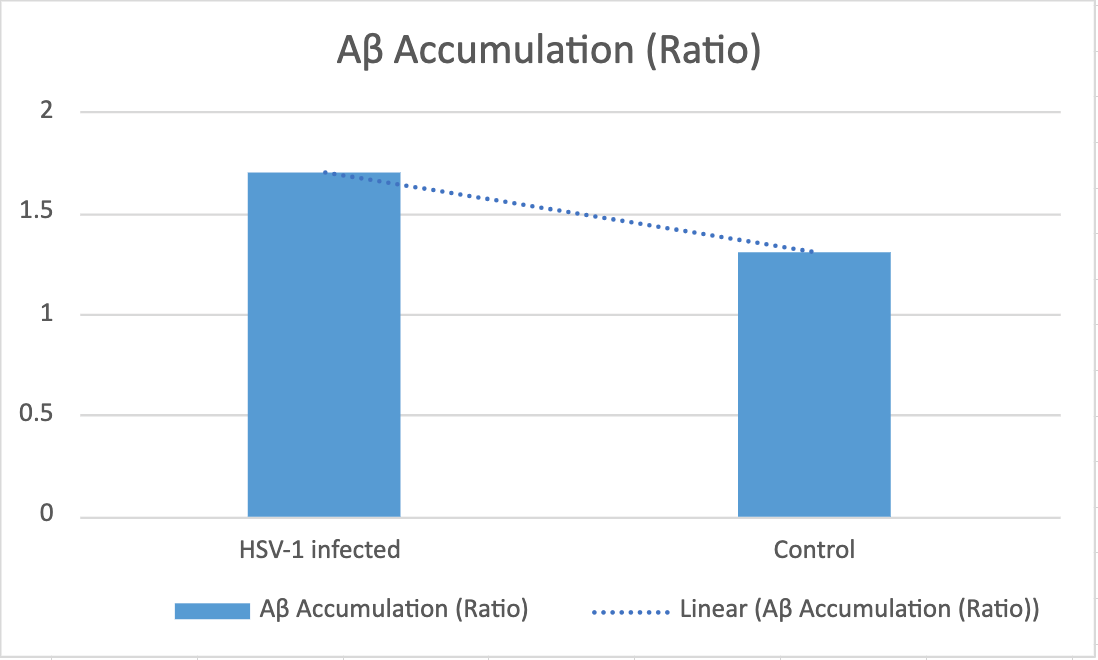
Figure 2. A higher level of Aβ42 oligomers accumulation in the brains of HSV-1 infected wild-type mice compared to the non-infected wild-type mice.
3.2. Experiment 2
The objective of our second experiment is to investigate whether APOE4 induces an accumulation of Aβ42, contributing to the initiation of Alzheimer's disease (AD) at early stages. To achieve this, we will use the 5xFAD mice model, which carries both APOE4 and APOE2 alleles, and the mice will be separated by sex. All mice will be 3 months old at the beginning of the study, equivalent to approximately 20 - 30 years in human age. The total number of mice involved in the experiment will be 60, with 15 in each group (experimental and control). The duration of this experiment will be approximately 4 months, the same as Experiment 1.
The division of mice by sex aims to gain a better understanding of sex-specific differences in Aβ42 accumulation and responses to HSV-1 interventions across different APOE alleles. We had considered that estrogen, a female sex hormone, might play a protective role against Aβ42, thereby resulting in potentially less severe Aβ42 accumulation in female mice. Additionally, the separation of mice into APOE4 and APOE2 allele groups will enable a direct comparison of Aβ42 oligomer accumulation within the 5xFAD mice model, helping to elucidate whether the presence of APOE4 may lead to early-stage Alzheimer's disease.
To initiate the experiment, the IC-0001 human neuronal cell line will be infected with non-activated HSV-1 in the media culture. Technologies includefunctional Magnetic Resonance Imaging (fMRI), western blot (WB), Immunofluorescence (IF) will be employed to ensure the HSV-1 is not activated by assessing the levels of certain biomarkers: glucocorticoid, cortisone, sgk1, MASP (Mitochondrial Antiviral-Signaling Protein), and Icp0 (Immediate Early Protein 0). Subsequently, the HSV-1 infected cell line will be injected into all the experimental groups. Our investigation into the effect of APOE4 on amyloid accumulation will employ both in vivo and in vitro approaches. Functional Magnetic Resonance Imaging (fMRI) will be performed three times a month over a total observation period of 3 months to monitor amyloid beta accumulation in the mice brain.
In the in vitro analysis, we will employ technologies such as Immunofluorescence (IF) and enzyme-linked immunosorbent assay (ELISA) to investigate the expression of amyloid beta protein in each group. These techniques will enable us to quantify and compare the levels of Aβ42 between the experimental and control groups, shedding light on the specific impact of APOE4 on amyloid accumulation. We will use a Threshold image analysis and statistical methods such as t-tests to quantify Aβ42 accumulation and assess the correlation between HSV-1 infectivity and Aβ42 oligomer accumulation in the 5xFAD mouse model.
Based on our hypothesis, we expect that HSV-1 infected mice with APOE4 will exhibit a greater Aβ accumulation, indicating that the presence of APOE4 in HSV-1 infected human neuronal cells leads to an accumulation of Aβ42 within the living 5xFAD mouse model. This finding will provide crucial evidence linking APOE4, HSV-1 infection, and Aβ42 deposition, contributing to a better understanding of the early stages of AD pathology.
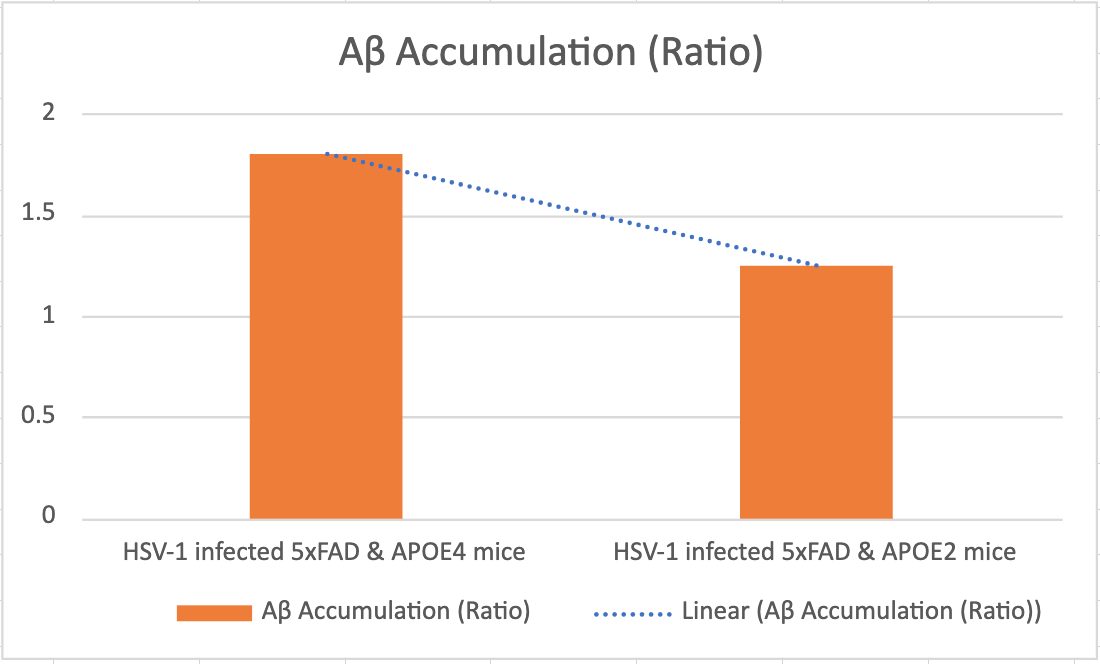
Figure 3. HSV-1 infected mice with APOE4 will exhibit a greater Aβ accumulation compared to HSV-1 infected mice with APOE2.
3.3. Experiment 3
The aim of our third experiment is to investigate the stage at which mice develop Alzheimer's disease (AD) within the amyloid plaque progression by assessing their spatial recognition memory and exploratory behavior. For this purpose, we will employ a Y-maze setup for the mice utilized in Experiment 2. The Y-maze consists of three arms arranged in a Y-shape, made of clear plastic, with a smooth and non-slip metal floor. The mice's arm entries will be recorded, including sequence, duration, and frequency. The duration of this experiment will be approximately 6 months, starting concurrently with Experiment 2, to capture significant changes in spatial recognition memory and exploratory behavior as amyloid plaque pathology progresses.
The experimental groups in Experiment 3 will be identical to those in Experiment 2, involving the 5xFAD mice model with both APOE4 and APOE2 alleles, separated by sex. Establishing baseline assessments of spatial recognition memory and exploratory behavior using the Y-maze will be crucial, as it will provide a reference point for comparison as the mice age and amyloid pathology develops under the influence of HSV-1 with APOE genes.
The initial step of the experiment will involve habituation, allowing the mice to adapt to the Y-maze by individually placing each mouse in the center for 5 minutes over several days. Subsequently, the spatial recognition memory test will be conducted by placing the mouse in the center of the Y-maze and observing their free exploration for 5 minutes. During this exploration, we will note the sequence of arm entries, and the percentage of alternation will be calculated using the formula: (Number of alterations / Total arm entries - 2) × 100. Higher percentages will indicate better spatial recognition memory.
In the exploratory behavioral test, the mouse will be positioned at the end of one arm and allowed to explore the maze for 5 minutes. We will record and analyze the time spent by the mouse in each arm. Increased time spent in open arms will reflect higher exploratory behavior, while more time in closed arms may indicate anxiety-like behavior.
Based on our hypothesis, we predict that the HSV-1 infected 5xFAD mice with APOE4 will demonstrate a lower percentage of alteration and spend a longer time in closed arms earlier than the HSV-1 infected 5xFAD mice with APOE2 allele. This observation would suggest that APOE4 can accelerate the progression of Alzheimer's disease, leading to an earlier appearance of AD-like symptoms in the mice. These symptoms may manifest through orientation difficulties and misregulation of exploratory behavior, providing important insights into the role of APOE4 and HSV-1 in AD pathogenesis.
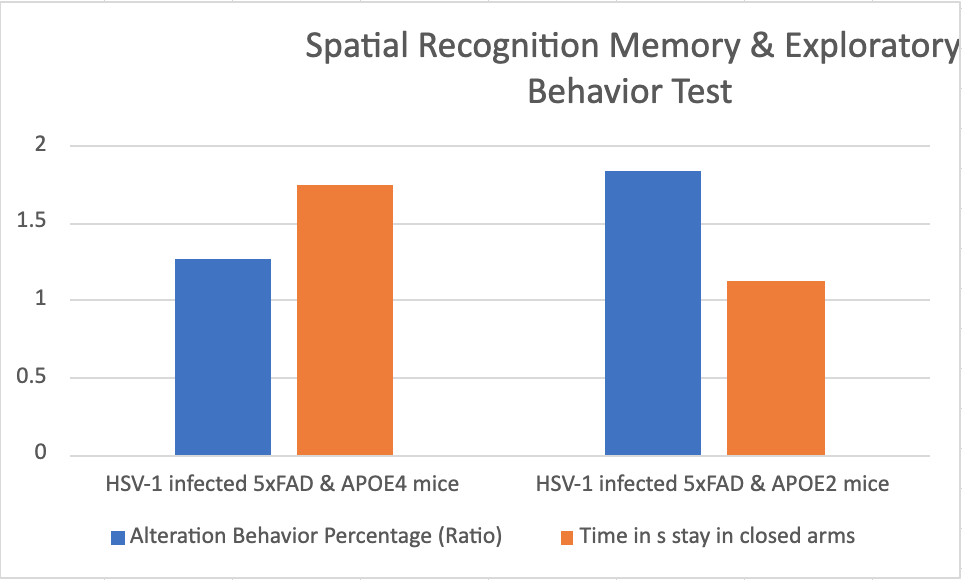
Figure 4. HSV-1 infected 5xFAD mice with APOE4 will demonstrate a lower percentage of alteration and spend a longer time in closed arms earlier than the HSV-1 infected 5xFAD mice with APOE2 allele.
3.4. Experiment 4
The fourth experiment will focus on investigating the role of APOE-ε4 in modulating HSV-1 reactivation. To achieve the activation of latent HSV-1 in mice, we will induce stress as an external factor in the mice models. The experiment will consist of four groups, which are the same as in Experiment 2, involving 5xFAD mice models with both APOE4 and APOE2 alleles, separated by sex. All groups will undergo stress induction to explore the effect of APOE4 on accelerating HSV-1 activation in comparison to the APOE2 allele. The study will include a total of 80 mice, with 20 mice in each group, all starting at the age of 3 months, and the duration of Experiment 4 is approximately 4-5 months.
For stress induction, the mice will be placed in well-ventilated, loose-fitting tubes for five cycles. They will be restrained for a 15-hour cycle, starting at 6:00 PM (lights out) and finishing at 9:00 AM (lights on at 6:00 AM). During this period, the control mice will be deprived of both water and food. This process will last for 3-4 weeks to ensure the activation of the virus in as many mice as possible.
Throughout the experiments, we will meticulously record the time from the onset of stress induction to the initial excitation of the virus in each mouse. Furthermore, we will closely monitor the mice's weight weekly, observe their behavior, and assess their skin condition to detect any signs indicating HSV-1 activation. Additionally, we will employ experimental techniques such as western blot to identify specific markers in the mice's blood, confirming the success or failure of HSV-1 activation.
Our hypothesis predicts that 5xFAD APOE4 mice will experience a quicker weight loss and exhibit more skin issues compared to 5xFAD APOE2 mice. Additionally, the biomarkers associated with HSV-1 activation, including glucocorticoid, cortisone, sgk1, MASP (Mitochondrial Antiviral-Signaling Protein), and Icp0 (Immediate Early Protein 0), will demonstrate a rapid increase in 5xFAD APOE4 mice compared to 5xFAD APOE2 mice, suggesting that APOE4 plays a critical role in accelerating HSV-1 activation.
To confirm the link between the activated form of HSV-1 and the accumulation of Aβ, we will measure the levels of Aβ in each group. As in Experiment 2, techniques including functional Magnetic Resonance Imaging (fMRI), western blot (WB), Immunofluorescence (IF), and enzyme-linked immunosorbent assay (ELISA) will be employed in this experiment to observe and investigate the expression of amyloid beta protein in the mice. We will utilize Threshold image analysis to quantify Aβ accumulation.
Based on the predictions, HSV-1 activated mice with APOE4 will exhibit greater Aβ accumulation, indicating that the presence of APOE4 in HSV-1 activated human neuronal cells leads to a faster accumulation of Aβ42 within the living 5xFAD mouse model. This finding will confirm that APOE4 accelerates the reactivation of HSV-1 in APOE4 mice in the 5xFAD model and speeds up AD progression once HSV-1 is activated, providing valuable insights into the interplay between APOE4, HSV-1, and Aβ accumulation, thus supporting our hypothesis.
4. Drug Treatments
To ensure the survival of the mice in Experiment 4 throughout our investigation, we plan to implement pharmacological interventions that can mitigate the toxic effects of HSV-1 and protect the mice. Once we confirm the activation of HSV-1 in the mice, we will administer acyclovir, an antiviral drug commonly used to treat HSV-1 infections. The aim of this treatment is to alleviate symptoms in the mice, thereby increasing their survival period while the virus remains active.
Within each group of Experiment 4, 15 out of 20 mice will receive the acyclovir drug, while the remaining 5 mice will serve as the control group without the drug treatment. The acyclovir drug will be orally administered by dissolving it in the drinking water at a concentration of 1.5 mg/ml. Given that mice typically consume about 4 to 5 ml of water daily, the mice in the experimental group will receive approximately 400 mg/kg of the drug per day for seven days. On the other hand, the control group, comprising a total of 20 mice, will not receive acyclovir.
Subsequently, we will closely observe changes in the mice's behavior, record their weight, and monitor their skin conditions throughout the experiment. Additionally, we will keep track of mortality rates and observe whether any of the activated mice revert to a latent state. This investigation will provide valuable insights into whether APOE4, when compared to APOE2, exerts a stronger activation effect on the virus.
The predicted outcomes suggest that the mice receiving the drug treatment will exhibit a higher average weight gain due to improved appetite recovery compared to those without the treatment. Furthermore, the negative skin symptoms, including ulcers, redness, and swelling, are expected to be reduced in the treated mice. Additionally, our hypothesis suggests that mice with the APOE4 allele may experience a less effective recovery compared to APOE2 mice, as we believe APOE4 may accelerate the reactivation and replication of HSV-1. Through these experiments, we aim to shed light on the impact of drug interventions and the role of APOE alleles in modulating HSV-1 activation, contributing to the advancement of our understanding in this area of research.
5. Conclusion
In conclusion, our study's findings align with our initial hypothesis, suggesting that the APOE e4 allele plays a role in expediting the activation of HSV-1, which in turn accelerates the accumulation of Aβ42 oligomers and contributes to faster Alzheimer's disease progression. We observed a higher level of Aβ42 oligomers in the brains of HSV-1 infected wild-type mice compared to their non-infected counterparts, indicating that HSV-1 may have the capacity to enhance Aβ42 oligomer buildup, potentially by triggering the antimicrobial response associated with Aβ42.
Firstly, our investigation examined the impact of APOE alleles on Aβ accumulation in the presence of unactivated HSV-1. Our results revealed that HSV-1 infected mice carrying the APOE4 allele exhibited greater Aβ accumulation, underscoring the connection between APOE4, HSV-1 infection, and Aβ42 deposition. This relationship may be attributed to the reduced ability of the APOE4 allele to break down Aβ42.
Additionally, our study explored the behavioral outcomes of these interactions, predicting that HSV-1 infected 5xFAD mice with APOE4 would display altered behavior, including spending more time in enclosed areas, compared to their APOE2 counterparts. This observation provides valuable insights into the roles of APOE4 and HSV-1 in influencing the progression of Alzheimer's disease, as evident through orientation difficulties and disruptions in exploratory behavior.
Lastly, our predictions were substantiated by observing that HSV-1 activated mice carrying the APOE4 allele indeed displayed increased Aβ accumulation. This confirmation underscores the role of APOE4 in expediting HSV-1 reactivation and consequently hastening Alzheimer's disease progression within the 5xFAD model. These findings collectively emphasize the intricate interplay between APOE4, HSV-1, and Aβ accumulation, supporting our initial hypothesis and shedding light on the mechanisms underlying Alzheimer's disease pathogenesis.
In summary, our research contributes to a better understanding of the intricate relationships between viral infections, genetic predisposition (APOE alleles), and Aβ42 accumulation in the context of Alzheimer's disease. These findings pave the way for further exploration of potential therapeutic interventions targeting these factors, with the aim of improving strategies for managing and preventing Alzheimer's disease.
This proposed scientific research has several limitations that need to be acknowledged and considered. First, it is crucial to recognize that mouse models of Alzheimer's disease (AD) have inherent limitations in fully replicating all aspects of human AD pathology. AD is a complex and multifactorial disease with intricate mechanisms, and the translation of findings from mouse models to human patients may not always be straightforward. While the 5xFAD mice model used in Experiments 2, 3, and 4 exhibits certain AD-like features, it may not fully recapitulate the entire spectrum of human AD pathogenesis. Therefore, caution should be exercised when extrapolating results from the mouse models to human AD patients.
Furthermore, when exploring the impact of sex hormones, such as estrogen, on AD progression, it is essential to recognize that estrogen is just one of numerous factors influencing the disease. There are likely other sex-specific factors and mechanisms that play a role in AD progression and Aβ42 accumulation. Therefore, further in-depth research is necessary to comprehensively understand the complex relationship between sex hormones, disease progression, and Aβ42 accumulation in AD. Additionally, considering the complex interplay between APOE alleles, sex hormones, and HSV-1 in AD, there might be other confounding factors that influence the observed outcomes.
Another important consideration is the sample size used in the experiments. The number of mice in each group can significantly influence the statistical power and the ability to detect significant differences between the experimental groups. While we have aimed for a sample size of 15 mice in each group in Experiments 1, 2, and 3, a larger sample size would enhance the reliability and generalizability of the research findings. Conducting power analysis before commencing the experiments and considering replication studies can improve the robustness of the results.
Moreover, the duration of the experiments, though carefully planned, may be relatively short in capturing the full spectrum of AD progression and HSV-1 reactivation. AD is a chronic and progressive disease, and longer-term studies may be needed to observe the complete impact of APOE alleles and HSV-1 on Aβ42 accumulation and AD pathogenesis. Additionally, the potential long-term effects of the administered acyclovir drug in Experiment 4 need to be monitored closely.
Finally, ethical considerations must be taken into account throughout the study. The use of animal models and human brain tissue raises ethical concerns that need to be addressed appropriately. Researchers must ensure that the experiments are conducted following all relevant ethical guidelines and regulations to safeguard the welfare of the animals and respect the principles of research ethics. Additionally, informed consent and proper approval from ethical review boards are essential when using human brain tissue for research purposes.
By acknowledging and addressing these limitations, we can enhance the rigor and validity of the proposed research, leading to a more comprehensive understanding of the investigated phenomena in Alzheimer's disease. Careful interpretation of the results in the context of these limitations will provide valuable insights and pave the way for future studies to advance our knowledge in AD pathogenesis and potential therapeutic interventions.
The future direction of this research proposal encompasses two pathways, each aimed at addressing critical aspects of the study: drug treatment and experimental investigations.
In the drug treatment pathway, our focus lies on exploring the potential of anti-HSV-1 agents as a promising avenue for further research. The development of antiviral drugs with specific targets against HSV-1 will be a primary objective. By inhibiting viral reactivation, we seek to reduce its impact on the progression of Alzheimer's disease. This approach holds significant potential in providing novel therapeutic interventions to address the intricate interplay between HSV-1 and AD. Additionally, considering the substantial association between APOE-ε4 and increased HSV-1 reactivation, the investigation of APOE-modulating drugs becomes crucial. We aim to explore drugs capable of modifying APOE expression or function, seeking potential strategies to mitigate the effects of APOE-ε4 on AD development and its interactions with HSV-1.
The experimental investigations pathway will be further enhanced by the implementation of clinical trials. These trials will play a vital role in advancing our research proposal. Rigorously designed and conducted, they will evaluate the efficacy of antiviral drugs and APOE4-targeted therapies. By assessing the potential of these interventions in preventing HSV-1 reactivation and its subsequent impact on the risk of Alzheimer's disease, we hope to pave the way for personalized prevention strategies. This would bring us closer to developing tailored treatments for individuals at risk of AD. Furthermore, we will conduct Human Brain Tissue Studies, an integral part of our experimental approach. Through in-depth studies on human brain tissue obtained from Alzheimer's patients with known APOE4 status and HSV-1 infection history, we aim to provide direct evidence of the relationship between these two factors within the context of the disease. These studies will offer valuable insights into the mechanistic aspects of HSV-1 and its interactions with APOE4 in the pathogenesis of AD.
In conclusion, the future directions of this scientific research proposal entail a comprehensive approach to tackle the complex interplay between HSV-1, APOE-ε4, and Alzheimer's disease. Our exploration of drug treatment strategies, along with rigorous experimental investigations, aims to advance our understanding of these mechanisms. Ultimately, we seek to pave the way for potential therapeutic breakthroughs in the prevention and management of AD. Through a multidimensional approach, this research proposal aspires to contribute to the broader field of Alzheimer's research, bringing us closer to addressing the challenges posed by this devastating neurodegenerative disorder.
References
[1]. Scheltens, P., De Strooper, B., Kivipelto, M., Holstege, H., Chételat, G., Teunissen, C. E., … van der Flier, W. M. (2021). Alzheimer’s disease. The Lancet, 397(10284), 1577–1590. doi:10.1016/s0140-6736(20)32205-4
[2]. Raulin, A.-C., Doss, S. V., Trottier, Z. A., Ikezu, T. C., Bu, G., & Liu, C.-C. (2022). ApoE in alzheimer’s disease: Pathophysiology and therapeutic strategies. Molecular Neurodegeneration, 17(1). https://doi.org/10.1186/s13024-022-00574-4
[3]. Graff-Radford, J., Yong, K. X., Apostolova, L. G., Bouwman, F. H., Carrillo, M., Dickerson, B. C., Rabinovici, G. D., Schott, J.M., Jones, D. T., & Murray, M. E. (2021). New insights into atypical alzheimer’s disease in the era of biomarkers. The Lancet Neurology, 20(3), 222–234. https://doi.org/10.1016/s1474-4422(20)30440-3
[4]. Masters, C. L., & Beyreuther, K. (1998). Science, medicine, and the future: Alzheimer’s disease. BMJ, 316(7129), 446–448. doi:10.1136/bmj.316.7129.446
[5]. Serrano-Pozo, A., Das, S., & Hyman, B. T. (2021). APOE and alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. The Lancet Neurology, 20(1), 68–80. doi:10.1016/s1474-4422(20)30412-9
[6]. Marcocci, M. E., Napoletani, G., Protto, V., Kolesova, O., Piacentini, R., Li Puma, D. D., … De Chiara, G. (2020). Herpes simplex virus-1 in the brain: The dark side of a sneaky infection. Trends in Microbiology, 28(10), 808–820. doi:10.1016/j.tim.2020.03.003
[7]. Wainberg, M., Luquez, T., Koelle, D. M., Readhead, B., Johnston, C., Darvas, M., & Funk, C. C. (2021). The viral hypothesis: How herpesviruses may contribute to alzheimer’s disease. Molecular Psychiatry, 26(10), 5476–5480. doi:10.1038/s41380-021-01138-6
[8]. Eimer, W. A., Vijaya Kumar, D. K., Navalpur Shanmugam, N. K., Rodriguez, A. S., Mitchell, T., Washicosky, K. J., … Moir, R. D. (2018). Alzheimer’s disease-associated β-amyloid is rapidly seeded by Herpesviridae to protect against brain infection. Neuron, 100(6), 1527–1532. doi:10.1016/j.neuron.2018.11.043
[9]. Lövheim, H., Gilthorpe, J., Johansson, A., Eriksson, S., Hallmans, G., & Elgh, F. (2015). Herpes simplex infection and the risk of alzheimer’s disease: A nested case-control study. Alzheimer’s & Dementia, 11(6), 587–592. doi:10.1016/j.jalz.2014.07.157
[10]. Linard, M., Letenneur, L., Garrigue, I., Doize, A., Dartigues, J.-F., & Helmer, C. (2020). Interaction between apoe4 and herpes simplex virus type 1 in alzheimer’s disease. Alzheimer’s & Dementia, 16(1), 200–208. doi:10.1002/alz.12008
Cite this article
Mo,W. (2024). APOE4, activation of latent form HSV-1, A beta accumulation and AD progression. Theoretical and Natural Science,44,36-44.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Scheltens, P., De Strooper, B., Kivipelto, M., Holstege, H., Chételat, G., Teunissen, C. E., … van der Flier, W. M. (2021). Alzheimer’s disease. The Lancet, 397(10284), 1577–1590. doi:10.1016/s0140-6736(20)32205-4
[2]. Raulin, A.-C., Doss, S. V., Trottier, Z. A., Ikezu, T. C., Bu, G., & Liu, C.-C. (2022). ApoE in alzheimer’s disease: Pathophysiology and therapeutic strategies. Molecular Neurodegeneration, 17(1). https://doi.org/10.1186/s13024-022-00574-4
[3]. Graff-Radford, J., Yong, K. X., Apostolova, L. G., Bouwman, F. H., Carrillo, M., Dickerson, B. C., Rabinovici, G. D., Schott, J.M., Jones, D. T., & Murray, M. E. (2021). New insights into atypical alzheimer’s disease in the era of biomarkers. The Lancet Neurology, 20(3), 222–234. https://doi.org/10.1016/s1474-4422(20)30440-3
[4]. Masters, C. L., & Beyreuther, K. (1998). Science, medicine, and the future: Alzheimer’s disease. BMJ, 316(7129), 446–448. doi:10.1136/bmj.316.7129.446
[5]. Serrano-Pozo, A., Das, S., & Hyman, B. T. (2021). APOE and alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. The Lancet Neurology, 20(1), 68–80. doi:10.1016/s1474-4422(20)30412-9
[6]. Marcocci, M. E., Napoletani, G., Protto, V., Kolesova, O., Piacentini, R., Li Puma, D. D., … De Chiara, G. (2020). Herpes simplex virus-1 in the brain: The dark side of a sneaky infection. Trends in Microbiology, 28(10), 808–820. doi:10.1016/j.tim.2020.03.003
[7]. Wainberg, M., Luquez, T., Koelle, D. M., Readhead, B., Johnston, C., Darvas, M., & Funk, C. C. (2021). The viral hypothesis: How herpesviruses may contribute to alzheimer’s disease. Molecular Psychiatry, 26(10), 5476–5480. doi:10.1038/s41380-021-01138-6
[8]. Eimer, W. A., Vijaya Kumar, D. K., Navalpur Shanmugam, N. K., Rodriguez, A. S., Mitchell, T., Washicosky, K. J., … Moir, R. D. (2018). Alzheimer’s disease-associated β-amyloid is rapidly seeded by Herpesviridae to protect against brain infection. Neuron, 100(6), 1527–1532. doi:10.1016/j.neuron.2018.11.043
[9]. Lövheim, H., Gilthorpe, J., Johansson, A., Eriksson, S., Hallmans, G., & Elgh, F. (2015). Herpes simplex infection and the risk of alzheimer’s disease: A nested case-control study. Alzheimer’s & Dementia, 11(6), 587–592. doi:10.1016/j.jalz.2014.07.157
[10]. Linard, M., Letenneur, L., Garrigue, I., Doize, A., Dartigues, J.-F., & Helmer, C. (2020). Interaction between apoe4 and herpes simplex virus type 1 in alzheimer’s disease. Alzheimer’s & Dementia, 16(1), 200–208. doi:10.1002/alz.12008









