1. Introduction
1.1. Background
Analgesics, also known as painkillers, are drugs that relieve pain selectively without blocking the transmission of nerve impulses, influencing consciousness, or markedly altering sensory perception [1]. This selectivity is what sets analgesics apart from anesthetics [2]. Analgesics can be classified into two types based on how they interact with the human body: nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids [1]. NSAIDs lessen pain by reducing mediators of inflammation in the human body while carrying no risk of addiction. Ibuprofen, aspirin, and naproxen are all typical examples of NSAIDs [3].
On the other hand, opioids work by interacting with the human central nervous system and carry a high risk of addiction. Opioid’s pain reducing effects are mediated by receptors on the cells of the brain and spinal cord [4]. Opioid molecules attach to these receptors, which in turn block any pain signals sent from the brain to the body while simultaneously releasing large amounts of dopamine [5]. Unfortunately, the neural pathways involved in opioids’ relief of pain and euphoria, which create the feelings of pleasure, are what ultimately cause opioids to be extremely addictive. Opioids can include legally available painkillers by prescription such as hydrocodone (Vicodin®), (OxyContin®), and morphine, but also include the illegal drug heroin and synthetic opioids such as fentanyl. But even if prescribed, over time one can be at risk of developing physical dependence to the drug, tolerance, and certain opioid use disorders [5]. When one develops a dependence it can quickly lead to addiction, for which each time larger doses are used to achieve the same effects as before. Opioid addiction is extremely dangerous as it puts individuals at a high risk of overdose and puts them through excruciating physical and psychological withdrawals when the drug’s pleasurable effects wear off. Opioids interact with the human in an extremely complex way and play a crucial role in modern medicine as both an acute and chronic pain reliever [6]. This paper will mainly focus on the opioid group of analgesics.
1.2. History of Opioid Use
Throughout history, humans have frequently applied opioids in pain management fields. The earliest attempt for human beings to utilize opioids can be traced back to around 5,000 years ago, in the Sumerian Civilization [7]. This civilization was located in southern Mesopotamia, and its residents were the first to cultivate opium poppies from which opiates, natural ingredients in opioids, were later derived. Nevertheless, the use of these substances, whether for medical or recreational aims, have been carried out throughout human history since then. For instance, Ancient Greeks, Indians, Chinese, Egyptians, Romans, and Arabs had all, sooner or later, realized the remarkable pain reducing effect of opioids. Additionally, throughout the renaissance period, the Medieval people and Europeans utilized morphine in their day-to-day lives . The use is mentioned in books passed down through the ages, including the Bible and Odyssey. Opioids were used by both well-known leaders and wounded soldiers who didn’t leave their names in history [8]. After the invention of the syringe and the first isolation of morphine done by Serterner in Germany in the early 19th century, its application range was further expanded. However, the opioid drugs in the precise definition only represent the drugs semi- or completely synthesized; thus, the actual common use did not begin until people could get it by artificial means. Still, it is reasonable to say that opioids, at least their predecessors, have left a non-negligible mark in the history of human drug use.
1.3. Finding the Synthesis of Opioids
In the past, the synthesis of morphine drugs was mainly concentrated in morphine, codeine, and intermediate dihydrocodeine, and the synthesis methods were mainly divided into two ways: total synthesis and formal total synthesis. In 1952, Gates’ group performed the first total synthesis of natural morphine, and Rice’s group also completed the total synthesis of morphine in 1980. In 1992, Parker’s group synthesized racemic dihydrocodeine using the free radical cyclization reaction of aryl bromide as a key step and completed the total synthesis of codeine and morphine. In 2008, Guillou’s group met the total synthesis of codeine using the Michael addition reaction of lactone ring opening and the Eschenmoser-Claisen rearrangement reaction as key steps. In 2015, Zhang Hongbin’s research group completed the total synthesis of the natural product codeine from the derivatives of the commercial raw material phenylboronic acid. In addition, in 2014, Fukuyama’s group semi-synthesized opioid analgesic (-)-Oxycodone with palladium-catalyzed the aryl coupling reaction of intramolecular bromobenzene and the intramolecular Michael addition reaction as key steps, and Hudlicky’s group reported the synthesis of enantiosis morphine with oxidative dearomatization and intramolecular DA reaction as key steps [9].
1.4. Aims and Objectives
In modern medicine, painkillers play an indispensable role. Among them, morphine opioids are the most common type of painkillers that have been widely and extensively used [10].
Morphine, a type of opioid, is extracted and processed from natural plants such as opium poppy [11]. However, due to the addictive nature of morphine opioids, plants such as opium poppy have been banned or strictly controlled in many countries, such as China, Afghanistan [12], Russia [13], and many more. These regulations lead to the inability to obtain morphine-like opium through natural purification. Due to this situation, scientists have been searching for efficient and diverse artificial synthesis methods to replace plant extraction methods in synthesizing morphine-like opium to meet the large demand of modern medicine [14]. In this paper, the goal is to find different and efficient synthesis methods of opioid analgesics to meet the needs of modern medicine.
2. Results and Discussion
In search of efficient yet effective means of synthesizing morphine opioid drugs, many routes have come about. This paper will be examining 3 different methods that have come about to synthesize morphine.
2.1. The Synthesis done by Gates’ Research Group
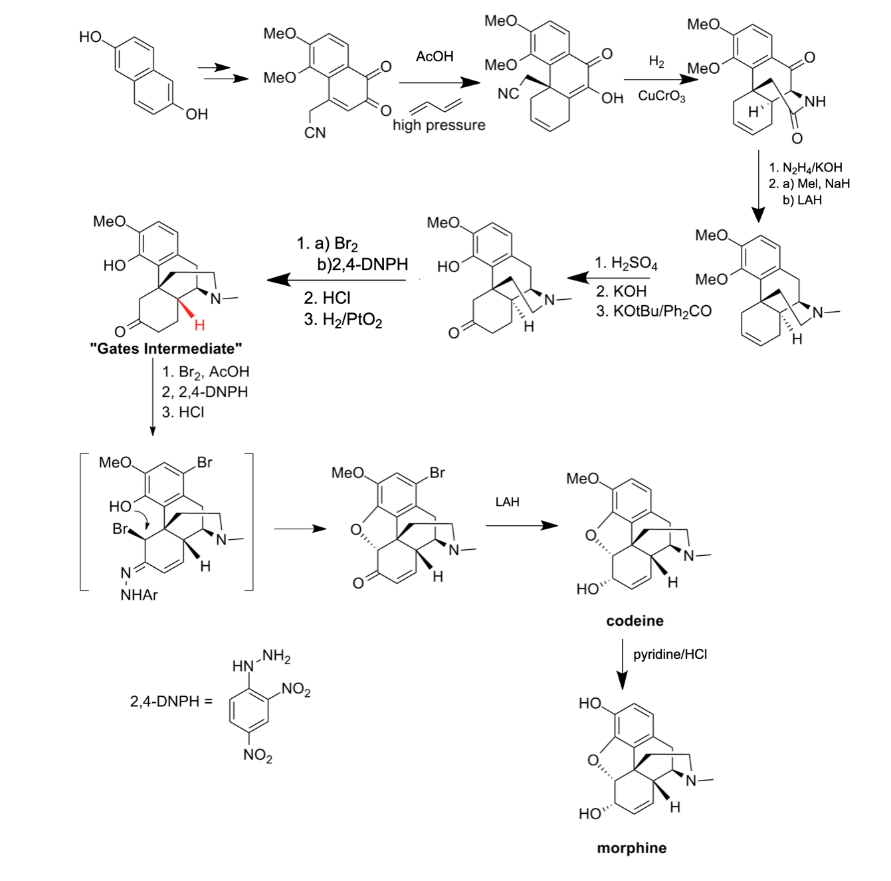
Figure 1. Synthesis Process done by Gates’ Research Group
2.1.1. Synthesis Process. In 1952, Gates and his group completed the total synthesis of morphine for the first time. In this method, the entire synthesis process is based on 1,5-naphthalene, also known as dihydroxy naphthalene, which is shown in Figure 1. Cyanoketone is then built on this basis. With dihydroxy naphthalene as a starting material, researchers synthesize precursors for the Diels-Alder reaction through a 9-step reaction. Additionally, Diels–Alder reaction is a chemical reaction taking place between a conjugated diene and a substituted alkene, resulting in the form of a substituted cyclohexene derivative. This compound reacts with butadiene under high-pressure conditions or high-temperature conditions, forming the compound and thus the center of the polycyclic system. After that, the key intermediate is obtained by copper-chromium-catalyzed hydrogenation [15]. This intermediate is also known as “Gates intermediate”, and “ [syntheses] published thereafter focused, for a time, on improvements to the synthesis of this intermediate as well as the final steps” [16]. The subsequent synthetic steps are to use the degradation products of natural Codeine for selective hydration, demethylation, and Oppenauer oxidation to obtain new compounds.
Furthermore, this compound undergoes a series of bromination reactions, hydrogenation reactions, and hydrolysis. These reactions taking place in different positions create changes in functional groups such as the removal of double bonds and the formation of bonds between carbon and brominate. Ultimately, these steps enabled the researchers to obtain the final compound, the natural morphine [17].
2.1.2. Significance and Deficiencies. The significance of this synthesis process which is a memorable step in the path of total synthesis is much worth mentioning. It has been the first complete total synthesis of Morphine. Just as the paper written by Marshall D. Gates claims, “In this [paper], [they] describe the completion of the first synthesis of morphine and therewith a complete confirmation of the Robinson formula” [17]. This synthesis is also proof of the structure of morphine proposed by Robinson in 1925. Additionally, it has been one of the first examples of the Diels-Alder reaction in the context of total synthesis [16]. Nevertheless, this route has its disadvantages at the same time. This synthesis process takes 31 steps yet the yield of 0.06% is very small in comparison, leading to the lack of economic benefits and efficiency [15].
2.2. The Synthesis Done by Varghese and Hudlicky
2.2.1. Synthesis Process
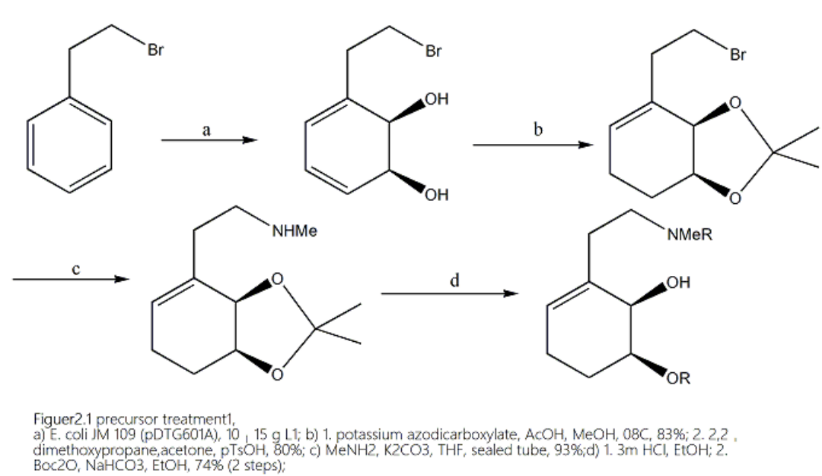
Figure 2. Precursor Treatment 1. a) E. coli JM 109 (pDTG601A), 10–15 g L1; b) 1. potassium azodicarboxylate, AcOH, MeOH, 08C, 83%; 2. 2,2-dimethoxypropane,acetone, pTsOH, 80%; c) MeNH2, K2CO3, THF, sealed tube, 93%;d) 1. 3m HCl, EtOH; 2. Boc2O, NaHCO3, EtOH, 74% (2 steps)
In 2014, Vimal Varghese and Tomas Hudlicky published an article discussing the use of 4+2 reactions to synthesize morphine opioid drugs. The authors started by synthesizing two subunits and connecting them to the precursors required for the synthesis process. The product is then dihydroxylated through whole-cell fermentation and selectively reduced with potassium azodicarboxylate. Furthermore, under the condition of protecting the glycol, the bromine in it is replaced with methylamine, and the secondary amine is hydrolyzed and protected with HCl. After selective silanization of the previously obtained product in a section far from the hydroxyl group, deprotection, and toluene sulfonylation are performed to obtain one of the monomers. For the other monomer, the author used the regioselective acetylation of 3,4-dixybenzaldehyde to generate its derivative, and then protected phenol for acetylation hydrolysis under mild alkaline conditions.
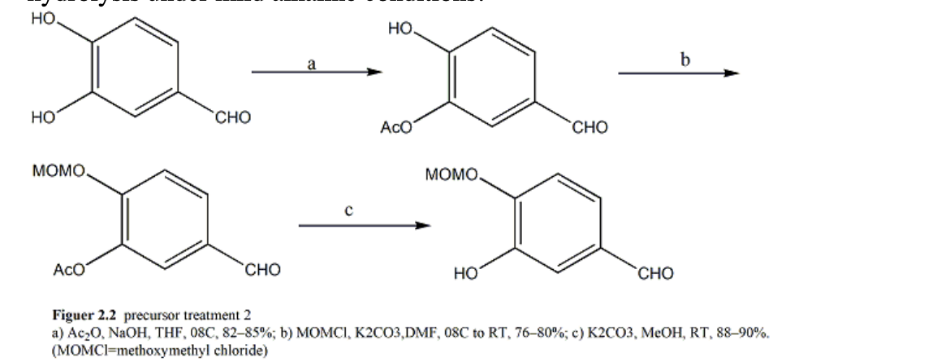
Figure 3. Precursor Treatment 2. a) Ac2O, NaOH, THF, 08C, 82–85%; b) MOMCl, K2CO3,DMF, 08C to RT, 76–80%; c) K2CO3, MeOH, RT, 88–90%. (MOMCl=methoxymethyl chloride)
After obtaining two monomers and coupling them, a series of reactions including 4+2 reactions were carried out. By Wittig reaction, the aldehyde group is converted to a vinyl group, and then the generated MOM group is removed under mild conditions. The substance is then exposed to lead tetraacetate in reflux dichloroethane resulting in a 50% yield of 4+2 adducts. It is worth noting that during the addition process, the quinone-like portion acts as both a diene and a dienophile, so different addition products may appear, resulting in a decrease in the final yield. Finally, the author utilized dissolved metal reduction tosylamide to achieve nitrogen center radical cyclization, established an ethylamine bridge, and ultimately synthesized the product. The most effective route of the method mentioned in the article is only completed in 7 steps, making it the shortest route for synthesizing morphine.
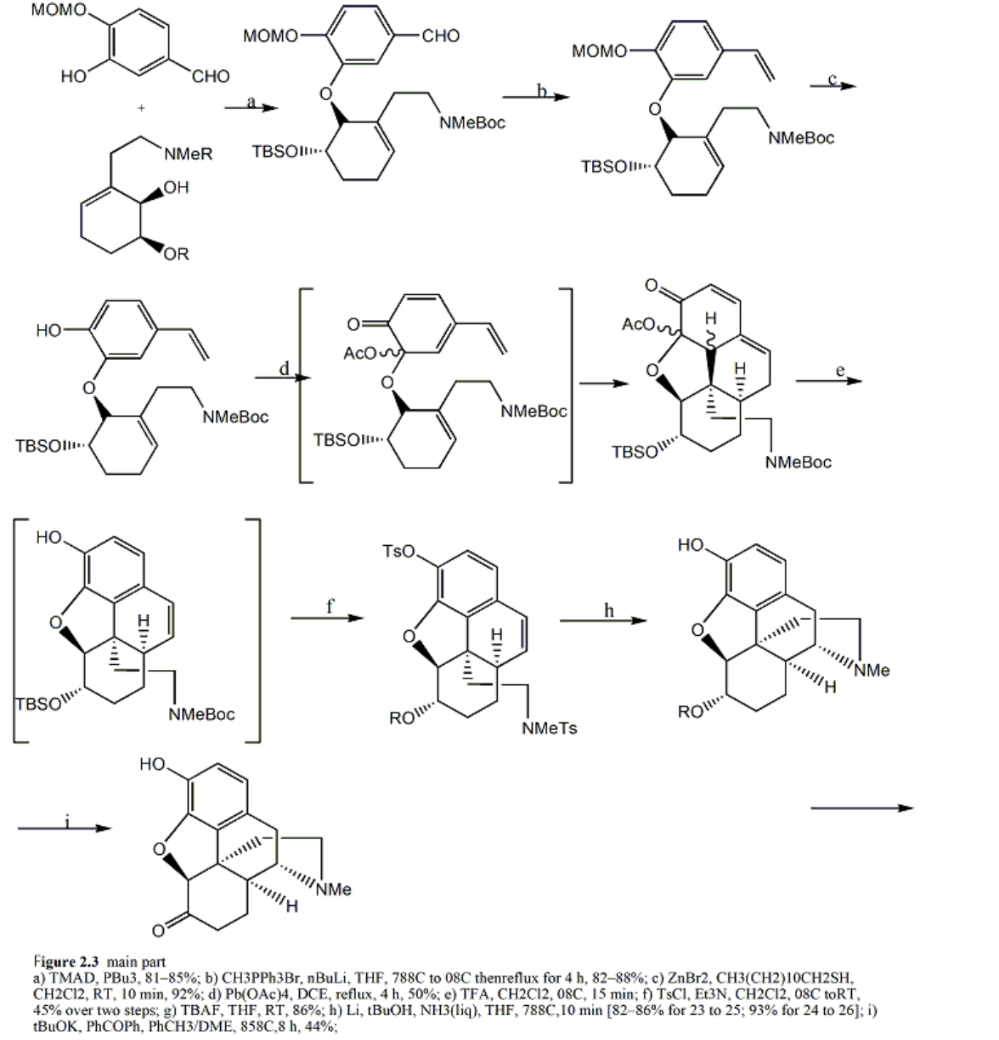
Figure 4. Main Part. a ) TMAD ,PBu3,81-85%: b )CH3PPh3Br, nBuLi , THF ,788C to 08C thenreflux for 4 h ,82-88%: c )ZnBr2,CH3(CH2)10CH2SH,CH2CI2, RT ,10 min ,92%: d ) Pb ( OAc )4. DCE , reflux ,4 h ,50%: e ) TFA ,CH2C12,08C,15 min : f ) TsCI .Et3N,CH2CI2,08C toRT ,45% over two steps ; g ) TBAF , THF , RT .86%; h ) Li , tBuOH ,NH3( lig ). THF ,788C,10 min [82-86% for 23 to 25:93% for 24 to 26]: i ) tBuOK , PhCOPh ,PhCH3/DME,858C.8 h .44%
2.2.2. Significance and Deficiencies. This method is currently the most effective and convenient method for synthesizing morphine opioid drugs. Compared to other synthesis methods, the main part can be completed in only seven steps, (atom economy/waste/use less hazardous chemicals) greatly improving the efficiency of drug synthesis and providing convenience and more possibilities for further research. On the other hand, this method also has drawbacks. However, since the diene and amphiphilic diene of the 42 reactions during the process are the same substance, Both intramolecular and intermolecular reactions may occur, which may lead to a decrease in yield and low utilization of reactants.
2.3. The Synthesis Done by He’s Research Group
2.3.1. Synthesis Process. In the 2022 Organic Chemistry Frontier, Chinese chemists Huang He, Fanglin Xue, and others proposed a method for synthesizing the four therapeutic eka-morphine opioid drugs. The reaction steps and overall yield of these drugs included the following: (-)-codeine (34% overall yield, 12 steps), (-)-oxycodone (20% overall yield, 14 steps), (-)-naloxone (16% overall yield, 15 steps), and (-)-naltrexone (17% overall yield, 15 steps). With the highest overall yields (16-34%) reported so far [2].
The development of using inexpensive phosphonium ligands during critical Pd-catalyzed arene coupling provides a more effective method of overcoming challenging regioselectivity issues by not using blocking groups.
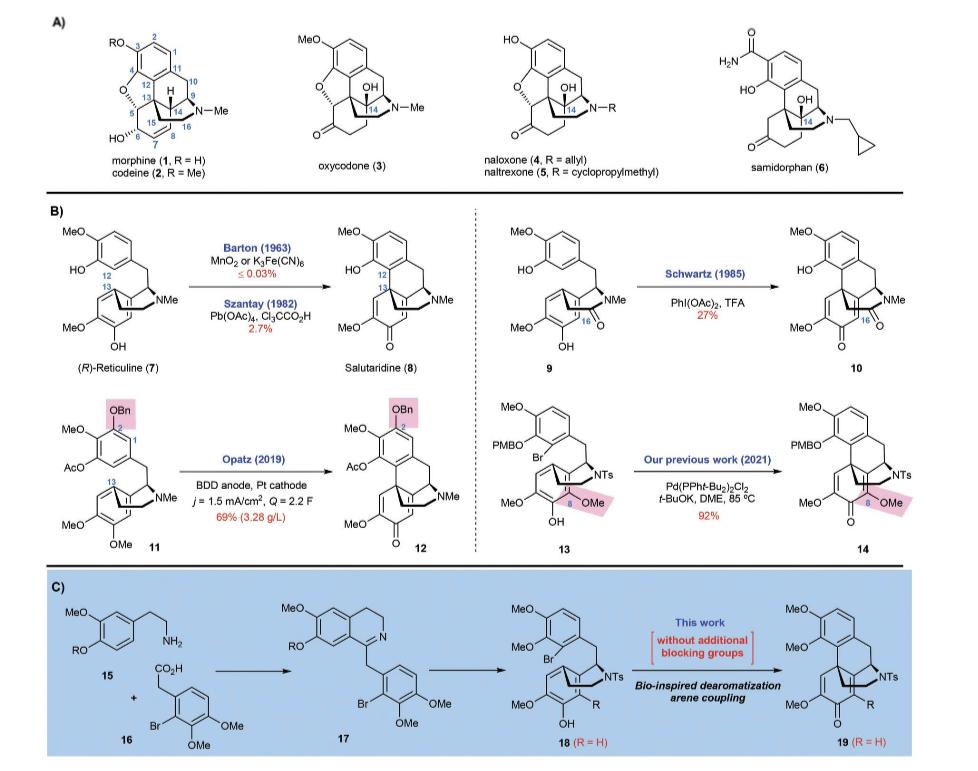
Figure 5. (A) Chemical structures of chosen natural morphine alkaloids (1 and 2) and semisynthetic opioids(3-6). (B)Literature methods for the assembly of the morphinan framework through bio-inspired arene couplings with problems of either the unsatisfied low output of the morphine or the excessive reliance on block groups. (C) The synthetic plan for the total synthesis of opioids by using no additional blocking groups [2].
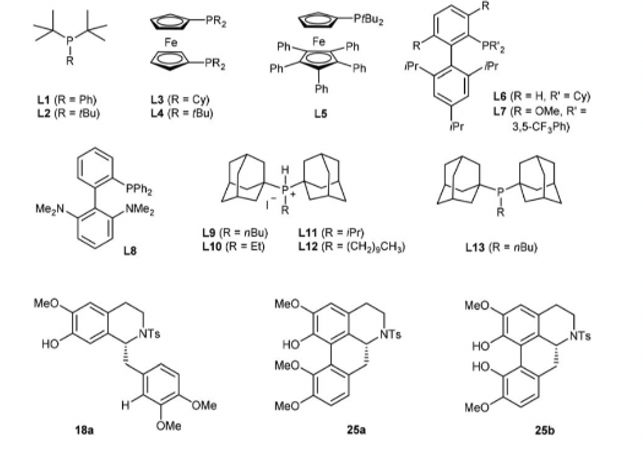
Figure 6. Subjects the letters in Table 1 and the article refers to.
The synthesis starts from two cognate subordinates of phenylethylamine and phenylacetic acid. The overall synthetic pathway would be uncomplicated, by the inspiration brought by the biosynthesis of morphinan alkaloid. Subsequently, continue with the steps above in the transformation of bromide 13 which contains a C8-OMe blocking group into the tetracycline morphinan core 14 (Fig. 5B). As a result, many ligands are tested, and they have failed to lead to any vital and remarkable results. Surprisingly, the desired coupling product 19 was isolated in low to moderate yields using phosphine ligands L1-19 (entries 1-9) (Table 1), respectively, together with different percentages of the undesired regioisomer 25a and its dimethyl derivative 25b.
Table 1. Select optimization for the arene coupling of bromide 18*.
ENTRY | L | BASE | \( x \) | \( y \) | T/℃ | TIME/ \( h \) | YIELD |
1 | L1 | \( {K_{2}}{CO_{3}} \) | 10 | 10 | 120 | 3 | 32% |
2 | L2 | \( {K_{2}}{CO_{3}} \) | 10 | 10 | 120 | 3 | 14% |
3 | L3 | \( {K_{2}}{CO_{3}} \) | 10 | 10 | 120 | 3 | 8% |
4 | L4 | \( {K_{2}}{CO_{3}} \) | 10 | 10 | 120 | 3 | 48% |
5 | L5 | \( {K_{2}}{CO_{3}} \) | 10 | 10 | 120 | 3 | 10% |
6 | L6 | \( {K_{2}}{CO_{3}} \) | 10 | 10 | 120 | 3 | 7% |
7 | L7 | \( {K_{2}}{CO_{3}} \) | 10 | 10 | 120 | 3 | 8% |
8 | L8 | \( {K_{2}}{CO_{3}} \) | 10 | 10 | 120 | 3 | 11% |
9 | L9 | \( {K_{2}}{CO_{3}} \) | 10 | 10 | 120 | 3 | 57% |
10 | L9 | \( {K_{2}}{CO_{3}} \) | 10 | 10 | 100 | 18 | 11% |
11 | L9 | \( {K_{2}}{CO_{3}} \) | 10 | 10 | 145 | 1 | 67% |
12 | L9 | \( {K_{3}}{PO_{4}} \) | 10 | 10 | 145 | 1 | 72% |
13 | L9 | \( {K_{3}}{PO_{4}} \) | 10 | 15 | 145 | 1 | 76% |
14 | L9 | \( {K_{3}}{PO_{4}} \) | 10 | 30 | 145 | 1 | 78% |
15 | L9 | \( {K_{3}}{PO_{4}} \) | 10 | 40 | 145 | 1 | 78% |
16 | L9 | \( {K_{3}}{PO_{4}} \) | 8 | 12 | 145 | 1 | 76% |
17 | L9 | \( {K_{3}}{PO_{4}} \) | 5 | 10 | 145 | 1 | 67% |
18 | L10 | \( {K_{3}}{PO_{4}} \) | 8 | 12 | 145 | 1 | 69% |
19 | L11 | \( {K_{3}}{PO_{4}} \) | 8 | 12 | 145 | 1 | 80% |
20 | L12 | \( {K_{3}}{PO_{4}} \) | 8 | 12 | 145 | 1 | 65% |
21 | L13 | \( {K_{3}}{PO_{4}} \) | 8 | 12 | 145 | 1 | 75% |
\( {22^{b}} \) | L11 | \( {K_{3}}{PO_{4}} \) | 8 | 12 | 145 | 1 | 78% |
Afterward, additional experiments were conducted to explore various reaction conditions for coupling compound 18 with di-(1-adamantyl])-n-butylphosphonium iodide (L9) as the ligand. In the initial test (entry 9), this yielded product 19 with a 57% yield. The change in temperature would significantly influence the yield of the 19. In the temperature of 100 °C, the reaction was significantly suppressed, giving out 19 in only 11% yield after 18 h (entry 10). On the contrary, increasing the temperature to 145 ° C can greatly accelerate the reaction rate, resulting in a 67% yield of 19 within 1 hour (entry 11). The solvents used in the reaction have a crucial impact on the reaction efficiency. DMF was proved to be the best choice to use. The de-bromo byproduct, 18a, is produced when sifting different bases in the reaction when KPO is used as the base proceeded with increased yield (72%, entry 12) and stronger bases. A further investigation was conducted of the loadings of PdCl, and 19 were conducted (entries 13-17). To be more specific, the improvement for the yield of 19 was not significant, if the ratio of 19/PdCh was over 1,5 (entries 13-15). The tetracyclic morphinan core is a crucial intermediate for the synthesis of opioid medicines. The synthesis begins with the condensation of amine 15 and acid 16, followed by the Bischler-Napieralski cyclization to form dihydroisoquinoline 17. The enantioselective reduction of the imine double bond in 17 was first initiated and the tosylation reaction followed next, resulting in the formation of sulfonamide 18. The tetracyclic morphinan core 19 is then assembled through the de-aromatization arene coupling of 18.
The synthesis starts with the primary amine 20 and carboxylic acid 16. The condensation of 20 and 16 using TBTU and Et3N results in the formation of amide 21 in 92% yield. The Bischler-Napieralski reaction of 21 with 2-fluoropyridine and Tf2O gives amine 22. Only use a small amount of of ruthenium complex 23 as the catalyst and amine L as the ligand allowed the catalytic enantioselective transfer hydrogenation of 22 to be successful after extensive checking of reaction conditions. The resulting secondary amine is masked with the tosyl group to output sulfonamide 24 in 84% yield over 3 steps from 21. Desilylation of 24 using KF in a mixed solvent of CH3CN and H2O yields phenol 18 in 97% yield.
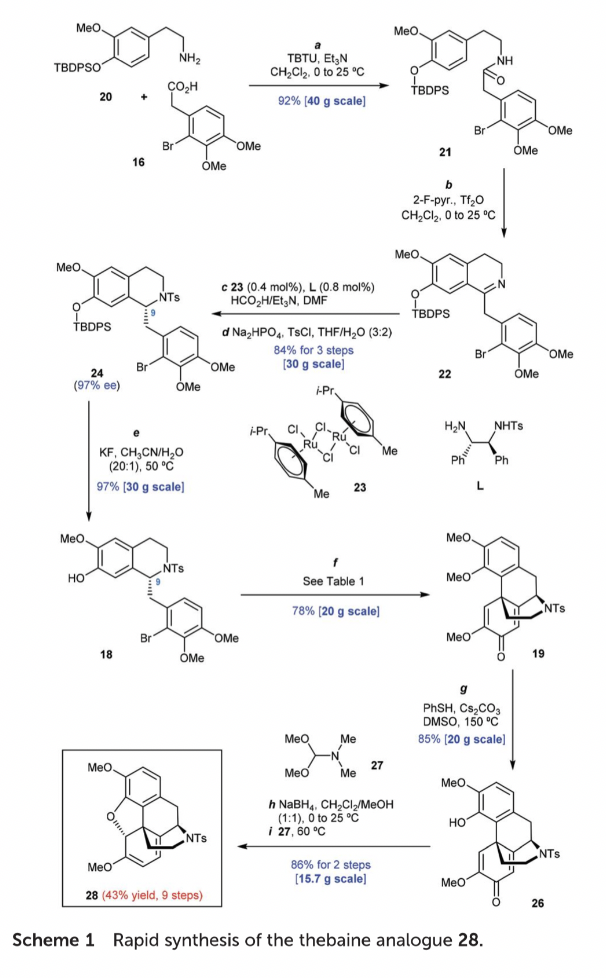
Figure 7. Rapid synthesis of thebaine analogue 29
The exploration of the arene coupling of 18 forming the tetracyclic morphinan core 19 has been delivered. Various phosphine ligands are tested, and it is found that di-(1-adamantyl)-n-butylphosphonium iodide (L9) is the optimal ligand, giving 19 in 57% yield at 120 °C. The reaction efficiency is improved by using DMF as the solvent and K3PO4 as the base, resulting in a yield of 72%. The loading of PdCl2 is also optimized, and it is found that reducing the loading to 8 mol% provides comparable efficiency. Further investigation of di-(1-adamantyl)-phosphine ligands reveals that di-(1-adamantyl)-iso-propylphosphonium iodide (L11) is the optimal ligand, giving 19 in 80% yield.
With the preparation of tetracycle 19, the synthesis is carried forward to access the thebaine analog 28. Heating 19 with PhSH and Cs2CO3 in DMSO at 150 °C achieved the regioselective O-demethylation of 19, bringing about the formation of product 26 in 85% yield. Reducing the ketone carbonyl group in 26 by using NaBH4, then followed by treatment with dimethylformamide dimethyl acetal (27), produced the desired product 28 with 86% yield over 2 steps (Figure 7).
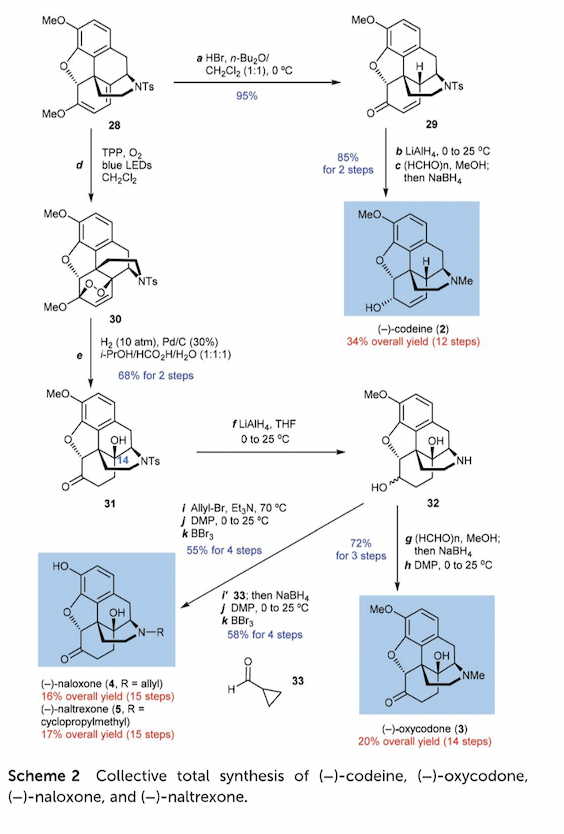
Figure 8. Collective total synthesis of (-)-codeine,(-)-oxycodone,(-)-naloxone,(-)-naltrexone
28 can be elaborated into four clinically useful opioids: codeine (2), oxycodone (3), naloxone (4), and naltrexone (5). Treatment of 28 with HCl hydrolyzes the dienol ether motif to produce enone 29 in 95% yield. Reduction of the N-Ts and ketone carbonyl groups in 29, followed by reductive amination, converts it into codeine (2) in 85% yield over 2 steps. For the synthesis of oxycodone (3), naloxone (4), and naltrexone (5), various functional group manipulations are performed on intermediate 32, which is obtained from 31. The desired opioids are obtained with yields ranging from 55% to 72% over three to four steps.
The synthesis described in the article provides an efficient route to the tetracyclic morphinan core and various opioid medicines.
2.3.2. Significance and Deficiencies. The synthesis’s primary highlights include a catalytic process for creating the initial C9 stereocenter through enantioselective transfer hydrogenation and a method for connecting aromatics to form the complex tetracyclic morphinan structure. Notably, when compared to prior syntheses, this approach utilizes a readily available, stable, and cost-effective phosphonium ligand to ensure both high reaction yield and the desired selectivity in the crucial aromatics coupling step, all without the need for blocking groups. However, this requires tedious installation and deprotection steps, leading to unsatisfactory overall efficacy. In other words, effective decarboxylation of C12-C13 bonds without the use of terminal groups on aromatic rings has always been highly challenging and has not been resolved in the synthesis of opioid drugs.
3. Conclusion
Currently, there are quite a few established synthetic pathways of morphine opioids. In this paper, 3 were reviewed. In terms of the Gates research group’s synthesis it is significant because it was the first complete total synthesis of morphine. It also provided proof of the structure of morphine in 1925, an extremely important precedent for the future synthesis methods that occurred after. Nevertheless, it is one of the most inefficient synthetic morphine methods. It has a 31 step process with a yield of only 0.06%, making it not very economically viable nor efficiently using resources. On the flipside, the synthesis done by Varghese and Hudlicky is a lot more efficient in terms of steps. This synthesis process only takes 7 steps for the main part. Compared to the Gates synthesis, it creates a much higher yield of around 50%, but due to potential simultaneous intermolecular and intramolecular processes, there is still pretty low utilization of materials. Finally, the synthesis done by He’s research group is significant compared to the others shown because of its accessibility. This method uses air stable, inexpensive and thus easily accessible phosphonium ligands to secure the reaction yield without using blocking groups. However, the yield still isn’t very high with the highest overall yields of 16-34%, still less than that of the Varghese and Hudlicky process, its tedious installation and deprotection steps contributing to the overall inefficiency.
Nowadays, the common issues associated with the synthesis of opioids are mainly about high synthesis costs, legal and regulatory restrictions as well as generation of byproducts during synthesis. The synthesis process of opioids involves complexity and requires expensive raw materials and equipment, resulting in elevated production costs. Additionally, opioids are controlled substances subject to legal and regulatory restrictions. These limitations encompass licensing requirements, quota restrictions, and other factors that increase the complexity of synthesis. Finally, during the synthesis of opioids, there may be byproducts generated, which can potentially cause environmental pollution, necessitating proper handling and treatment [14].
In the process of researching this topic and consulting relevant literature, we have always maintained following relatively clear and concise thinking and writing way of thinking. At the same time, our research target is clear. These make our concluding work much easier. However, due to time and article length constraints and the abundance of research on morphine opioids, we have not been able to read through more works in this area of research and include a more complete picture of the synthetic pathways and their features in the article.
With the progress and development of science and technology, and the improvement of technology, we hope to achieve more efficient ways and methods of synthesizing morphine drugs one day in the future. This is an important process for the large demand for morphine drugs. Hopefully, this will happen in the near future as it would be a great milestone in human history.
References
[1]. Rogers, K. (2023, June 29). opioid. Encyclopedia Britannica. https://www.britannica.com/ science/opioid (last visited Aug 11th, 2023).
[2]. Winacoo JN, Maykel JA. Operative anesthesia and pain control. Clin Colon Rectal Surg. 2009 Feb;22(1):41-6. doi: 10.1055/s-0029-1202885. PMID: 20119555; PMCID: PMC2780232.
[3]. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018 Feb 1;9(1):143-150. doi: 10.14336/AD.2017.0306. PMID: 29392089; PMCID: PMC5772852
[4]. Li Qilin, Zhang Hongbin. Research Progress on the Synthesis of Morphine Alkaloids [J]. Chin. J. Org. Chem., 2017, 37(7): 1629-1652.
[5]. Ancient Analgesics: A brief history of opioids Ancient Analgesics: A brief history of opioids – Yale Scientific Magazine (last visited Aug 11th, 2023).
[6]. Zhang Lu, Huang Xingxing, Liu Keke, et al. Research progress on opioid management in China [J]. Oncology Pharmaceutics, 2023,13(1):17-22. DOI: 10.3969/j.issn.2095-1264.2023.01.03.
[7]. An 8,000-year History of Use and Abuse of Opium and Opioids: How That Matters For A Successful Control Of The Epidemic? (P4.9-055)Sankar Bandyopadhyay, Neurology Apr 2019, 92 (15 Supplement) P4.9-055;)
[8]. Li Qilin, Zhang Hongbin. Research Progress on the Synthesis of Morphine Alkaloids [J]. Chin. J. Org. Chem., 2017, 37(7): 1629-1652.
[9]. Zaslansky, R., Schramm, C., Stein, C. et al. Topical application of morphine for wound healing and analgesia in patients with oral lichen planus: a randomized, double-blind, placebo-controlled study. Clin Oral Invest 22, 305–311 (2018).
[10]. Mustafa Bilici, Adem Zengin, Elvan Ekmen, Demet Cetin, Nahit Aktas, Efficient and selective separation of metronidazole from human serum by using molecularly imprinted magnetic nanoparticles, Journal of Separation Science, 10.1002/jssc.201800428, 41, 14, (2952-2960), (2018).
[11]. Mark R. Hutchinson, Benjamen D. Coats, Susannah S. Lewis, Yingning Zhang, David B. Sprunger, Niloofar Rezvani, Eric M. Baker, Brian M. Jekich, Julie L. Wieseler, Andrew A. Somogyi, David Martin, Stephen Poole, Charles M. Judd, Steven F. Maier, Linda R. Watkins, Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia, Brain, Behavior, and Immunity, Volume 22, Issue 8, 2008, Pages 1178-1189, ISSN 0889-1591
[12]. Schiermeier, Q. Outcry over jailed Russian chemist. Nature (2012).
[13]. Gjermund Henriksen, Frode Willoch, Imaging of opioid receptors in the central nervous system, Brain, Volume 131, Issue 5, May 2008, Pages 1171–1196
[14]. Graham Farrell, John Thorne, Where have all the flowers gone?: evaluation of the Taliban crackdown against opium poppy cultivation in Afghanistan, International Journal of Drug Policy, Volume 16, Issue 2, 2005, Pages 81-91
[15]. Marshall D. Gates Jr., https://en.wikipedia.org/w/index.php?title=Marshall_D._Gates_Jr. &oldid=1070811664 (last visited Aug. 13, 2023)
[16]. Gates, M. and Tschudi, G., 1956. The synthesis of morphine. Journal of the American Chemical Society, 78(7), pp.1380-1393.
[17]. 10.1002/anie.201400286 Vimal Varghese and Tomas Hudlicky Short, Chemoenzymatic Total Synthesis of ent-Hydromorphone: An Oxidative Dearomatization/Intramolecular [4+2] Cycloaddition/ Amination Sequence
Cite this article
Wang,X.;Liu,Y.;Liu,Y.;Suo,C.;Huang,Y. (2024). Several synthetic pathways of morphine opioids. Theoretical and Natural Science,44,7-17.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Rogers, K. (2023, June 29). opioid. Encyclopedia Britannica. https://www.britannica.com/ science/opioid (last visited Aug 11th, 2023).
[2]. Winacoo JN, Maykel JA. Operative anesthesia and pain control. Clin Colon Rectal Surg. 2009 Feb;22(1):41-6. doi: 10.1055/s-0029-1202885. PMID: 20119555; PMCID: PMC2780232.
[3]. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018 Feb 1;9(1):143-150. doi: 10.14336/AD.2017.0306. PMID: 29392089; PMCID: PMC5772852
[4]. Li Qilin, Zhang Hongbin. Research Progress on the Synthesis of Morphine Alkaloids [J]. Chin. J. Org. Chem., 2017, 37(7): 1629-1652.
[5]. Ancient Analgesics: A brief history of opioids Ancient Analgesics: A brief history of opioids – Yale Scientific Magazine (last visited Aug 11th, 2023).
[6]. Zhang Lu, Huang Xingxing, Liu Keke, et al. Research progress on opioid management in China [J]. Oncology Pharmaceutics, 2023,13(1):17-22. DOI: 10.3969/j.issn.2095-1264.2023.01.03.
[7]. An 8,000-year History of Use and Abuse of Opium and Opioids: How That Matters For A Successful Control Of The Epidemic? (P4.9-055)Sankar Bandyopadhyay, Neurology Apr 2019, 92 (15 Supplement) P4.9-055;)
[8]. Li Qilin, Zhang Hongbin. Research Progress on the Synthesis of Morphine Alkaloids [J]. Chin. J. Org. Chem., 2017, 37(7): 1629-1652.
[9]. Zaslansky, R., Schramm, C., Stein, C. et al. Topical application of morphine for wound healing and analgesia in patients with oral lichen planus: a randomized, double-blind, placebo-controlled study. Clin Oral Invest 22, 305–311 (2018).
[10]. Mustafa Bilici, Adem Zengin, Elvan Ekmen, Demet Cetin, Nahit Aktas, Efficient and selective separation of metronidazole from human serum by using molecularly imprinted magnetic nanoparticles, Journal of Separation Science, 10.1002/jssc.201800428, 41, 14, (2952-2960), (2018).
[11]. Mark R. Hutchinson, Benjamen D. Coats, Susannah S. Lewis, Yingning Zhang, David B. Sprunger, Niloofar Rezvani, Eric M. Baker, Brian M. Jekich, Julie L. Wieseler, Andrew A. Somogyi, David Martin, Stephen Poole, Charles M. Judd, Steven F. Maier, Linda R. Watkins, Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia, Brain, Behavior, and Immunity, Volume 22, Issue 8, 2008, Pages 1178-1189, ISSN 0889-1591
[12]. Schiermeier, Q. Outcry over jailed Russian chemist. Nature (2012).
[13]. Gjermund Henriksen, Frode Willoch, Imaging of opioid receptors in the central nervous system, Brain, Volume 131, Issue 5, May 2008, Pages 1171–1196
[14]. Graham Farrell, John Thorne, Where have all the flowers gone?: evaluation of the Taliban crackdown against opium poppy cultivation in Afghanistan, International Journal of Drug Policy, Volume 16, Issue 2, 2005, Pages 81-91
[15]. Marshall D. Gates Jr., https://en.wikipedia.org/w/index.php?title=Marshall_D._Gates_Jr. &oldid=1070811664 (last visited Aug. 13, 2023)
[16]. Gates, M. and Tschudi, G., 1956. The synthesis of morphine. Journal of the American Chemical Society, 78(7), pp.1380-1393.
[17]. 10.1002/anie.201400286 Vimal Varghese and Tomas Hudlicky Short, Chemoenzymatic Total Synthesis of ent-Hydromorphone: An Oxidative Dearomatization/Intramolecular [4+2] Cycloaddition/ Amination Sequence









