1. Introduction
Implantable devices are medical devices designed to be surgically implanted inside the human body to diagnose, monitor or treat various medical conditions. These devices are a critical part of advanced medicine as they serve targeted and localized therapies, improve patient outcomes and enhance the quality of life for individuals with specific health issues. The first cardiac pacemaker was successfully implanted into a 77-year-old man in 1960 by William Chardack and his team; the patient lived for 10 months after the procedure [1]. Nine additional patients also received the devices that year, and several of them lived for more than two decades after their operations. Over 3 million individuals worldwide benefit from cardiac pacemakers, which have been frequently implanted as a result of the rising incidence rate of arrhythmia [2]. The advancement of implantable medical devices began with this groundbreaking device, which also sparked developments in implanted materials, electronics, integration of systems, miniaturization, and biointegration. Subsequent to this breakthrough, implantable neural interfaces, implantable drug delivery systems, and implantable orthopedic devices have continued to follow this technological trajectory. It is noticeable that these implantable devices contact human tissues directly with intermediate species. Conventional materials applied in these devices are usually alloys that have a possibility of foreign body reaction (FBR) and are relatively dense which could be a concern for certain applications [3]. Moreover, FBR outcomes from ongoing dislocation and stress at the implant-tissue interfaces might be worsened by mechanical mismatch, generally referring to the difference in mechanical characteristics between alloys and human tissues [2].
FBR managed by the immune system targets implantable devices and can be separated into two categories (acute FBR and chronic FBR) [3]. When any foreign material is inserted into living tissues, it triggers an inflammatory and fibrotic response. The immune system’s cells are drawn to the foreign materials, attempting to break it down. If this breakdown process is unsuccessful, fibroblasts enclose the material which creates a physical barrier to separate it from the surrounding tissues. As a result, FBR weakens the chronic performance of bioelectronics implants and especially limits the application of miniaturized and recording bioelectronic implants [4-6].
Biomaterials surface properties play an important role in modulating FBR at the tissue/material interface which lasts for the in vivo lifetime of the medical device, particularly in the first two to four weeks after implantation [4]. As used in implantable devices, hydrogels—three-dimensional (3D) polymer networks with a high water content—display more desirable features as contrasted with conventional materials [6]. These hydrophilic polymeric materials, which are based on water, closely resemble real tissues. Moreover, their mechanical characteristics can be manipulated to imitate the target tissue. Additionally, therapeutic substances like medications, cells, or anti-inflammatory compounds can be created to release from hydrogels [7]. Furthermore, the investigation of electrically conductive hydrogels is made possible in a number of ways, facilitating charge transmission between the bioelectronic devices and the target tissue while lowering FBR [8]. In brief, conductive hydrogels can be used as soft and stretchable bioelectronics that acquire electrophysiological and physical signals, empowering the utilization of these signals for the diagnosis and treatment of different medical conditions.
Here, we provide the benefits of incorporating hydrogels in bioelectronics and examine the cutting-edge techniques employed to improve hydrogel properties. These methods are developed to enhance their characteristics which include achieving conductivity, reducing swelling and enabling self-healing capabilities, and satisfying the need for advanced implantable bioelectronics devices.
2. Benefits of hydrogels in bioelectronics
2.1. Structure of hydrogels
Hydrogels are water-absorbing hydrophilic polymers, possessing a three-dimensional structure that can retain a significant amount of water thanks to the inclusion of hydrophilic groups [9]. Hydrogels exist as a solid with a network of polymer chains interconnected through cross-linking. The 3D network structure gives hydrogels an infinite molecular weight. At the molecular level, key factors defining hydrogels’ structure include the mesh size and the molecular weight of the polymer chains between the crosslink [10]. Hydrogel crosslinking can be achieved through either chemical-like covalent interactions or physical interactions such as hydrogen bonding and entanglement as Figure 1 shows.
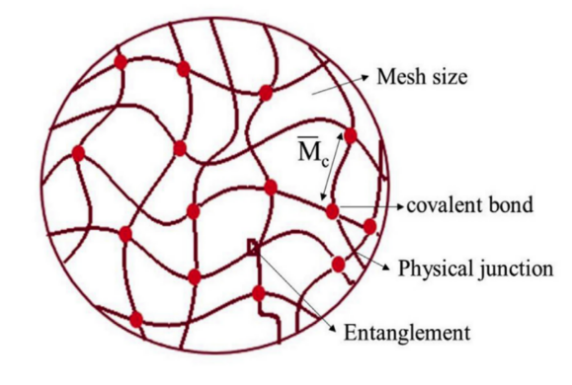
Figure 1. The molecular-level structure of hydrogels [11]
2.2. Benefits of hydrogels in bioelectronics
For medical devices and implants, biocompatibility, which refers to the ability of a material or substance to interact with living tissues or biological systems without causing harm, adverse reactions, or toxic effects, is a critical consideration to ensure that the materials do not trigger rejection or complications when implanted inside the body [12]. From this point of view, hydrogels have unique properties that could be useful to apply within implantable devices and control or mitigate FBR.
Due to the crosslinked networks of hydrophilic polymers that have a high affinity for water, hydrogels have the ability to absorb and retain water which makes them versatile materials with numerous applications in various fields, including medicine, and biotechnology. Both natural and synthetic hydrogels have found extensive use in biomedical applications. Common hydrogel examples include collagen, alginate, fibrin, and hyaluronic acid (HA) [2]. In water, the 3D polymer mesh does not obviously dissolve. Importantly, gelation is a crucial step in the creation of hydrogels. It can involve chemical interactions between monomers that have been covalently linked together to form polymer chains and condensed via cross-linkers, initiators, or reactive substances, or it can involve the physical interlocking of polymer chains by means of ionic gelation, electrostatic interactions, or hydrogen bonds through molecular entanglements and hydrophobic associations [13]. Overall, hydrogels possess a multi-scale structure, encompassing features ranging from millimeters to sub-nanometers.
The three-dimensional structure of hydrogels mimics the mechanics, porosity, and water content of the extracellular matrix (ECM). Hydrogels are highly sought-after for incorporation into implantable biomimetic devices due to their mechanical properties. Moreover, hydrogels can be made to have soft tissue-like qualities, such as softness between 1 Pa and 1 MPa and high stretchability (about 20-75% reversible elongation), by modifying both the cross-linking ratio and molecular weight of the polymer chains [14]. Hydrogels exhibit controlled swelling and degradability features, which means they display stress relaxation when subjected to deformation [15]. Furthermore, in comparison with physically cross-linked hydrogels, covalently cross-linked hydrogels display higher viscoelastic behavior, with a stress relaxation duration of roughly 10 to 200 s. As a result, hydrogels can mimic the fundamental mechanical properties of tissues, which can also lessen shear strains at the tissue interface that result from micromotion and tethering [16].
The combination of these factors including water-based structure, hydrophilic nature, minimal toxicity, similarity to ECM, and outstanding mechanical properties we mentioned above makes hydrogels biocompatible. Currently, hydrogels are predominantly utilized as a surface coating for various bioelectronic implants. Simultaneously, recent researches focus on their usage as electrical and electronic/ionic interface material. Until now, a significant challenge lies in creating hydrogel with high electrical conductivity while maintaining its mechanical flexibility and processability [17].
3. Cutting-edge research areas of hydrogels for bioelectronics
3.1. Conductive hydrogels
Recent progress in cardiac engineering, neural engineering, and microtechnology has improved the integration of electronic devices with living tissues. This advancement enables precise electrical stimulation and accurate recording of biological signals from living tissues [18]. Electrical signals originating from biological tissues are either stimulated or monitored using electrical conductors that carry signals to and from the reading platform. The desired electrical conductors for this bioelectronics should possess high conductivity, mechanical properties similar to tissues, and noncytotoxicity to mitigate the likelihood of adverse immune responses [19]. Due to their flexibility, high tensile properties, and biocompatibility, conductive hydrogels have attracted great interest [17]. Electrical conductivity can be mediated either ionically or electronically. Owing to their ability to absorb biological fluids where ionic interactions are predominant, hydrogels can indirectly act as ionic conductors [20]. However, such build-in conductivity cannot meet the requirements of most implantable devices which are used to carry signals. Here are several methods to further improve the conductivity of hydrogels:
1) Salt doping
Chemical doping involves introducing conductive molecules or dopants into the hydrogels during their synthesis. The ionic conductivity of the hydrogels can be further enhanced by adding salt as a dopant such as NaCl, LiCl, FeCl3, KCl, or CaCl2. However, it is essential to exercise caution as excessive concentrations of salts lead to tissue damage limiting the potential increase in ionic conductivity [21].
2) Doping conductive polymers
An alternative approach is to utilize conductive polymers like Polypyrrole (PPy), Poly(3,4-ethylenedioxythiophene) (PEDOT), and polyaniline (PANi). These polymers contain aromatic groups with \( π \) -conjugation, which comprises alternating single and double covalent bonds that provide free electrons, resulting in electronic conductivity [22,23].
3) Incorporation of conductive fillers
The hydrogel matrix’s electrical conductivity can be increased by mixing conductive fillers like metallic nanoparticles, carbon nanotubes, or nanotubes of graphene into it. These conductive materials create pathways for electron transport within the hydrogel, enhancing its overall conductivity. Notably, hydrogels doped with silver particles have exhibited conductivities within the range of 1.36 to 374 S/cm as significant examples [24,25]. In addition, by incorporating graphene or carbon nanotubes, it has been possible to achieve conductivities of approximately \( 4×{10^{-5}} \) to \( 4.2×{10^{-3}} \) S/cm [26,27]. and 0.01 to 10 S/cm [28,29], respectively.
There are also other methods to make hydrogels achieve conductive. By using conductive crosslinkers to physically link the hydrogel polymer chains, hydrogels can be conductive because of the created conductive network within themselves. Yang et al. reported conductive polymer hydrogels by using Poly (3,4-ethylene dioxythiophene) /poly (styrene sulfonate) (PEDOT: PSS) as conductive dopant to crosslink various conductive polymers including PANi, PPy, and Pin-X-NH2 (X=4, 5, 6, 7). These modified hydrogels showed conductivity ranging from 11.72 to 70.54 S/m with good biocompatibility [30]. Andrew R.S. et al. also added PEDOT: PSS to synthesize electroconductive gelatin methacryloyl hydrogels [31]. Their results indicated that characteristics of hydrogels including mechanics, swelling, and degradation, could be controlled by the influence of the concentration of PEDOT: PSS. Moreover, biological-based approaches may be the proper method to achieve conductivity, which involves incorporating conductive biomaterials like conductive biomolecules into hydrogels. Iman N. et al. reported a new kind of conductive hydrogel by functionalizing non-conductive polymers with a conductive choline-based bio-ionic liquid (Bio-IL) [32].
Overall, when thinking about conductivity in biointerfaces, it is essential to recognize that bioelectronic devices necessitate localized electrically conductive regions in contact with tissues (e.g., electronic contacts). The remaining device surface must be electrically insulated to ensure specific and precise electrical interfacing. Since hydrogels absorb ionically conductive fluids from the surrounding environment [31], they cannot serve as insulators for electrodes and conductors. Therefore, careful device design, incorporating suitable barrier layers, is imperative to ensure proper functionality. Additionally, optimizing the conductive properties while preserving the desirable mechanical and biological properties of hydrogels is crucial for successful integration into various bioelectronics and biomedical applications.
3.2. Non-swelling hydrogels exhibiting tough tissue adhesion
Electrically conductive hydrogels contain a hydrophilic polymer network with a three-dimensional crosslinked structure, incorporating conductive polymers or conductive nanomaterials as additives. The inclusion of these conductive components enhances the functional versatility and mechanical toughness of the hydrogels. Because of their likeness to the ECM, distinct biocompatibility, and tunable mechanical and biochemical properties, electrically conductive hydrogels are ideally suited to fulfill the complex requirements of materials in bioelectronics [32].
However, an essential yet overlooked aspect concerning the inherent characteristic of electrically conductive hydrogels is their long-term water swelling due to osmotic pressure differences. An outstanding volume change can be observed when swelling (see Fig 2). The extent of hydrogel swelling primarily depends on the water diffusion into the hydrogel [33]. Influenced by swelling, their practical use in implantable scenarios is limited since all existing hydrogels inevitably undergo swelling under physiological conditions, resulting in significant degradation of their mechanical properties [34]. Swelling in implanted electrically conductive hydrogels can exert pressure on surrounding tissues, leading to severe side effects over time. Moreover, the swelling weakens mechanical strength and reduces adhesion, which can result in interfacial delamination. Additionally, the electrical conductivity of these hydrogels may also decrease due to swelling, making it challenging to employ them as long-term, stable bioelectronic electrodes [18].
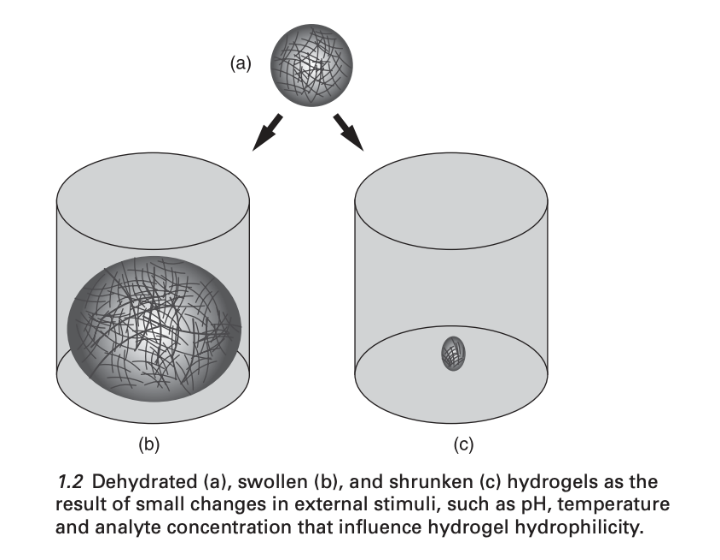
Figure 2. Dehydrated (a), swollen (b), and shrunken (c) hydrogels [33]
Furthermore, rapid response plays a crucial role in capturing signals promptly. To achieve this quick response, it is vital for substrates to strongly adhere to conductive materials, preventing slipping at the substrate-conductive materials interfaces and accelerating sensor responses effectively. In other words, strong substrate-tissue adhesion is essential for stable electrical signal transmission at the electrode-tissue interfaces during dynamic nerve stimulation and electrophysiological signal recording [35]. Hence, it is important to create non-swelling hydrogels that possess regenerable and robust tissue adhesion. This development is vital for enhancing the accuracy and reliability of signal transmission at bioelectronic-tissue interfaces.
The most common method to restrict hydrogels from swelling is crosslinking density control [36]. Adjusting the crosslinking density of hydrogels can reduce their swelling behavior. Increasing the degree of crosslinking leads to a more compact and rigid network, which limits water absorption. Moreover, chemical modification is also a useful way to limit swelling. Introducing specific hydrophobic chemical groups or moieties into hydrogels can alter their hydrophilic nature and reduce water uptake. Tian et al. introduced hydrophobic poly (vinyl butyral) (PVB) to poly (acrylic acid) (PAA)-based hydrogel [37]. The tissue adhesion strength of this hydrogel reached 211.4 kPa, approximately ten times greater than that of the current non-swelling hydrogels, despite having a 1.2 swelling ratio. They claimed that the hydrophobic PVB is capable of efficiently limiting water penetration without impeding the migration of adhesive groups within the hydrogel toward the hydrogel-tissue interface. Another traditional approach to reducing swelling involves incorporating a thermoresponsive polymer with a lower critical solution temperature (LCST) into the hydrogel network, which mitigates water absorption at higher temperatures. Nevertheless, adjusting the mechanical strength of the gel through changes in the network structure frequently impacts water absorption characteristics. Therefore, a preferred goal is to develop a non-swelling platform with adjustable mechanical properties, catering to diverse biomedical applications. Vinh X. T. et al. reported a non-swelling click-cross-linked gelatin and PEG hydrogels with limited swelling at approximately physiological temperature by applying the commercially available triblock PEG-PPG-PEG (Pluronic) as a cross-linker [38]. Using non-swelling fillers or nanoparticles in the hydrogel is another option that can act as a barrier to water absorption and prevent excessive swelling [39]. However, the key problem of this method is that it may compromise the hydrogel’s overall mechanical properties and flexibility because non-swelling fillers influence the structure and porosity of the hydrogel, affecting its ability to interact with surrounding tissues of biological systems.
Managing adhesion to both components becomes essential to take into account when hydrogels act as interface materials between a bioelectronic device and biological tissue. Numerous functional groups, such as hydroxyl, carboxylic acid, thiol, and amino groups, are present in biological tissues and provide opportunities for chemical or physical bonds that could be used to attach the hydrogel [40]. Physical binding primarily arises from electrostatic, Van der Waals, and hydrogen bonds, which are inherently more fragile than covalent bonding. Hydrogels can be chemically functionalized with moieties that can bind to the functional groups naturally present in tissues to increase adhesion. Both Michaelson’s addition and Schiff’s base reaction are frequently used chemical processes to create covalent bonds with tissues [41]. Moreover, nature-inspired approaches have been explored. For instance, by emulating the adhesive capabilities of muscles in seawater, polydopamine has been employed as a plaster to enhance adhesion to wet surfaces [42]. Because polydopamine contains catechol, imine, and amine groups, it can adhere to a variety of surfaces, including paper, PI, glass, and metals, through \( π \) - \( π \) stacking, hydrogen bonds, and covalent bonds [42].42 Mechanical intermeshing is another choice, which is made possible by surface roughness or microstructured patterns [43].
3.3. Self-healing
Due to abrasion and bending fatigue, the implanted hydrogel can be broken at a high percentage. As a result, the implanted hydrogel is hoped to be improved to be self-healing, which refers to the capability to repair and recover from damage or physical stress autonomously [44]. Self-healing mechanisms, which draw inspiration from or imitate natural systems, are being integrated into synthetic polymeric materials (hydrogels) [45]. Just like how our skin heals from minor cuts or wounds, self-healing hydrogels can mend themselves when subjected to mechanical damage. Self-healing involves the material reestablishing its initial shape or condition through reconnection, such as cutting a gel and subsequently enabling it to rejoin seamlessly (shown in Fig. 3). The material’s ability to recover results from the presence of reversible bonds in its architecture [45]. This is primarily due to the inherent capability of chemical molecules to reestablish bonds after they have been broken.
Research into self-healing hydrogels can be categorized into two primary methods: dynamic covalent reactions, involving chemical crosslinking, and noncovalent reactions, based on physical crosslinking [46]. In hydrogel self-healing through covalent reactions, the reapplication of polymerization conditions or an external stimulus such as pH, alternating current, or UV light is often necessary [45]. Less prevalent are hydrogels that employ dynamic covalent reactions which do not necessitate external intervention. On the other hand, autonomous self-healing hydrogels predominantly employ noncovalent interactions, either individually or in conjunction. These interactions encompass ionic bonding, hydrogen bonding, supramolecular interactions, hydrophobic bonding, as well as molecular diffusion and chain entanglement [47]. When the hydrogel experiences damage, these reversible bonds or interactions can reassemble or rearrange, enabling the material to regain its original structure and functionality. For instance, hydroxyl or carboxyl groups and \( {Fe^{3+}} \) can form and break ionic or hydrogen bonds [48]. Reversible covalent bond breakage and formation have also been observed involving disulfide bonds and imine bonds [49].
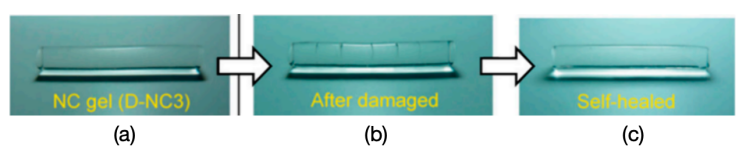
Figure 3. The self-healing proceeds of hydrogels [39].
4. Conclusion
Over the past few decades, due to the similarity between living tissues and hydrogels, hydrogels have garnered substantial attention as an ideal engineering material that can be used in numerous fields, encompassing biomedicine, biomechanics, and bioelectronics. In earlier times, with limited insights into biological systems and their interactions with electronics, traditional engineering materials, and devices such as metal-based and silicon-based probes were commonly used in implantable devices, which were highly related to FBR and mechanical mismatch, etc., leading to potential danger for a long-term cure. As our comprehension of the intricate dynamics of tissue-electrode interactions expands, our awareness of the origins of various adverse outcomes and failures in bioelectronic devices concurrently deepens.
To overcome the limitations of conventional materials, hydrogels with distinct biological and mechanical properties have drawn great attention and are expected to alter the traditional materials used in living tissues. In this review, we provide an overview of the advantages of hydrogels to demonstrate that their outstanding characteristics meet the requirement of specific materials applied in vivo. In addition, we introduce cutting-edge research areas to improve common hydrogels to be conductive, non-swelling, and self-healing, which adds more highlights to hydrogels making them more suitable in biological tissues. In the future, we believe that the forthcoming advancements in hydrogels bioelectronics will derive their benefits from well-informed and guided design principles rooted in a comprehensive grasp of the fundamental mechanisms governing the interactions between electrodes and tissues and the comprehensive control of the approaches to tuning the properties of hydrogels to reach better mechanical properties and biocompatibility. Despite these daunting challenges and troubles faced by the development of hydrogel bioelectronics, simultaneously it will surely pave an efficient way to achieve the combination of biology and electronics, which is expected to solve the health problems relevant to the public.
References
[1]. Chardack, W. M., Gage, A. A. & Greatbatch, W. A transistorized, self-contained, implantable pacemaker for the long-term correction of complete heart block. Surgery 48, 643-654 (1960).
[2]. Sagdic, K., Fernández-Lavado, E., Mariello, M., Akouissi, O. & Lacour, S. P. Hydrogels and conductive hydrogels for implantable bioelectronics. MRS Bulletin 48, 495-505 (2023). https://doi.org:10.1557/s43577-023-00536-1
[3]. Carnicer-Lombarte, A., Chen, S. T., Malliaras, G. G. & Barone, D. G. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Front Bioeng Biotechnol 9, 622524 (2021). https://doi.org:10.3389/fbioe.2021.622524
[4]. Anderson, J. M., Rodriguez, A. & Chang, D. T. Foreign body reaction to biomaterials. Semin Immunol 20, 86-100 (2008). https://doi.org:10.1016/j.smim.2007.11.004
[5]. Barone, D. G. et al. Prevention of the foreign body response to implantable medical devices by inflammasome inhibition. Proc Natl Acad Sci U S A 119, e2115857119 (2022). https://doi.org:10.1073/pnas.2115857119
[6]. Chen, A. et al. Zwitterionic Polymer/Polydopamine Coating of Electrode Arrays Reduces Fibrosis and Residual Hearing Loss after Cochlear Implantation. Adv Healthc Mater 12, e2200807 (2023). https://doi.org:10.1002/adhm.202200807
[7]. Li, J. & Mooney, D. J. Designing hydrogels for controlled drug delivery. Nat Rev Mater 1 (2016). https://doi.org:10.1038/natrevmats.2016.71
[8]. Park, S. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat Commun 12, 3435 (2021). https://doi.org:10.1038/s41467-021-23802-9
[9]. Habib, M. A., Akter, A., Alam, M. E., Karim, Z. & Joarder, M. T. A.
[10]. Ganji, F., Vasheghani-Farahani, S. & Vasheghani-Farahani, E.
[11]. Aswathy, S. H., Narendrakumar, U. & Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 6, e03719 (2020). https://doi.org:10.1016/j.heliyon.2020.e03719
[12]. Black, J. Biological Performance of Materials: Fundamentals of Biocompatibility, Fourth Edition (4th ed.). (CRC Press, 2005).
[13]. Burdick, J. A. & Stevens, M. M. in Biomaterials, Artificial Organs and Tissue Engineering (eds Larry L. Hench & Julian R. Jones) 107-115 (Woodhead Publishing, 2005).
[14]. Zhang, Y. S. & Khademhosseini, A. Advances in engineering hydrogels. Science 356, eaaf3627 (2017). https://doi.org:10.1126/science.aaf3627
[15]. Kong, H. J., Kaigler, D., Kim, K. & Mooney, D. J. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules 5, 1720-1727 (2004). https://doi.org:10.1021/bm049879r
[16]. Spencer, K. C., Sy, J. C., Ramadi, K. B., Graybiel, A. M., Langer, R. & Cima, M. J. Characterization of Mechanically Matched Hydrogel Coatings to Improve the Biocompatibility of Neural Implants. Sci Rep 7, 1952 (2017). https://doi.org:10.1038/s41598-017-02107-2
[17]. Yuk, H., Lu, B. & Zhao, X. Hydrogel bioelectronics. Chem Soc Rev 48, 1642-1667 (2019). https://doi.org:10.1039/c8cs00595h
[18]. Han, I. K. et al. Electroconductive, Adhesive, Non-Swelling, and Viscoelastic Hydrogels for Bioelectronics. Adv Mater 35, e2203431 (2023). https://doi.org:10.1002/adma.202203431
[19]. Lacour, S. P., Courtine, G. & Guck, J. Materials and technologies for soft implantable neuroprostheses. Nature Reviews Materials 1 (2016). https://doi.org:10.1038/natrevmats.2016.63
[20]. Saghir, S., Imenes, K. & Schiavone, G. Integration of hydrogels in microfabrication processes for bioelectronic medicine: Progress and outlook. Front Bioeng Biotechnol 11, 1150147 (2023). https://doi.org:10.3389/fbioe.2023.1150147
[21]. Peng, Q. et al. Recent advances in designing conductive hydrogels for flexible electronics. InfoMat 2, 843-865 (2020). https://doi.org:10.1002/inf2.12113
[22]. Gao, C., Song, S., Lv, Y., Huang, J. & Zhang, Z. Recent Development of Conductive Hydrogels for Tissue Engineering: Review and Perspective. Macromol Biosci 22, e2200051 (2022). https://doi.org:10.1002/mabi.202200051
[23]. Nezakati, T., Seifalian, A., Tan, A. & Seifalian, A. M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem Rev 118, 6766-6843 (2018). https://doi.org:10.1021/acs.chemrev.6b00275
[24]. Devaki, S. J., Narayanan, R. K. & Sarojam, S. Electrically conducting silver nanoparticle– polyacrylic acid hydrogel by in situ reduction and polymerization approach. Materials Letters 116, 135-138 (2014).
[25]. Ohm, Y., Pan, C., Ford, M. J., Huang, X., Liao, J. & Majidi, C. Publisher Correction: An electrically conductive silver–polyacrylamide–alginate hydrogel composite for soft electronics. Nature Electronics 4, 313-313 (2021). https://doi.org:10.1038/s41928-021-00571-3
[26]. Park, J., Choi, J. H., Kim, S., Jang, I., Jeong, S. & Lee, J. Y. Micropatterned conductive hydrogels as multifunctional muscle-mimicking biomaterials: Graphene-incorporated hydrogels directly patterned with femtosecond laser ablation. Acta Biomater 97, 141-153 (2019). https://doi.org:10.1016/j.actbio.2019.07.044
[27]. Zhou, J. et al. Injectable OPF/graphene oxide hydrogels provide mechanical support and enhance cell electrical signaling after implantation into myocardial infarct. Theranostics 8, 3317-3330 (2018). https://doi.org:10.7150/thno.25504
[28]. Cho, Y. & Borgens, R. B. The effect of an electrically conductive carbon nanotube/collagen composite on neurite outgrowth of PC12 cells. J Biomed Mater Res A 95, 510-517 (2010). https://doi.org:10.1002/jbm.a.32841
[29]. Liu, X. W. et al. Conductive carbon nanotube hydrogel as a bioanode for enhanced microbial electrocatalysis. ACS Appl Mater Interfaces 6, 8158-8164 (2014). https://doi.org:10.1021/am500624k
[30]. Yang, T. et al. Conductive polymer hydrogels crosslinked by electrostatic interaction with PEDOT:PSS dopant for bioelectronics application. Chemical Engineering Journal 429, 132430 (2022). https://doi.org:https://doi.org/10.1016/j.cej.2021.132430
[31]. Spencer, A. R., Primbetova, A., Koppes, A. N., Koppes, R. A., Fenniri, H. & Annabi, N. Electroconductive Gelatin Methacryloyl-PEDOT:PSS Composite Hydrogels: Design, Synthesis, and Properties. ACS Biomater Sci Eng 4, 1558-1567 (2018). https://doi.org:10.1021/acsbiomaterials.8b00135
[32]. Noshadi, I. et al. Engineering Biodegradable and Biocompatible Bio-ionic Liquid Conjugated Hydrogels with Tunable Conductivity and Mechanical Properties. Sci Rep 7, 4345 (2017). https://doi.org:10.1038/s41598-017-04280-w
[33]. Holback, H., Yeo, Y. & Park, K. in Biomedical Hydrogels 3-24 (2011).
[34]. Kamata, H., Akagi, Y., Kayasuga-Kariya, Y., Chung, U. I. & Sakai, T. “Nonswellable” hydrogel without mechanical hysteresis. Science 343, 873-875 (2014). https://doi.org:10.1126/science.1247811
[35]. Liang, C. et al. Strategies for interface issues and challenges of neural electrodes. Nanoscale 14, 3346-3366 (2022). https://doi.org:10.1039/d1nr07226a
[36]. Cates, R. S. <Influence of Crosslink Density on Swelling and Conformation of Su.pdf>. (2010).
[37]. Tian, G. et al. A Nonswelling Hydrogel with Regenerable High Wet Tissue Adhesion for Bioelectronics. Adv Mater 35, e2212302 (2023). https://doi.org:10.1002/adma.202212302
[38]. Truong, V. X., Tsang, K. M. & Forsythe, J. S. Nonswelling Click-Cross-Linked Gelatin and PEG Hydrogels with Tunable Properties Using Pluronic Linkers. Biomacromolecules 18, 757-766 (2017). https://doi.org:10.1021/acs.biomac.6b01601
[39]. Dannert, C., Stokke, B. T. & Dias, R. S. Nanoparticle-Hydrogel Composites: From Molecular Interactions to Macroscopic Behavior. Polymers (Basel) 11 (2019). https://doi.org:10.3390/polym11020275
[40]. Yang, Y., Ren, Y., Song, W., Yu, B. & Liu, H. Rational design in functional hydrogels towards biotherapeutics. Materials & Design 223, 111086 (2022). https://doi.org:https://doi.org/10.1016/j.matdes.2022.111086
[41]. Cong, Y. & Fu, J. Hydrogel–Tissue Interface Interactions for Implantable Flexible Bioelectronics. Langmuir 38, 11503-11513 (2022). https://doi.org:10.1021/acs.langmuir.2c01674
[42]. Chalmers, E., Lee, H., Zhu, C. & Liu, X. Increasing the Conductivity and Adhesion of Polypyrrole Hydrogels with Electropolymerized Polydopamine. Chemistry of Materials 32, 234-244 (2020). https://doi.org:10.1021/acs.chemmater.9b03655
[43]. Fan, H. & Gong, J. P. Bioinspired Underwater Adhesives. Advanced Materials 33, 2102983 (2021). https://doi.org:https://doi.org/10.1002/adma.202102983
[44]. Binder, W. H. Self-Healing Polymers: From Principles to Applications. (Wiley-VCH Verlag GmbH, 2013).
[45]. Taylor, D. L. & In Het Panhuis, M. Self-Healing Hydrogels. Adv Mater 28, 9060-9093 (2016). https://doi.org:10.1002/adma.201601613
[46]. Blaiszik, B. J., Kramer, S. L. B., Olugebefola, S. C., Moore, J. S., Sottos, N. R. & White, S. R. Self-Healing Polymers and Composites. Annual Review of Materials Research 40, 179-211 (2010). https://doi.org:10.1146/annurev-matsci-070909-104532
[47]. Zhang, A., Liu, Y., Qin, D., Sun, M., Wang, T. & Chen, X. Research status of self-healing hydrogel for wound management: A review. Int J Biol Macromol 164, 2108-2123 (2020). https://doi.org:10.1016/j.ijbiomac.2020.08.109
[48]. Devi, V. K. A., Shyam, R., Palaniappan, A., Jaiswal, A. K., Oh, T. H. & Nathanael, A. J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers (Basel) 13 (2021). https://doi.org:10.3390/polym13213782
[49]. Talebian, S. et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Advanced Science 6, 1801664 (2019). https://doi.org:https://doi.org/10.1002/advs.201801664
Cite this article
Xia,X. (2024). The application of advanced hydrogel in bioelectronics. Theoretical and Natural Science,45,53-61.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Chardack, W. M., Gage, A. A. & Greatbatch, W. A transistorized, self-contained, implantable pacemaker for the long-term correction of complete heart block. Surgery 48, 643-654 (1960).
[2]. Sagdic, K., Fernández-Lavado, E., Mariello, M., Akouissi, O. & Lacour, S. P. Hydrogels and conductive hydrogels for implantable bioelectronics. MRS Bulletin 48, 495-505 (2023). https://doi.org:10.1557/s43577-023-00536-1
[3]. Carnicer-Lombarte, A., Chen, S. T., Malliaras, G. G. & Barone, D. G. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Front Bioeng Biotechnol 9, 622524 (2021). https://doi.org:10.3389/fbioe.2021.622524
[4]. Anderson, J. M., Rodriguez, A. & Chang, D. T. Foreign body reaction to biomaterials. Semin Immunol 20, 86-100 (2008). https://doi.org:10.1016/j.smim.2007.11.004
[5]. Barone, D. G. et al. Prevention of the foreign body response to implantable medical devices by inflammasome inhibition. Proc Natl Acad Sci U S A 119, e2115857119 (2022). https://doi.org:10.1073/pnas.2115857119
[6]. Chen, A. et al. Zwitterionic Polymer/Polydopamine Coating of Electrode Arrays Reduces Fibrosis and Residual Hearing Loss after Cochlear Implantation. Adv Healthc Mater 12, e2200807 (2023). https://doi.org:10.1002/adhm.202200807
[7]. Li, J. & Mooney, D. J. Designing hydrogels for controlled drug delivery. Nat Rev Mater 1 (2016). https://doi.org:10.1038/natrevmats.2016.71
[8]. Park, S. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat Commun 12, 3435 (2021). https://doi.org:10.1038/s41467-021-23802-9
[9]. Habib, M. A., Akter, A., Alam, M. E., Karim, Z. & Joarder, M. T. A.
[10]. Ganji, F., Vasheghani-Farahani, S. & Vasheghani-Farahani, E.
[11]. Aswathy, S. H., Narendrakumar, U. & Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 6, e03719 (2020). https://doi.org:10.1016/j.heliyon.2020.e03719
[12]. Black, J. Biological Performance of Materials: Fundamentals of Biocompatibility, Fourth Edition (4th ed.). (CRC Press, 2005).
[13]. Burdick, J. A. & Stevens, M. M. in Biomaterials, Artificial Organs and Tissue Engineering (eds Larry L. Hench & Julian R. Jones) 107-115 (Woodhead Publishing, 2005).
[14]. Zhang, Y. S. & Khademhosseini, A. Advances in engineering hydrogels. Science 356, eaaf3627 (2017). https://doi.org:10.1126/science.aaf3627
[15]. Kong, H. J., Kaigler, D., Kim, K. & Mooney, D. J. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules 5, 1720-1727 (2004). https://doi.org:10.1021/bm049879r
[16]. Spencer, K. C., Sy, J. C., Ramadi, K. B., Graybiel, A. M., Langer, R. & Cima, M. J. Characterization of Mechanically Matched Hydrogel Coatings to Improve the Biocompatibility of Neural Implants. Sci Rep 7, 1952 (2017). https://doi.org:10.1038/s41598-017-02107-2
[17]. Yuk, H., Lu, B. & Zhao, X. Hydrogel bioelectronics. Chem Soc Rev 48, 1642-1667 (2019). https://doi.org:10.1039/c8cs00595h
[18]. Han, I. K. et al. Electroconductive, Adhesive, Non-Swelling, and Viscoelastic Hydrogels for Bioelectronics. Adv Mater 35, e2203431 (2023). https://doi.org:10.1002/adma.202203431
[19]. Lacour, S. P., Courtine, G. & Guck, J. Materials and technologies for soft implantable neuroprostheses. Nature Reviews Materials 1 (2016). https://doi.org:10.1038/natrevmats.2016.63
[20]. Saghir, S., Imenes, K. & Schiavone, G. Integration of hydrogels in microfabrication processes for bioelectronic medicine: Progress and outlook. Front Bioeng Biotechnol 11, 1150147 (2023). https://doi.org:10.3389/fbioe.2023.1150147
[21]. Peng, Q. et al. Recent advances in designing conductive hydrogels for flexible electronics. InfoMat 2, 843-865 (2020). https://doi.org:10.1002/inf2.12113
[22]. Gao, C., Song, S., Lv, Y., Huang, J. & Zhang, Z. Recent Development of Conductive Hydrogels for Tissue Engineering: Review and Perspective. Macromol Biosci 22, e2200051 (2022). https://doi.org:10.1002/mabi.202200051
[23]. Nezakati, T., Seifalian, A., Tan, A. & Seifalian, A. M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem Rev 118, 6766-6843 (2018). https://doi.org:10.1021/acs.chemrev.6b00275
[24]. Devaki, S. J., Narayanan, R. K. & Sarojam, S. Electrically conducting silver nanoparticle– polyacrylic acid hydrogel by in situ reduction and polymerization approach. Materials Letters 116, 135-138 (2014).
[25]. Ohm, Y., Pan, C., Ford, M. J., Huang, X., Liao, J. & Majidi, C. Publisher Correction: An electrically conductive silver–polyacrylamide–alginate hydrogel composite for soft electronics. Nature Electronics 4, 313-313 (2021). https://doi.org:10.1038/s41928-021-00571-3
[26]. Park, J., Choi, J. H., Kim, S., Jang, I., Jeong, S. & Lee, J. Y. Micropatterned conductive hydrogels as multifunctional muscle-mimicking biomaterials: Graphene-incorporated hydrogels directly patterned with femtosecond laser ablation. Acta Biomater 97, 141-153 (2019). https://doi.org:10.1016/j.actbio.2019.07.044
[27]. Zhou, J. et al. Injectable OPF/graphene oxide hydrogels provide mechanical support and enhance cell electrical signaling after implantation into myocardial infarct. Theranostics 8, 3317-3330 (2018). https://doi.org:10.7150/thno.25504
[28]. Cho, Y. & Borgens, R. B. The effect of an electrically conductive carbon nanotube/collagen composite on neurite outgrowth of PC12 cells. J Biomed Mater Res A 95, 510-517 (2010). https://doi.org:10.1002/jbm.a.32841
[29]. Liu, X. W. et al. Conductive carbon nanotube hydrogel as a bioanode for enhanced microbial electrocatalysis. ACS Appl Mater Interfaces 6, 8158-8164 (2014). https://doi.org:10.1021/am500624k
[30]. Yang, T. et al. Conductive polymer hydrogels crosslinked by electrostatic interaction with PEDOT:PSS dopant for bioelectronics application. Chemical Engineering Journal 429, 132430 (2022). https://doi.org:https://doi.org/10.1016/j.cej.2021.132430
[31]. Spencer, A. R., Primbetova, A., Koppes, A. N., Koppes, R. A., Fenniri, H. & Annabi, N. Electroconductive Gelatin Methacryloyl-PEDOT:PSS Composite Hydrogels: Design, Synthesis, and Properties. ACS Biomater Sci Eng 4, 1558-1567 (2018). https://doi.org:10.1021/acsbiomaterials.8b00135
[32]. Noshadi, I. et al. Engineering Biodegradable and Biocompatible Bio-ionic Liquid Conjugated Hydrogels with Tunable Conductivity and Mechanical Properties. Sci Rep 7, 4345 (2017). https://doi.org:10.1038/s41598-017-04280-w
[33]. Holback, H., Yeo, Y. & Park, K. in Biomedical Hydrogels 3-24 (2011).
[34]. Kamata, H., Akagi, Y., Kayasuga-Kariya, Y., Chung, U. I. & Sakai, T. “Nonswellable” hydrogel without mechanical hysteresis. Science 343, 873-875 (2014). https://doi.org:10.1126/science.1247811
[35]. Liang, C. et al. Strategies for interface issues and challenges of neural electrodes. Nanoscale 14, 3346-3366 (2022). https://doi.org:10.1039/d1nr07226a
[36]. Cates, R. S. <Influence of Crosslink Density on Swelling and Conformation of Su.pdf>. (2010).
[37]. Tian, G. et al. A Nonswelling Hydrogel with Regenerable High Wet Tissue Adhesion for Bioelectronics. Adv Mater 35, e2212302 (2023). https://doi.org:10.1002/adma.202212302
[38]. Truong, V. X., Tsang, K. M. & Forsythe, J. S. Nonswelling Click-Cross-Linked Gelatin and PEG Hydrogels with Tunable Properties Using Pluronic Linkers. Biomacromolecules 18, 757-766 (2017). https://doi.org:10.1021/acs.biomac.6b01601
[39]. Dannert, C., Stokke, B. T. & Dias, R. S. Nanoparticle-Hydrogel Composites: From Molecular Interactions to Macroscopic Behavior. Polymers (Basel) 11 (2019). https://doi.org:10.3390/polym11020275
[40]. Yang, Y., Ren, Y., Song, W., Yu, B. & Liu, H. Rational design in functional hydrogels towards biotherapeutics. Materials & Design 223, 111086 (2022). https://doi.org:https://doi.org/10.1016/j.matdes.2022.111086
[41]. Cong, Y. & Fu, J. Hydrogel–Tissue Interface Interactions for Implantable Flexible Bioelectronics. Langmuir 38, 11503-11513 (2022). https://doi.org:10.1021/acs.langmuir.2c01674
[42]. Chalmers, E., Lee, H., Zhu, C. & Liu, X. Increasing the Conductivity and Adhesion of Polypyrrole Hydrogels with Electropolymerized Polydopamine. Chemistry of Materials 32, 234-244 (2020). https://doi.org:10.1021/acs.chemmater.9b03655
[43]. Fan, H. & Gong, J. P. Bioinspired Underwater Adhesives. Advanced Materials 33, 2102983 (2021). https://doi.org:https://doi.org/10.1002/adma.202102983
[44]. Binder, W. H. Self-Healing Polymers: From Principles to Applications. (Wiley-VCH Verlag GmbH, 2013).
[45]. Taylor, D. L. & In Het Panhuis, M. Self-Healing Hydrogels. Adv Mater 28, 9060-9093 (2016). https://doi.org:10.1002/adma.201601613
[46]. Blaiszik, B. J., Kramer, S. L. B., Olugebefola, S. C., Moore, J. S., Sottos, N. R. & White, S. R. Self-Healing Polymers and Composites. Annual Review of Materials Research 40, 179-211 (2010). https://doi.org:10.1146/annurev-matsci-070909-104532
[47]. Zhang, A., Liu, Y., Qin, D., Sun, M., Wang, T. & Chen, X. Research status of self-healing hydrogel for wound management: A review. Int J Biol Macromol 164, 2108-2123 (2020). https://doi.org:10.1016/j.ijbiomac.2020.08.109
[48]. Devi, V. K. A., Shyam, R., Palaniappan, A., Jaiswal, A. K., Oh, T. H. & Nathanael, A. J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers (Basel) 13 (2021). https://doi.org:10.3390/polym13213782
[49]. Talebian, S. et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Advanced Science 6, 1801664 (2019). https://doi.org:https://doi.org/10.1002/advs.201801664









