1. Introduction
Food packaging includes items that protect or contain food. In the increasingly urbanized population, the transportation, storage, and consumption of food require food packaging. Most food packaging is directly processed after use (95%), with over one-third of food packaging not entering the recycling system [1]. Food packaging protects food from physical, chemical, and biological pollution. At the same time, it improves the lifespan of food and maintains its quality. Nowadays, petroleum based products are the most widely used material in the food packaging industry due to their excellent performance and relatively low prices. However, the extensive use of petroleum based materials can have negative impacts on the environment as they are not from sustainable sources, are not recyclable, compostable, or biodegradable [2].
Aimed at reducing the pollution of the ocean caused by plastic waste. some significant efforts have focused on biomaterials such as tannic acid, nano chitin, and chitosan. Biomaterials are substances designed to interact with biological systems and are used for medical purposes, whether for treatment (treatment, enhancement, repair, or replacement of the body’s tissue function) or diagnosis. At the same time, biomaterials have the advantages of waste minimization, recyclability, biodegradability, and sustainability, which can effectively reduce pollution. For example, tannic acid can be used as a natural crosslinking agent for biopolymer food packaging. Tannic acid undergoes crosslinking with polymers, including physical and chemical covalent crosslinking. Importantly, tannic acid cross-linked biopolymer based food packaging films/coatings have shown astonishing results in preventing food from contamination [3]. Meanwhile, in recent years, chitin and some of its mixture can act as functional food. But, the effect of chitin cannot be ignored. As it always has really great affinity in chemistry and relatively large specific surface area [4]. In addition, chitosan (CS) is a polysaccharide with positive charge and has great antibacterial activity and good biocompatibility, making it a promising alternative biopolymer molecule for plastic food packaging. Nevertheless, compared to the traditional plastic packaging, it has inferior aspects in barrier and mechanical properties. What is more, some ingredients from plants may be added to CS as additional support.[5]
The purpose of this review is to specifically introduce three kinds of biomaterials, including tannic acid, nano-chitin and CS in the utilization of food packaging.
2. Tannic Acid
Biopolymer food packaging films are assembled from polymers in nature. There are several types, such as protein and polysaccharides. Therefore, the biopolymer food packaging films effectively solves the problems of plastic food packaging films that are not environmentally friendly and sustainable, and also frees people from the potential side effects of microplasticparticles in food. Another benefit of using biopolymers as food packaging materials is that some biopolymer molecules have owned antibacterial and antioxidant properties, which can enhance the ability of protecting food in food packaging materials. Nevertheless, there are some inferior aspects of this biopolymer, such as bad water sensitivity and mechanical properties. To deal with these several problems, some method can be applied. For example, in food packaging materials containing deep eutectic solvents (DES), DES is a relatively secure chemical substance that has recently got great potential capacity in the manufacturing of biopolymer films [6]. Biopolymers forms a structural network when making the film. A firm 3D network structure is made through the interaction of functional groups between or within the chains of biopolymer molecules. Crosslinking agent molecules can chemically interact with many natural polymer molecules to form a more intense and structural 3D network structure in membranes, and improve the ability of biopolymer membranes by cross-linking the network structure within the matrix. According to the types of chemical bonds between crosslinking agents and biopolymers, the major types of crosslinking agents include covalent bond in chemical crosslinking agents, non-covalent bond in physical crosslinking agents, for instance, there are hydrogen bonding, ion interactions, intermolecular forces, and enzyme catalyzed enzymatic crosslinking agents. The high reactivity and strong crosslinking properties of chemically synthesized crosslinking agents (such as glutaraldehyde) are considered to have outstanding properties for natural polymer molecules and distinctive crosslinking agents. However, the toxic released from some materials may prevent it from food packaging, such as glutaraldehyde. Therefore, natural crosslinking agents are more proper for use in biopolymer based food packaging. As shown in Figure 1. According to reports, tannic acid, as a natural crosslinking agent molecule, can improve the performance of various biopolymer based food packaging industry, including casein and chitosan.
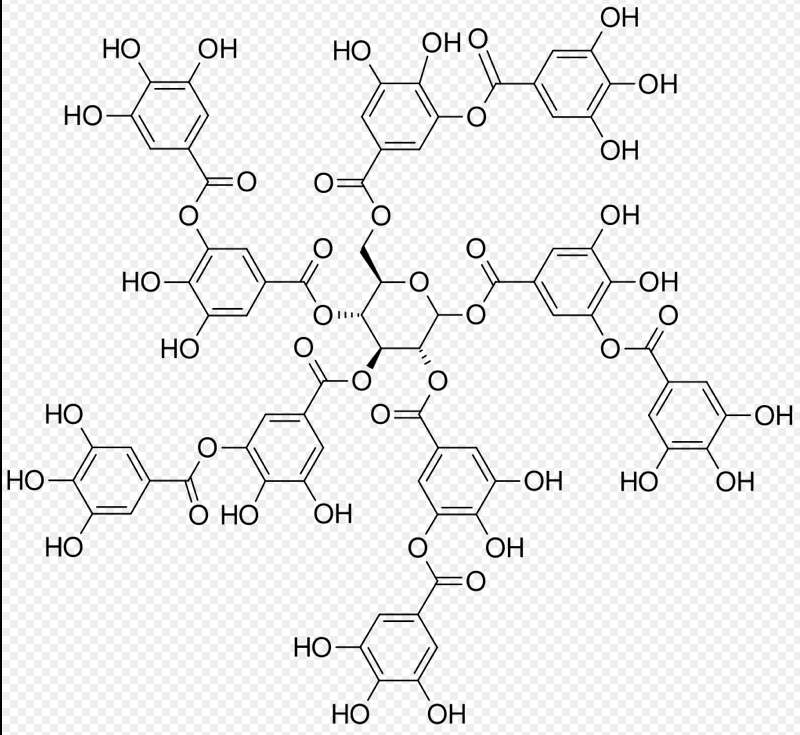
Figure 1. Structural formula of tannic acid
Crosslinking agents can play a physical crosslinking role, such as hydrogen bonding and π - π stacking. Most water-soluble polymers typically do not contain some ideal functional groups. Usually, tedious post functionalization methods are used to meet the requirements of physical crosslinking by covalently bonding useful parts onto polymer chains. Therefore, researchers have turned to discovering natural building blocks, resulting in a batch of supramolecular materials with the advantages of simple preparation, robustness, and low cost.
In addition, chemical crosslinking can also be mentioned. People are increasingly interested in phenolic molecules due to their extensive chemical properties and multivalent reaction sites. For instance, in food packaging, tannic acid crosslinks protein based food packaging films. Natural proteins are very effective for producing food packaging films because they have many functional chemical and physical properties. However, before thin films can be used for daily applications, some defects such as poor water resistance need to be addressed. This content explores the production of plasticized casein films using low-cost plant derived phenolic compound tannic acid as a crosslinking agent. Fourier transform infrared (FTIR) and rheological measurements accounts for crosslinking reaction between the amino group of casein and tannic acid [7].
After the results come out, it is clear that tannic acid is an effective crosslinking agent for casein, and the prepared film has good physical and chemical properties, which can be used for food packaging.
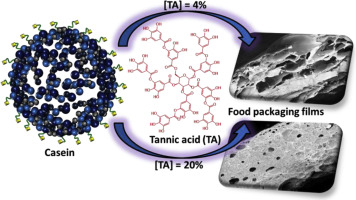
Figure 2. Casein crosslinked by tannic acid
3. Nano-chitin
There are two main types of chitin: beta chitin and alpha chitin. Beta chitin is mainly derived from some mollusk bones, relatively low content in nature, insoluble in common simple solvents such as water, and its intermolecular hydrogen bond force is weak, and chitin nanofibers can be obtained by low-energy mechanical force treatment such as ultrasound. Alpha chitin is mainly derived from shrimp, crabs and other hard shell animal shell, which has a wide range of sources and large yield, so it has more research value. However, its intermolecular hydrogen bond force is strong, it is difficult to dissolve in common solvents, and low energy mechanical force is difficult to open its molecular chain, so it is necessary to use high-tech research alpha chitin.
Chitin nanocrystals are crystalline regions of chitin, which have high mechanical strength and can be used as material strengthening agents and also for regulating food texture. It was found that the mechanical properties of multifunctional konjac glucomannan membrane could be enhanced by adding chitin oxide nanocrystals, and the nano-chitin had strong interaction with polyphenols. It can be used as a fixed carrier of anthocyanins to control the release rate of anthocyanins in the film, and then prepare intelligent food packaging film materials [8]. The study showed that the gel temperature of gelatin has experienced a rise, an increase of 11.7 °C, when chitin nanocrystals were added to gelatin. The gel network structure of gelatin is denser. This is because there is a strong hydrogen bond and electrostatic interaction between chitin nanocrystals and gelatin, resulting in the formation of a denser gel network structure of chitin, thereby enhancing the gel strength and gel ability of gelatin. The study also found that the salt resistance and acid and alkali resistance of gelatin were improved after adding chitin nanocrystals to gelatin [9]. By adding chitin nanofibers to soybean protein and cross-linking with glutamine transaminase, a new soybean protein gel with adjustable texture properties was obtained. This is due to the high mechanical properties of chitin nanofibers and their strong interaction with soy protein [10].
4. Chitosan (CS)
As a kind of natural bioactive substance with abundant reserves, Marine polysaccharide has attracted great attention in the fields of food industry in recent years due to its unique biological activity. Marine polysaccharides can be roughly divided into three categories: Marine animal polysaccharides/Marine algae polysaccharides and Marine microbial polysaccharides, among which the Marine animal and plant polysaccharides represented by chitin, CS, alginic acid and carrageenan are the most commonly studied, mainly due to their unique antibacterial properties, biocompatibility, biodegradability, permeability and processing properties. CS is one of the most widely studied and abundant Marine polysaccharides. CS is the product of deacetylation of chitin (which exists in large quantities in shrimp shell and crab shell). It is the only cationic polysaccharide in nature and has excellent film forming and spinnable properties. Most CS soluble in dilute acid, insoluble in medium and alkaline solution. In terms of molecular structure, CS is similar to cellulose, but the difference is that the group on the C2 position of cellulose is a hydroxyl group, and the C2 position of CS is an amino group, which gives CS many unique properties. CS is safe, non-toxic, biodegradable and non-immunogenic.It is widely used in food, biology, medicine and other fields. Studies have shown that CS has many physiological functions, such as antibacterial, tumor inhibitory, blood lipid lowering, blood glucose lowering, immune enhancement and wound healing promotion [11-16].
Natural polysaccharides come from a wide range of sources and are cheap, and some polysaccharides have good compatibility with CS. The blending of CS with these polysaccharides can improve part of the physical properties of CS film on the one hand and reduce the production cost on the other.In recent years, there have been many researches on CS polysaccharide composite membrane, among which the commonly used polysaccharides mainly include starch, glucomannan, modified cellulose or its derivatives. CS was prepared with corn starch, potato starch and cassava starch respectively. The properties of the composite membrane were related to the ratio of CS and starch, but not to the type of starch. CS composite films were prepared with waxy starch and common starch respectively. The tensile strength (TS), elongation at break (E) and water vapor transmission coefficient (WVTR) of the films were studied. The experiment used X-ray and Fourier infrared spectroscopy to analyze the interaction between the two main compounds. With the increase of starch content, the WVTR of the composite film decreased, while TS and E increased first and then decreased, and the composite film made of common starch showed higher TS and E than waxy starch. The infrared spectrum showed good compatibility between the two compounds [17]. Using ferulic acid as crosslinking agent, the starch-chitosan composite membrane has strong barrier properties and can reduce the formation of lipid peroxides [18]. The cassava starch/chitosan composite film prepared by solution blending has good compatibility between its components. Compared with the cassava starch film, the mechanical properties of the composite film are improved after the addition of CS, and the antibacterial properties, moisture resistance and oxygen resistance properties are also improved to a certain extent. Similarly, tapioca starch was used as raw material to prepare edible composite film by adding different contents of CS and glycerol. The response surface method was used for analysis, and the results showed that the contents of both had different degrees of influence on the properties of tapioca starch film [19]. Konjac glucomannan has film forming properties and is also a biodegradable film material. The composite membrane was prepared by blending CS with konjac glucomannan, which showed good intermolecular compatibility and certain internal action. Compared with pure konjac glucomannan film and pure CS film, the light transmittance, mechanical properties and water vapor barrier properties of the two blended films were improved under different proportions. The properties of CS membrane can be improved by blending the modified cellulose derivatives with CS. For example, the water vapor permeability coefficient of the chitosan-methyl cellulose composite film is higher than that of the pure CS film, the transparency is reduced, and the water solubility and tensile strength increase with the increase of methyl cellulose content.
5. Conclusion
Biological materials composed of polysaccharides and proteins have good degradability and safety, which can reduce the use of a large number of non-degradable plastics in the field of food packaging. The use of appropriate biological packaging films can also guarantee the shelf life, texture, quality and flavor of food. Due to immature technology and cost reasons, it is not yet popular. However, food packaging films composed of biological materials must be the direction of future development of the food industry.
References
[1]. Versino F, Ortega F, Monroy Y, et al. Sustainable and bio-based food packaging: a review on past and current design innovations[J]. Foods, 2023, 12(5): 1057.
[2]. María Flo ́rez, Esther Guerra-Rodríguez, Patricia Cazo ́n *, Manuel V ́azquez
[3]. Zhang W, Roy S, Ezati P, et al. Tannic acid: A green crosslinker for biopolymer-based food packaging films[J]. Trends in Food Science & Technology, 2023.
[4]. Liao J, Zhou Y, Hou B, et al. Nano-chitin: Preparation strategies and food biopolymer film reinforcement and applications[J]. Carbohydrate Polymers, 2023: 120553.
[5]. Zhang W, Hadidi M, Karaca A C, et al. Chitosan-grafted phenolic acids as an efficient biopolymer for food packaging films/coatings[J]. Carbohydrate Polymers, 2023: 120901.
[6]. Román-Hidalgo C, López-Pérez G, Villar-Navarro M, et al. Green electromembrane extraction procedure based on biodegradable chitosan films for determination of polyphenolic compounds in food samples: Greenness assessment of the sample preparation approach[J]. Talanta, 2023, 253: 124034.
[7]. Picchio M L, Linck Y G, Monti G A, et al. Casein films crosslinked by tannic acid for food packaging applications[J]. Food Hydrocolloids, 2018, 84: 424-434.
[8]. WU Chun-hua, LI Yao-ling, SUN Ji-shuai, et al. Novel konjac glucomannan films with oxidized chitin nanocrystals immobilized red cabbage anthocyanins for intelligent food packaging[J]. Food Hydrocolloids, 2020, 98: 105245.
[9]. GE Sheng-ju, LIU Qing, LI Man, et al. Enhanced mechanical properties and gelling ability of gelatin hydrogels reinforced with chitin whiskers[J]. Food Hydrocolloids, 2018.75: 1-12.
[10]. YUAN Yang, SUN Ying-en, WAN Zhi-li, et al. Chitin microfibers reinforce soy protein gels cross-linked by transglu-taminase[J]. Journal of Agricultural and Food Chemistry, 2014, 62(19): 4 434-4 442.
[11]. Yao H T,Chiang M T.Effect of chitosan on plasma lipids,hepatic lipids,and fecal bile acid in hamsters.Journal of Food and Drug Analysis,2006,14:183-189.
[12]. Yao H T,Huang S Y,Chiang M T.comparative study on hypoglycemic and hypocholesterolemic effects of high and low molecular weight chitosan in streptozotocin—induced diabetic rats.Food and Chemical Toxicology,2008,46(5):1525-1534.
[13]. No H K,Park N Y,Lee S H,et a1.Antibacterial activity of chitosans and chitosan oligomers with different molecular weights.International Journal of Food Microbiology,2002,74(1-2):65-72.
[14]. Maeda Y,Kimura Y.Antimmor effects of various low-molecular.weight chitosans are due to increased natural killer activity of intestinal intraepithelial lymphocytes in sarcoma 180-bearing mice.Journal of Nutrition,2004,134(4):945-950.
[15]. Zaharoff D A,Rogers C J,Hance K W,et al.Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination.Vaccine,2007,25(11):2085-2094.
[16]. Dai T,Tanaka M,Huang Y Y,et al.Chitosan preparations for wounds and bums:antimicrobial and wound—healing effects.Expert Review of Anti-infective Therapy,2011,9(7):857-879.
[17]. XU Y X, Kim K M, Hanana M A, et al. Chitosan–starch composite film: Preparation and characterization[J]. Industrial Crops and Products, 2005, 21:185-192.
[18]. Sindhu Mathew, T. Emilia Abraham. Characterisation of ferulic acid incorporated starch-chitosan blend films [J]. Food Hydrocolloids, 2008, 22(5):826-835.
[19]. JS. Chillo, S. Flores,M. Mastromatteo, et al. Influence of glycerol and chitosan on tapioca starch-based edible film properties[J]. Journal of Food Engineering,2008,88(2):159-168.
Cite this article
Tan,Y. (2024). The application of different biomaterials in food package. Theoretical and Natural Science,45,62-67.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Versino F, Ortega F, Monroy Y, et al. Sustainable and bio-based food packaging: a review on past and current design innovations[J]. Foods, 2023, 12(5): 1057.
[2]. María Flo ́rez, Esther Guerra-Rodríguez, Patricia Cazo ́n *, Manuel V ́azquez
[3]. Zhang W, Roy S, Ezati P, et al. Tannic acid: A green crosslinker for biopolymer-based food packaging films[J]. Trends in Food Science & Technology, 2023.
[4]. Liao J, Zhou Y, Hou B, et al. Nano-chitin: Preparation strategies and food biopolymer film reinforcement and applications[J]. Carbohydrate Polymers, 2023: 120553.
[5]. Zhang W, Hadidi M, Karaca A C, et al. Chitosan-grafted phenolic acids as an efficient biopolymer for food packaging films/coatings[J]. Carbohydrate Polymers, 2023: 120901.
[6]. Román-Hidalgo C, López-Pérez G, Villar-Navarro M, et al. Green electromembrane extraction procedure based on biodegradable chitosan films for determination of polyphenolic compounds in food samples: Greenness assessment of the sample preparation approach[J]. Talanta, 2023, 253: 124034.
[7]. Picchio M L, Linck Y G, Monti G A, et al. Casein films crosslinked by tannic acid for food packaging applications[J]. Food Hydrocolloids, 2018, 84: 424-434.
[8]. WU Chun-hua, LI Yao-ling, SUN Ji-shuai, et al. Novel konjac glucomannan films with oxidized chitin nanocrystals immobilized red cabbage anthocyanins for intelligent food packaging[J]. Food Hydrocolloids, 2020, 98: 105245.
[9]. GE Sheng-ju, LIU Qing, LI Man, et al. Enhanced mechanical properties and gelling ability of gelatin hydrogels reinforced with chitin whiskers[J]. Food Hydrocolloids, 2018.75: 1-12.
[10]. YUAN Yang, SUN Ying-en, WAN Zhi-li, et al. Chitin microfibers reinforce soy protein gels cross-linked by transglu-taminase[J]. Journal of Agricultural and Food Chemistry, 2014, 62(19): 4 434-4 442.
[11]. Yao H T,Chiang M T.Effect of chitosan on plasma lipids,hepatic lipids,and fecal bile acid in hamsters.Journal of Food and Drug Analysis,2006,14:183-189.
[12]. Yao H T,Huang S Y,Chiang M T.comparative study on hypoglycemic and hypocholesterolemic effects of high and low molecular weight chitosan in streptozotocin—induced diabetic rats.Food and Chemical Toxicology,2008,46(5):1525-1534.
[13]. No H K,Park N Y,Lee S H,et a1.Antibacterial activity of chitosans and chitosan oligomers with different molecular weights.International Journal of Food Microbiology,2002,74(1-2):65-72.
[14]. Maeda Y,Kimura Y.Antimmor effects of various low-molecular.weight chitosans are due to increased natural killer activity of intestinal intraepithelial lymphocytes in sarcoma 180-bearing mice.Journal of Nutrition,2004,134(4):945-950.
[15]. Zaharoff D A,Rogers C J,Hance K W,et al.Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination.Vaccine,2007,25(11):2085-2094.
[16]. Dai T,Tanaka M,Huang Y Y,et al.Chitosan preparations for wounds and bums:antimicrobial and wound—healing effects.Expert Review of Anti-infective Therapy,2011,9(7):857-879.
[17]. XU Y X, Kim K M, Hanana M A, et al. Chitosan–starch composite film: Preparation and characterization[J]. Industrial Crops and Products, 2005, 21:185-192.
[18]. Sindhu Mathew, T. Emilia Abraham. Characterisation of ferulic acid incorporated starch-chitosan blend films [J]. Food Hydrocolloids, 2008, 22(5):826-835.
[19]. JS. Chillo, S. Flores,M. Mastromatteo, et al. Influence of glycerol and chitosan on tapioca starch-based edible film properties[J]. Journal of Food Engineering,2008,88(2):159-168.









