1. Background
1.1. Situation and Clinical Features
Discovered as a cause of pneumonia in the 1880s, streptococcus pneumoniae is still a major respiratory pathogen until today. The bacteria could be found in 5-10% of healthy adults and 20-40% of children, adhering to the epithelium of the nasopharynx [1]. Pneumococcal infection is most common in young or older people, and except for respiratory tract infection, Otitis media, meningitis, septicaemia, and other diseases can also be a result of severe infection.
Inhabiting in respiratory tract, adult S. pneumoniae carriers have a rate of asymptomatic between 5% to 10%, while that for school-age children is 20% to 60% [2].
Pneumococcal disease is a global issue, more common during winter and early spring. There’s no animal or insect vector, so S. pneumoniae is only transmitted between human hosts, via respiratory droplets or autoinoculation in carriers’ upper respiratory tract.
Clinical report from Centres for Disease Control and Prevention (CDC) shows, for children under 2, S. pneumoniae is the cause of approximately 25% of invasive pneumonia and 40% of bacteraemia. It’s also the major cause of bacterial meningitis among children under 5. Among adults, the incubation period for S. pneumoniae infection is 1 to 3 days, leading to fever, chills, cough, hypoxia, and other symptoms. The complicating bacteraemia and meningitis have fatality ratio of 12% and 14% respectively [2,3]. Other components are either toxic or inflammation inducer, which are important virulence factors.
1.2. Pathogenic Feature
S. Pneumoniae is gram-positive spherical bacteria, with approximately 1 μm diameter. It belongs to diplococci, which means the bacteria is usually found in pairs. When observed under microscope after staining, the bacteria show no flagella or spores, and toxic strains are wrapped by transparent capsules. The pathogen could be easily killed by heating under 56℃ for 15-30 minutes. It’s also sensitive to common disinfectants. Meanwhile, capsular strains commonly have resistance to drying.
S. pneumoniae grows small and round colonies, with smooth surface. if grown on blood agar plates, α-haemolytic ring could be observed around the colonies. Typically, S. Pneumoniae is optochin sensitive, although optochin resistance is being developed. Plus, next-generation sequencing also provide a reliable method to detect and identify S. Pneumoniae based on genomics comparison.
1.3. Virulence Factor
As previously mentioned, as a type of opportunistic pathogen, S. pneumoniae infection occurs among susceptible individuals rather than healthy adults. Normally, the bacteria are not found in the bronchitis, but in patients with chronic bronchitis and other diseases, the pathogen could be found even in the lower respiratory tract. Many protein adhesins are identified playing a role binding to the galactose-based disaccharides, which presents on type II lung cells and vascular endothelial cells in the alveoli. This attaching ability is essential for the following infectious process of the disease, in which the virulence factors can be harmful to the host through various mechanism.
1.3.1. Polysaccharide Capsule. Distinct in serotype, the surface polysaccharides of S. Pneumoniae itself is not toxic. Instead, the capsule gives the bacteria resistance against phagocytosis, so the alveolar macrophages are not able to kill them effectively without antibodies. This makes the pathogen especially dangerous to children with underdeveloped plasma immunity. On the other hand, developing antibodies targeting specific serotype of capsular polysaccharide is the basis of many current vaccines against S. Pneumoniae. The capsule also plays an important role adhering and invading to the host cell. Plus, it is able to defend antibiotic attacks, which will be discussed later. For the reasons listed above, capsular polysaccharide is an important virulence factor, and only encapsulated strains of S. Pneumoniae are isolated from clinical material, while unencapsulated forms don’t have considerable pathogenicity. In fact, most pneumococcal infections are caused by seven most common serotypes among the 100 serotypes documented until 2020.
1.3.2. Cell Wall. The cell wall polysaccharide is a potent inflammation inducer, with a possible mechanism through activating complement and the induction of cytokines [3]. One unusual component of the polysaccharide is phosphorylcholine, which mediates binding between pneumococcal proteins and alveolar cell protein. After that, the target is shifted to platelet activating factor (PAF). Adhering and invading ability rises during this process, and this may be an important and reversible step for asymptomatic colonisation transition to invasive disease [4, 5].
Also, other researches show phosphorylcholine is a possible target for antibodies, but not that effective as those targeting capsular polysaccharide
1.3.3. Other Toxic Proteins. Pneumolysin has the ability to lyse cholesterol-containing cell membranes and activates complement. This toxin is only released when cell wall undergoes lysis. Previous study on mice shows highly pathogenic of pneumolysin, causing severe lobar pneumonia. Compared to wild type, laboratory mutated strain with pneumolysin deficient have reduced virulence, and immunisation against penumolysin could protect the host against virulent pneumococci [1].
This intracytoplasmic toxin needs to be released on lysis of cell wall, and this is processed through autolysin, which will break cell wall when growth of bacteria is ceased by drugs or other factors. The presence of autolysin makes it more efficient to release pneumolysin.
Pneumococcal surface protein A (PspA) is another protein with proved toxicity using similar method. The protein is immunogenic, but specific function is still unclear.
2. Augmentin treatment (β-lactam antibiotics)
β-lactam antibiotics are one of the most mature and widely used class antibiotic drug nowadays. The low toxicity, broad spectrum, effectiveness and low cost makes β-lactam antibiotics popular since discovered and applied in the 1970s, saved countless lives from bacterial infections. Meanwhile, β-lactam antibiotics also face arising bacterial resistance emergence, which has been prevalently and thoroughly studied.
In the following part of this chapter, I will introduce Augmentin, a combination of amoxicillin and clavulanic acid as a modern treatment against S. pneumoniae.
Started with Penicillin G, a lot of varied structure of β-lactam antibiotics are created, and amoxicillin is one of them. They share a same structure that inhibits bacterial cell wall synthesis, which is essential to the growth of bacteria. As a representative broad-spectrum antibiotic, amoxicillin more effective against gram-positive bacteria with thinner cell wall, since the drug molecule need to penetrate inside the bacteria to function.
Clinically administration of amoxicillin is usually oral because of the high bioavailability (approximately 60%). A common strategy to avoid antibiotic resistance problem is combining β-lactamase inhibitors like clavulanic acid with amoxicillin, which could be effective to a wide range of bacteria, especially those causing respiratory tract infection, including S. Pneumoniae. This is still one of the most popular therapies against this pathogen until today.
2.1. Structure and Mechanism
Amoxicillin is an inhibitor of cell wall synthesis. Cell wall is a rigid structure covering the cytoplasmic membrane, structurally support and protect the bacteria. This wall is made of peptidoglycan (PGN), a heteropolymer. Crosslinking aminoglycosides gives the cell wall mechanical stability and rigidness, and different bacteria have different composition of this network.
The biosynthesis process is complexed involving about 30 enzymes during three stages. Amoxicillin selectively inhibits the bacterial transpeptidases, which is involved in the last crosslinking step between amino acid residues that strengthening the cell wall. Normally, cell wall supports and maintain the bacteria in a regular shape. Once the crosslinking between PGN polymers is interrupted, the cell wall become fragile and easily broken. After that, bacteria can absorb water spontaneously in a low osmotic pressure environment and blow up to death.
β-lactam antibiotics are absolutely safe to eukaryotes as transpeptidases are not present in eukaryote cells. This is the basis of selectively toxicity of the drug, makes it safe to humans and animals.
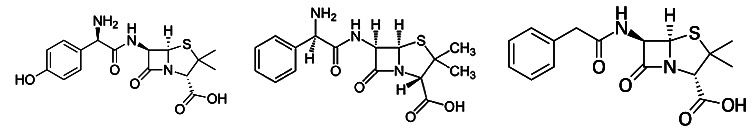
Figure 1. amoxicillin, ampicillin and penicillin G structure. Source: Wikipedia
There are four structures of transpeptidases, which are also called penicillin binding proteins (PBPs). As a Penicillin-G derivative, Amoxicillin binds to PBP 1A, and the C-terminal domain of that enzyme is acylated when the β-lactam ring opens. The drug molecule is similar to the D-Ala, D-Ala termini of polypeptides, so it can fit in the binding pocket of PBPs. However, when the β-lactam ring opens, the drug molecule forms a stable intermediate that occupies the enzyme for minutes, greatly reducing the catalytic activity and nearly cease the synthesis of cell wall.
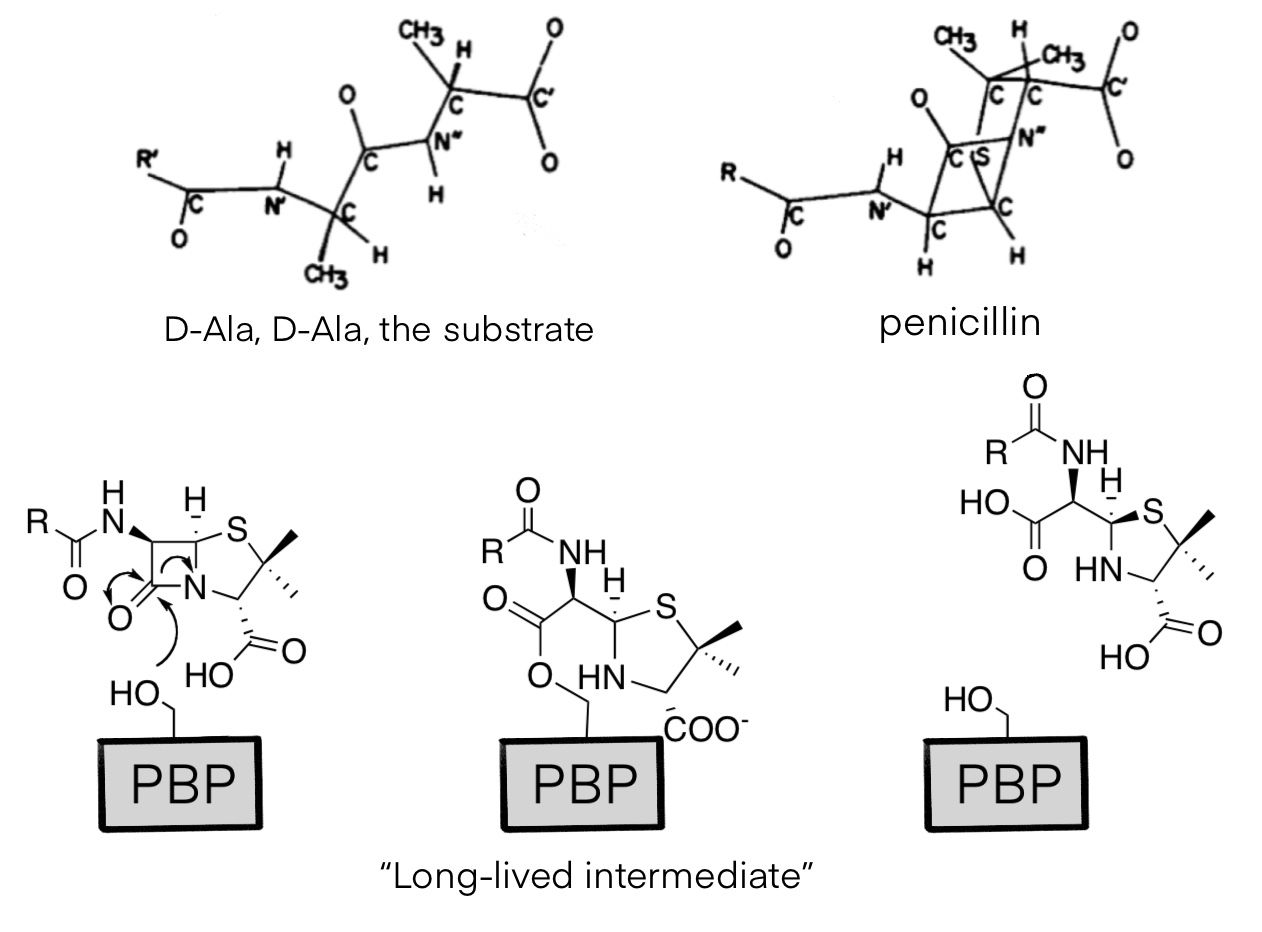
Figure 2. structural similarity between D-Ala, D-Ala and penicillin (above) and the mechanism of drug molecule (below).
Until today, Augmentin is still one of the first line antibiotic drugs. However, bacteria are developing a strategy against β-lactam antibiotics simply through a new enzyme called β-lactamase. This enzyme can hydrolyse the drug bound to PBP and release the occupied enzyme, and this is where the resistance comes from. The various of improved β-lactams are mostly aimed to make it more difficult to be hydrolysed by making the molecule larger and bulkier, so it is too big to bind β-lactamases and be degraded. Another trick is using β-lactamase inhibitors to cut this pathway and make β-lactam antibiotics able to block PBPs again. Representative β-lactamase inhibitors include clavulanic acid and sulbactam/trizobactam.
2.2. Amoxicillin Synthetic Pathway
Industrial production pathway of amoxicillin is semi-synthetic, which means a second product synthesized by living organism is extracted for following chemical modification. This method avoided many difficulties in chemical synthesis of peptides, including purification, low yield and so on, while conserving further structural improvement. 6-aminopenicilanic acid(6-APA) is the material for many β-lactam antibiotics, and different side chains are introduced to produce distinct drugs.
The most prevalent way to acquire 6-APA is through penicillin acylase. genetically modified bacteria are lysed, and penicillin G undergoes series of hydrolysation to remove the original sidechain. Compared to chemical synthesis of 6-APA, this enzyme-dependent pathway is more efficient, environmentally friendly and low-cost. Approximately 90% of penicillin G can be preserved during this process [6].
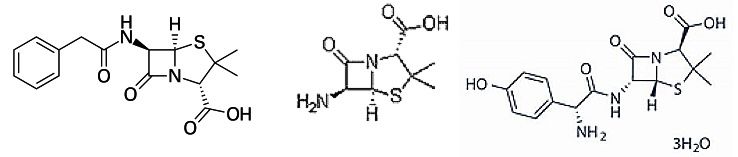
Figure 3. structure of penicillin G, 6-APA and amoxicillin trihydrate. [6]
6-APA is silylated and acylated under a basic environment to add a side chain. The other ingredient D-(-)-2-para-hydroxyphenylglycine chloride hydrochloride is the precursor of the amoxicillin side chain. After that, hydrolysed and neutralized product is ready to be extracted as amoxicillin trihydrate, the molecule with antibiotic activity.
2.3. Problems (Antibiotic Resistance)
Bacterial genome is much more flexible than eukaryotes. They don’t have a karyotheca wrapping the chromatin, and DNA molecules are free to bind, exchange and transfer between individuals through plasmid and transposon system. Rapid reproduction also makes antibiotic resistant strains easier selected and reproduced, spreading the resistant gene wider. Amoxicillin and other improved semi-synthesized β-lactam antibiotics still share the same function and part of the structure, and we can only alter the other part of the molecule to make it bulkier and harder to be degenerated, or combine β-lactamase inhibitors.
Known variation against β-lactam antibiotics includes:
β-lactamase. Most β-lactamase coding genes are located on plasmids that could be transferred easily. Until today, many bacteria have multiple β-lactamase that can hydrolyse certain type of β-lactam rings.
alternating PBPs structure. Mutated PBPs are still enzymatic active, but less sensitive to β-lactam.
Altering porin. Porin is the entrance of hydrolytic compounds, usually located on the plasma membrane as trimers. If the number and structure of porin changes, β-lactam antibiotics can’t enter the cell effectively.
Efflux pump. The efflux pump system discharges toxic and harmful compounds, including drug molecules.
As an example, resistance of S. Pneumoniae against β-lactam antibiotics is increasing throughout the world, particularly common in Europe, South America, and South Africa. A 30%-50% resistance on penicillin is commonly reported, and approximately 40% strains also have resistance to vancomycin, ceftriaxone, and others. To fight against this urgent global issue, standardized medication and new drug development are urgent needs worldwide.
3. Macrolide treatment (azithromycin)
Macrolides are natural products with antibiotic or antifungal activity that are used as pharmaceutical drugs. They consist of a large macrocyclic lactone ring, usually 14-, 15- or 16-membered, with one or more deoxy sugars attached on it. Erythromycin is the first macrolide drug discovered, which was widely used as an alternative choice of penicillin, when there’s resistance or allergy to penicillin. Other modified macrolides, like azithromycin and clarithromycin are designed to avoid side effects and easier to absorb.
Compared to β-lactam antibiotics, macrolides are relatively new type of drug, with a relatively high cost. Except for gastrointestinal upset, allergic reaction, rash, and other side effects that they share, high dose and long-term use of erythromycin might also cause cardiac problems.
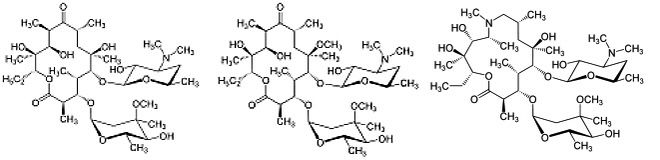
Figure 4. structure of erythromycin, clarithromycin and azithromycin. Notice the difference in shape of their lactone ring.
Azithromycin is one of the macrolides, with a 15-membered lactone ring. Discovered in 1980, this drug is sued to treat various of bacterial infection including otitis media, streptococcal pharyngitis, pneumonia and so on. Azithromycin is usually demonstrated orally or through IV, with only one dose per day.
Macrolides including azithromycin are protein synthesis inhibitors, which is effective against majorly gram-positive bacteria. Azithromycin has lower activity against gram-positive bacteria than erythromycin, but the spectrum is broader, including some gram-negative bacteria, mycoplasma and protozoa.
Other reports show macrolides also have immunomodulatory effect by reducing the production of cytokines. Plus, it has direct effect on respiratory tract epithelial cells to reduce mucus secretion. Due to these reasons, azithromycin is also demonstrated against some chronic respiratory system disease.
3.1. Structure and Mechanism
Azithromycin and other macrolides inhibit bacterial protein synthesis by binding the 50S subunit of the ribosome, the one responsible for polymerizing amino acids into protein. The drug molecule recognizes a site near peptidyl transferase centre, and partially blocks the nascent peptide exit tunnel (NPET), a structure that can sense the growth of protein and regulate ribosomal function. The methyl groups on the lactone ring of the drug are the key structure, allowing it to rest on a surface formed by three bases. Also, the 2’ OH group stabilizes the molecule on its position by forming hydrogen bond. These interactions, together with Van Der Waals contact of the amino acid group of P-site tRNA, occludes the tunnel [6].
Traditionally, macrolides are considered stopping translation by clotting the NEPT, so the newly synthesized polypeptides cannot pass through it once it grows to 3-10 amino acids in length. Result of structural studies partially supported this idea, as Cryo-EM image gives a clear view of partially blocked tunnel, and a small number of proteins is still allowed to be synthesized. Also, accumulated peptidyl tRNA was observed in macrolide-treated cells. In fact, a more accurate description on macrolides could be “selectively interfering the production of a subset of protein, rather than being global inhibitors of translation.”
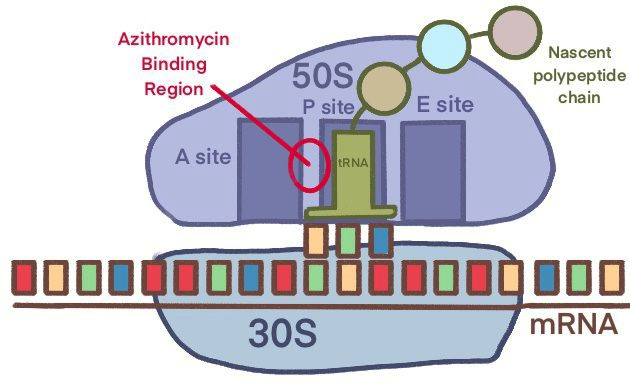
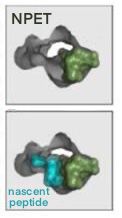
Figure 5. Cartoon representation and Cryo-EM figure of Azithromycin binding ribosome [6]
Recent studies also show there’s a series of mRNA codons that azithromycin prefers arresting the ribosome. There’s a distinctive Macrolide-Arrest Motifs (MAMs) for these drugs, and the bounded ribosome will stop polymerizing the amino acid sequences of an MAM. This explains the presence of polypeptides synthesized even longer than the NPET. What’s more, it provides a possibility to adjust and rationally design a macrolide drug terminating translation at sites we desire.
When given orally, the bioavailability of azithromycin can reach 37%. However, this number could be easily halved when the drug is taken with meal. The elimination half-life of azithromycin is nearly 68h. New microsphere formulation of azithromycin gives it longer term release, gives it a long-lasting effect.
3.2. Azithromycin Synthetic Pathway
Azithromycin is prepared from erythromycin A. treated in acetone with O-mesitylene-sulfonylhydroxylamine and aqueous base (sodium bicarbonate) at 0 °C. To acquire an intermediary (6,9-iminoether), the product then undergoes Beckmann’s rearrangement, which extends the macrocyclic lactone ring from 14- to 15-membered ring. This iminoether molecule then undergoes reductive methylation to obtain azithromycin [7].
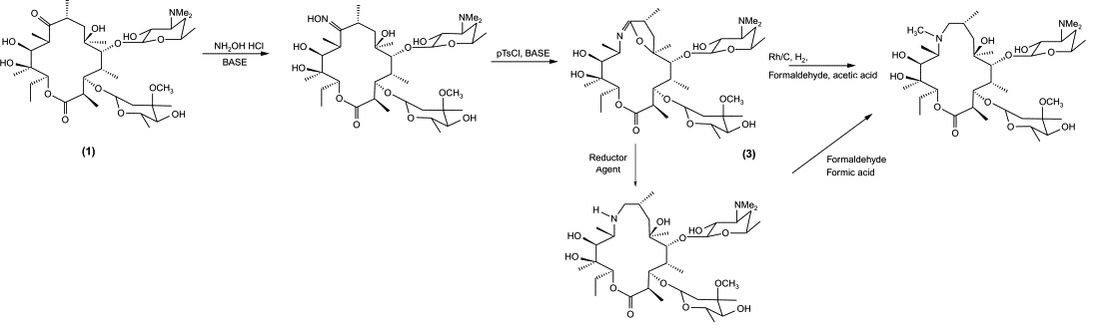
Figure 6. Beckmann’s Rearrangement
3.3. Problems (Antibiotic Resistance)
Different pathogens have distinctive mechanism to express azithromycin. Neisseria gonorrhoeae and pseudomonas aeruginosa over express efflux pump and decrease antimicrobial affinity by mutating gene responsible for 23S ribosomal subunit to resist azithromycin. Enterobacteriaceae on the other hand, decreases uptake through efflux pumps, alter membrane and make shorter peptides. Azithromycin resistant strains of multiple diseases has been reported in many different countries and regions worldwide, and similar to β-lactam antibiotics, the misuse and improper administration of azithromycin is considered the major cause of resistance. Although when combined treatment against some of the diseases, azithromycin can also have an ideal therapeutic effect, decisions still should be made with discretion when used as an antibiotic.
4. Conclusion
The discovery of antibiotics is an exhaustive revolution for human being. With these powerful natural products and their derivants, many severe diseases are no longer life threatening. Still, we need to re-consider how to use this formidable weapon against our enemy, which evolve endlessly and get stronger every second. Fortunately, antibiotics resistance problem has caused widespread concern. The prudent administration, elaborate study about underlying mechanisms and awareness of the public all promotes the development of medicine, pharmacy, and biochemistry.
References
[1]. Cherazard, Regine MD1; Epstein, Marcia MD2; Doan, Thien-Ly PharmD3; Salim, Tanzila MBBS1; Bharti, Sheena DO2; Smith, Miriam A. MD, MBA1,*. Antimicrobial Resistant Streptococcus pneumoniae: Prevalence, Mechanisms, and Clinical Implications. American Journal of Therapeutics 24(3): p e361-e369, May 2017. | DOI: 10.1097/MJT.0000000000000551
[2]. atterall JR. Streptococcus pneumoniae Thorax 1999; 54:929-937. Epidemiology and Prevention of Vaccine-Preventable Diseases (The Pink Book) - 14th Edition (2021)
[3]. George RC. The epidemiology of pneumococcal disease. In: Mayon-White RT, ed. The clinical impact of pneumococcal disease and strategies for its prevention. London: Royal Society of Medicine, 1995: 1–7.
[4]. Gil E, Noursadeghi M, Brown JS. Streptococcus pneumoniae interactions with the complement system. Front Cell Infect Microbiol. 2022 Jul 28; 12:929483. doi: 10.3389/fcimb.2022.929483. PMID: 35967850; PMCID: PMC9366601.
[5]. Lima LM, Silva BNMD, Barbosa G, Barreiro EJ. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur J Med Chem. 2020 Dec 15; 208:112829. doi: 10.1016/j.ejmech.2020.112829. Epub 2020 Sep 16. PMID: 33002736.
[6]. Heidary M, Ebrahimi Samangani A, Kargari A, Kiani Nejad A, Yashmi I, Motahar M, Taki E, Khoshnood S. Mechanism of action, resistance, synergism, and clinical implications of azithromycin. J Clin Lab Anal. 2022 Jun;36(6): e24427. doi: 10.1002/jcla.24427. Epub 2022 Apr 21. PMID: 35447019; PMCID: PMC9169196.
[7]. Ahmed H.H. Bakheit, Badraddin M.H. Al-Hadiya, Ahmed A. Abd-Elgalil, Chapter One -Azithromycin, Editor(s): Harry G. Brittain, Profiles of Drug Substances, Excipients and Related Methodology, Academic Press, Volume 39, 2014, Pages 1-40, ISSN 1871-5125, ISBN 9780128001738, https://doi.org/10.1016/B978-0-12-800173-8.00001-5.
Cite this article
Yue,L. (2024). Streptococcus pneumoniae: Virulence factor, pathogenesis, and two typical drugs with distinctive mechanism. Theoretical and Natural Science,45,122-129.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Cherazard, Regine MD1; Epstein, Marcia MD2; Doan, Thien-Ly PharmD3; Salim, Tanzila MBBS1; Bharti, Sheena DO2; Smith, Miriam A. MD, MBA1,*. Antimicrobial Resistant Streptococcus pneumoniae: Prevalence, Mechanisms, and Clinical Implications. American Journal of Therapeutics 24(3): p e361-e369, May 2017. | DOI: 10.1097/MJT.0000000000000551
[2]. atterall JR. Streptococcus pneumoniae Thorax 1999; 54:929-937. Epidemiology and Prevention of Vaccine-Preventable Diseases (The Pink Book) - 14th Edition (2021)
[3]. George RC. The epidemiology of pneumococcal disease. In: Mayon-White RT, ed. The clinical impact of pneumococcal disease and strategies for its prevention. London: Royal Society of Medicine, 1995: 1–7.
[4]. Gil E, Noursadeghi M, Brown JS. Streptococcus pneumoniae interactions with the complement system. Front Cell Infect Microbiol. 2022 Jul 28; 12:929483. doi: 10.3389/fcimb.2022.929483. PMID: 35967850; PMCID: PMC9366601.
[5]. Lima LM, Silva BNMD, Barbosa G, Barreiro EJ. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur J Med Chem. 2020 Dec 15; 208:112829. doi: 10.1016/j.ejmech.2020.112829. Epub 2020 Sep 16. PMID: 33002736.
[6]. Heidary M, Ebrahimi Samangani A, Kargari A, Kiani Nejad A, Yashmi I, Motahar M, Taki E, Khoshnood S. Mechanism of action, resistance, synergism, and clinical implications of azithromycin. J Clin Lab Anal. 2022 Jun;36(6): e24427. doi: 10.1002/jcla.24427. Epub 2022 Apr 21. PMID: 35447019; PMCID: PMC9169196.
[7]. Ahmed H.H. Bakheit, Badraddin M.H. Al-Hadiya, Ahmed A. Abd-Elgalil, Chapter One -Azithromycin, Editor(s): Harry G. Brittain, Profiles of Drug Substances, Excipients and Related Methodology, Academic Press, Volume 39, 2014, Pages 1-40, ISSN 1871-5125, ISBN 9780128001738, https://doi.org/10.1016/B978-0-12-800173-8.00001-5.









