1. Introduction
Splenic marginal band lymphoma (SMZL) is a rare, low-grade B-cell non-Hodgkin lymphoma characterized by splenomegaltis with moderate lymphocytosis. Patients with SMZL may have symptoms such as enlarged lymph nodes, weight loss, and decreased appetite, which brings huge psychological and economic burdens to patients and their families. Because SMZL is a rare disease and there is a lack of randomized prospective trials, there is currently no standard treatment. Previously, people managed the disease through splenectomy, but now splenectomy is no longer the only option, and a combination of chemotherapy and immunotherapy is more often used [1]. This paper discusses the possibility of applying Lipid Nanoparticles (LNPs) as drug carrier of chemotherapeutic drug rituximab using animal model.
Rituximab is a monoclonal antibody drug that is used in the treatment of SMZL and some other autoimmune diseases. It works by targeting and binding to specific proteins on the surface of cells, such as B cells, which are involved in immune responses [2]. Being able to control the activity of these cells and modulate immune responses, it is now also commonly used to treat conditions like non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, and certain autoimmune disorders.
Lipid Nanoparticles (LNPs) is a type of popular application of liposomes in medicine. The extensive medical application of lipids dates back decades [3]. It was initially employed for the preparation of colloids and suspensions, and also found as a minor component in both soft and hard capsules. Nowadays LNPs have been found to have great potentials in drug delivery. LNPs feature a bilayer phospholipid structure and nanoscale particle size. These carriers can encapsulate DNA or RNA via ionizable cationic lipids while maintaining low toxicity. Other benefits include improved stability and eco-friendly production. Presently, the LNPs were found having extensive applications in cancer therapy, antimicrobial agents, central nervous system treatment, and localized therapies. The pharmacokinetic profiles of nearly all disease treatments are improved through drug delivery via LNPs [4]. Furthermore, LNPs are capable of encapsulating a wide range of chemotherapeutic drugs. Ongoing research explores their potential as carriers for central nervous system disorders and antibiotics, expanding their utility beyond conventional applications.
For animal model, SMZL have been found to occur at a high frequency in NFS.N mice (Transmissible spongiform encephalopathy in the gray tremor mutant mouse) congenic for high-expressing ecotropic murine leu- kemia virus (MuLV) genes from AKR and C58 mice [5]. The spleen, a vital organ of the immune system, plays a crucial role in the production and maturation of lymphocytes. It serves as a reservoir for immune cells and is involved in the initiation of immune responses. It has been investigated that the efficiency of transduction, or the transfer of genetic material in the spleen is higher compared to other organs.
In recent years, lipid-based nanoparticles (LNPs) have gained attention as a promising drug delivery system. These nanoparticles offer several advantages for spleen-targeted drug delivery. Their small size enables efficient uptake and penetration into spleen tissue [6]. The lipid bilayer structure provides stability and protection for the therapeutic agents. LNPs can be modified for specific targeting, enhancing their efficacy. Moreover, LNPs can encapsulate various therapeutic agents, allowing for diverse treatment modalities like targeted chemotherapy or gene therapy in the spleen.
In conclusion, the high frequency of MZLs in NFS.N mice provides a unique model for studying the disease and exploring potential therapeutic approaches. Therefore, in the present study, we designed experiments to inject LNPs with Rituximab in a mouse model to detect the efficacy of this combination treatment for SMZL. The use of LNPs in targeting and delivering therapeutic agents to the spleen holds promise for improving the treatment of MZLs. This hypothesis-driven research aims to assess the effectiveness of LNPs and Rituximab in suppressing tumor growth and enhancing therapeutic outcomes in SMZL. The results of this study could contribute to the development of more effective and targeted therapies for this specific type of lymphoma.
2. Method
To investigate whether using LNPs to deliver Rituximab will have an enhanced effect for delivering Rituximab, a mice model with SMZL is established with the following Technology Roadmap. (Figure 1) And the effect of LNPs delivery on SMZL is determined using relevant standard of recovery.
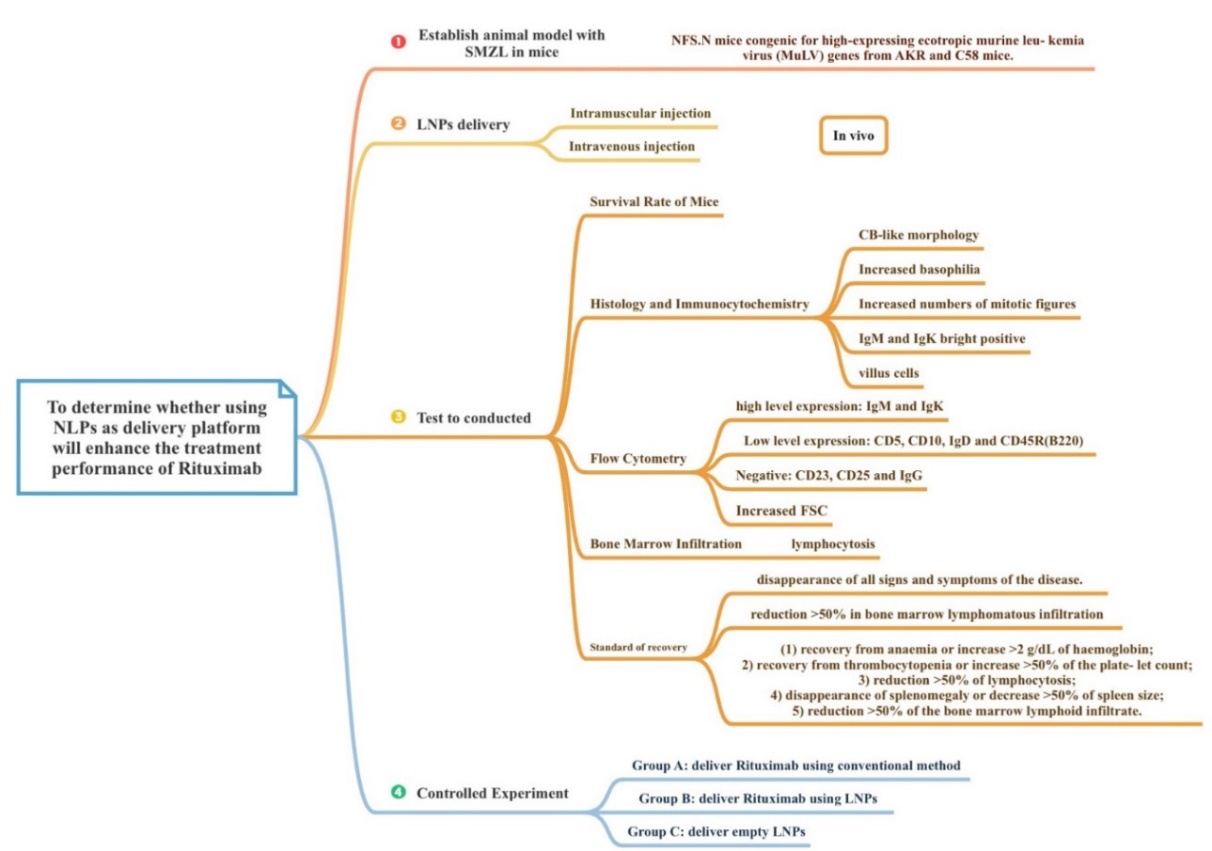
Figure 1. Technology road map
3. Animal model of SMZL on Mice
In order to better investigate the pathogenic mechanisms and pathological characteristics of SMZL established a mouse model. To establish a mouse model with SMZL, a systematic approach can be followed. Initially, it is crucial to select an appropriate mouse strain with a high predisposition to develop SMZL, such as NFS.V mice. These mice should be carefully bred and housed under controlled conditions to ensure their well-being and minimize external variables.
Next, the mice can be infected with a high-expressing ecotropic murine leukemia virus (MuLV) strain, such as AKR and C58 MuLV [5]. This infection can be achieved through various methods, including injection or other suitable routes. Regular monitoring and observation of the mice are essential to detect any pathological features associated with SMZL development, such as splenic marginal zone expansion and changes in cell morphology.
Once potential SMZL-like symptoms manifest in the mice, the diagnosis can be confirmed through rigorous histopathological examination, immunohistochemistry, or flow cytometry techniques, ensuring the accuracy of the model.
Following the establishment of the SMZL mouse model, further investigations can be conducted to explore the underlying mechanisms, clinical characteristics, and potential treatment methods for SMZL. This may involve in-depth studies such as gene expression analysis, protein studies, and drug treatment trials, contributing to a comprehensive understanding of SMZL and its potential therapeutic interventions. In this study, several key research parameters were employed to assess the development of SMZL in our mouse model, including splenic marginal zone expansion, alterations in cell morphology, and the presence of lymphoma-like features within the spleen tissues.
Additionally, for comparative purposes, a control group of mice was subjected to normal administration, while another group received LNP (Lipid Nanoparticle) treatment, and a third group received empty LNPs. The sample size included a balanced ratio of male and female mice, ensuring statistical robustness and gender parity within the study.
4. Successful Construction of the SMZL Mouse Model
As we described early, the following tests were conducted to determine the successful construction of the SMZL mouse model.
Assessment of Pathological Characteristics: We extracted spleens from the mouse models and closely examined the expansion of the marginal zone, as well as changes in cell morphology, to determine the presence of pathological characteristics resembling SMZL.
Detection of Molecular Markers: Techniques such as immunohistochemistry and flow cytometry were employed to detect molecular markers associated with SMZL in mouse spleens, including CD20 and CD79a [7].
Gene Expression Analysis: Utilizing gene expression analysis technology, we conducted a comparative study of gene expression variances between the spleens of SMZL mouse models and those of normal mice, aiming to unveil potential mechanisms underlying SMZL development.
Drug Treatment Assessments: Various drugs and treatment modalities were administered to the SMZL mouse model to evaluate their impact on tumor growth and mouse survival, thus gauging therapeutic efficacy.
5. The detection of effectiveness of LNPs delivery and toxicity
In order to know the effectiveness of drug therapy for SMZL, we need to measure and record the survival rate of mice. After reading the literature, it is known that the most effective method to study the survival rate of mice is every 10 mice in a group, and on this basis, a controlled test is conducted [8]. First, we need to classify the mice into male and female categories, so as not to interfere with gender factors. Then you can change the dosage and test it on different groups. During the trial, the mice were continuously observed and recorded every 24 hours. Finally, the test results were analyzed by SPSS. There are two ways to calculate survival rates.
1. \( Simple survival rate =\frac{Number of surviving individuals}{number} \) of all individuals
2. \( Survival by date =\frac{number of surviving individuals at the end of the observation period}{number of individuals at the beginning of the observation period} \) calculating the survival rate can help judge the effectiveness of this drug treatment.
Due to the complex nature of SMZL, it is very hard to determine whether the subject has this disease or not by a single standard, and therefore it is important to use multiple characterisation method to cross verify the condition of such subject (mice).
6. To directly observe the lesion tissue cells
One of the most intuitive ways to look at SMZL is by directly looking at the cells at spleen and to do this, one needs to carry out Histology and Immunocytochemistry study. By obtaining spleen tissue from NFS.N mice and comparing with normal mice spleen tissue some morphological appearance of the lesion cells in the infected mouse should be found. This method also allows us to look at the expression of certain surface marker of B-cells that are related to this disease via fluorescence microscope.
Materials to prepare includes mice spleen sample, cryoprotectant (e.g., sucrose solution), cryostat, microscope slides, antibodies (primary and secondary), Blocking solution, Mounting medium with DAPI (for nuclear staining), Fluorescence microscope.
The method begins with sample preparation. Tissue sample is fixed in buffered formalin and embedded in paraffin or plastic depending on specific case. Tissue sample is then dehydrated through a graded series of alcohol solutions (i.e. 100% EtOH, 95%EtOH & 70%EtOH). Next, the tissue sample in paraffin is sectioned using a microtome before being stained with hematoxylin and eosin (H&E) or Giemsa [9].
For immunocytochemistry, tissue should be fixed in tissue-freezing medium and quick frozen using dry ice and 2-methylbutane. In this case, tissue should be stores at -70 \( °C \) and sectioned using a cryostat instead.
Immunostaining is then performed using the following biotinylated mouse monoclonal antibodies against lineage-specific differentiation antigens: CD4, CD8, and CD45R (B220) and IgM, IgK, and IgD. A blocking solution, usually serum from the same species, is required to perform the next step in order to block any non-specific binding. The tissue sample is incubated in the blocking solution for about 20 mins to minimise the background staining [10].
Next, the sample is taken out and excess wetness is wiped off using Kim-wipe followed by the addition of primary antibody, incubate for about 1h. The sections should be washed several times with a wash buffer to remove unbound primary antibody before secondary antibody incubation starts and incubate for approximately 40 mins.
Last step before microscopy is mounting. An anti-fade mounting medium is applied to preserve fluorescence and prevent photobleaching, and a coverslip should be placed on the mounted sections. Now the sample is ready to be examined using a fluorescence microscope followed by further analysis.
Some expected results as literatures indicates might demonstrate e.g. Centro blast-like cells, villous cells, increased number of mitotic figure and increased basophilia etc.,
7. To detect expression of cell surface markers related to SMZL
There are several surface markers (Table 1) on B-cells that are believed to have connection with SMZL and therefore to detect the expression level of these markers Flow Cytometry needed to be applied. The expression level of these markers are compares between NFS.N mice and normal mice.
Materials to prepare include SMZL mouse samples (slpeen), antibodies for cell surface markers, buffer solution (e.g., Phosphate-Buffered Saline (PBS) with 1-3% Bovine Serum Albumin (BSA)), flow cytometer, tubes for sample preparation, pipettes and tips, centrifuge and other relevant experimental equipments etc.,
The method includes mainly 5 parts: sample preparation, sample incubation, antibody staining, separation by washing & centrifugation and last but not least data collection & analysis.
During the first step spleen cells are prepared according to laboratory animal protocol. Mouse Spleen should be inspected and examined for necrotic region, enlargement and discolouration. Then, excess tissues such as fat and connection tissue are trimmed off, before putting into a Petri dish with a mixture of PBS and BSA buffer solution. Afterwards spleen is minced carefully into small pieces.
Next, the spleen sample is incubated in the dark for around 30 minutes at room temperature or 4°C after Ethylenediaminetetraacetic acid (EDTA) of 1mM have been added to the Petri dish.
After incubation is completed, cells are strained using 70 \( μ \) m cell strainer and mash further with the plunger end of a syringe. 5-10 ml of DBPS can be added to aid wash down the cells.
Use appropriate cell surface markers as shown in Table 1:
Table 1. Antibodies tested for flow cytometry in SMZL characterization
Antibody tested | Expected results |
CD5 | Positive at low level |
CD10 | Positive at low level |
CD45R(B220) | Positive at low level |
IgD | Positive at low level or negative |
IgK | Positive |
IgG | Negative |
IgM | Positive |
CD23 | Negative |
CD25 | Negative |
All above markers are labelled with biotin (e.g. animohexanoyl-biotin-N-hydroxysuccinimide ester) or fluorochromes before reacting with respective antibodies. One thing to note is that one tube needs to be a negative control (no antibodies) and another one as a compensation control (single-strained samples for compensation adjustments).
DBPS solution is then added to each tube to wash away unbound antibodies, followed by Centrifuge the tubes to pellet the cells and discard the supernatant. After gently resuspend the cell pellets in buffer solution, the solution is ready for analysis. The flow cytometer needs to be set up according to the manufacturer’s instructions after which instrument can then be calibrated using the compensation controls. The negative control is to be analysed first to set appropriate gating to exclude non-specific binding events. The antibody-stained samples are then run through the flow cytometer and acquire data for each marker.
In the final step, flow cytometry analysis software is used to analyse data by gating the population of interest based on the marker expression pattern. Distribution of the marker expression in the gated population is analysed and compared with appropriate controls and literature to identify SMZL cells based on marker expression patterns, before the percentage of SMZL cells within the gated population is quantified. Some expected results are reported in the table above.
Nonviable cells are stained with propidium iodide and combined gating on forward angle light scatter (FSC). In this case an increased FSC is expected for cells from which mice have SMZL than those that are normal.
8. Observation of cell’s morphology via Bone Marrow Infiltration
Bone marrow infiltration is a common finding in SMZL, occurring in approximately 83-100% of carriers of this disease. Pathological features of bone marrow infiltration are the presence in the bone marrow of mature B-cells of small and medium size with round or oval nuclei and concentrated chromatin, basophilic cytoplasm and, in most cases, typical uneven membrane protrusions (villous cells).
The detection of bone marrow infiltration includes bone marrow puncture and bone marrow biopsy. A bone marrow puncture is performed by inserting a thin needle into the bone marrow cavity and drawing fluid from the bone marrow for examination. A bone marrow biopsy is performed by removing a small piece of bone marrow tissue for pathological analysis. On examination of bone marrow fluid and bone marrow tissue, pathological cells of SMZL, as well as other characteristic cell morphology and immunophenotypes, can be observed. Therefore, through the analysis of bone marrow fluid and bone marrow tissue, the presence of bone marrow infiltration can be detected [11].
We need to use a dry syringe with a capacity of 20ml, withdraw the internal plug from 1cm and pull out the needle core, then connect the syringe and slowly aspirate with appropriate force. When a small amount of red bone marrow fluid is seen into the syringe, the aspirate volume of bone marrow fluid should preferably be 0.1-0.2ml. Finally, the syringe was removed, the bone marrow liquid was dropped onto the slide, and 5 to 6 smears were quickly prepared for cell morphology and cytochemical staining.
Through we read some reference, we find a better way to extraction the marrow that aspiration of bone marrow cells from the femur [12]. Because all blood cells are creat from hematopoietic stem cells. With this method, cells from mouse bone marrow can be continuously collected and studied for cytological analysis, flow cytometry and cell culture. This technique can be used to detect the presence of exogenous cells in mouse bone marrow, as well as to detect cell types such as hematopoietic stem cells and progenitor cells that are abundant in the bone marrow. Compared with traditional bone marrow collection methods, this method does not require the sacrifice of mice and allows long-term bone marrow research. The main finding of this article is that this femoral bone marrow puncture technique can effectively obtain mouse bone marrow cells and be used for different types of studies.
Using these methods to extracte spinal fluid. When bone marrow infiltration occurs, bone marrow aspiration should be performed as soon as possible, and the cause should be understood and treated in time.
9. Results and discussion
In our study, we aimed to evaluate the therapeutic potential of Lipid Nanoparticles (LNPs) in a Splenic Marginal Zone Lymphoma (SMZL) mouse model. To achieve this, we utilized a total of 60 mice, ensuring a balanced gender ratio. These mice were divided into three groups: Group A (treatment group) comprising 20 mice infected with a high-expressing SMZL strain and receiving LNPs treatment, Group B (positive control group) with 20 mice infected with the SMZL strain but not receiving LNPs treatment, and Group C (negative control group) consisting of 20 mice neither infected with the SMZL strain nor treated with LNPs. The treatment regimen for Group A involved weekly administration of LNPs at a dose of X mg/kg for a duration of Y weeks. Throughout the treatment period, we regularly monitored and observed the mice for changes in body weight, splenic marginal zone expansion, cell morphology alterations, and any other pathology related to SMZL. These observations should be meticulously recorded for subsequent analysis.
The recovery standard of SMZL for mice have not yet been established before and such disease due to its chronic nature is very hard to define a full recovery therefore the following standard of recovery is summarized and reported as shown in Table 2
Table 2. Standards of recovery for mice with SMZL [13]
Extend of recovery | Detailed description |
Fully recovered | Disappearance of all signs and symptoms of the disease |
Recovered | Reduction of > 50% in bone marrow infiltration without cytopenia |
Partly recovered (at least meet one of the criteria on the right) | Recovery from anaemia or increase > 2g/dL of Haemoglobin |
Recovery from thrombocytopenia or increase >50% of platelet count | |
Reduction of >50% of lymphocytosis | |
Disappearance of splenomegaly or decrease in >50% spleen size | |
Reduction >50% of the bone marrow lymphoid infiltrate |
Our expected results should show that Group A exhibited a noticeable deceleration in SMZL progression during treatment, with significantly reduced splenic marginal zone expansion and cell morphology changes compared to Group B. Group B displayed typical SMZL pathology features, while Group C, serving as the negative control, did not exhibit SMZL-related pathology. In conclusion, our design provides the theoretical support for using LNPs as delivery platform for Rituximab and is expected to enhance the treatment effect of such drug. This highlights LNPs as a promising avenue for SMZL treatment, although further in-depth research is necessary to confirm their clinical viability.
10. Conclusion
In light of current study and development this study investigated LNPs as a drug in the treatment of SMZL. In animal models, we found that SMZL has a high incidence in NFS, while LNPs has been widely concerned and studied as a promising drug delivery system in recent years. Finally, we decided to test and compare the mice with intramuscular injection and intravenous injection. The survival rate of mice was observed and recorded during the experiment, and SMZL was observed by looking at spleen cells, so we conducted histological and immunocytochemical studies. We also used flow cytometry to detect the expression levels of markers. Finally, we looked at the morphology of the cells by the degree of bone marrow infiltration. The meaning of the research:
By establishing a mouse model, it is possible to better study the pathogenesis, pathological features, and molecular markers of SMZL, providing important clues for further understanding and treating human SMZL.
Evaluation of treatment methods: By conducting drug treatment experiments on mouse models, the efficacy of different treatment methods for SMZL can be evaluated, providing guidance and new treatment strategies for clinical treatment.
Exploration of new treatment targets: Through techniques such as gene expression analysis, key genes and signaling pathways involved in the development of SMZL can be discovered, providing clues for finding new treatment targets.
References
[1]. Santos TSD, Tavares RS, Farias DLC. Splenic marginal zone lymphoma: a literature review of diagnostic and therapeutic challenges. Rev Bras Hematol Hemoter. 2017 Apr-Jun;39(2):146-154. doi: 10.1016/j.bjhh.2016.09.014. Epub 2016 Dec 22. PMID: 28577652; PMCID: PMC5457460.
[2]. Amhaz G, Bazarbachi A, El-Cheikh J. Immunotherapy in indolent Non-Hodgkin’s Lymphoma. Leuk Res Rep. 2022 May 18; 17:100325. doi: 10.1016/j.lrr.2022.100325. PMID: 35663281; PMCID: PMC9160834.
[3]. de Blaey, C. J., and Polderman, J. (1980). “Rationales in the design of rectal and vaginal delivery forms of drugs,” in Medicinal Chemistry, ed. E. J. Ariens (London: Academic Press), 237. doi: 10.1016/B978-0-12-060309-1.50011-2
[4]. Scioli Montoto S, Muraca G, Ruiz ME. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front Mol Biosci. 2020 Oct 30;7:587997. doi: 10.3389/fmolb.2020.587997. PMID: 33195435; PMCID: PMC7662460
[5]. Fredrickson TN, Lennert K, Chattopadhyay SK, Morse HC 3rd, Hartley JW. Splenic marginal zone lymphomas of mice. Am J Pathol. 1999 Mar;154(3):805-12. doi: 10.1016/S0002-9440(10)65327-8. PMID: 10079258; PMCID: PMC1866400.
[6]. Algarni A, Pilkington EH, Suys EJA, Al-Wassiti H, Pouton CW, Truong NP. In vivo delivery of plasmid DNA by lipid nanoparticles: the influence of ionizable cationic lipids on organ-selective gene expression. Biomater Sci. 2022 May 31;10(11):2940-2952. doi: 10.1039/d2bm00168c. PMID: 35475455.
[7]. [7] Characterization of a New Antigen Expressed by B and Myeloid Lineage Cells Identified by the Monoclonal Antibody LIP-6,by KEVIN L. HOLMES,*,2 LARRY M. LANTZ,* JOON S. LEE,* HENDRICK G. BEDIGIAN,† AND JEFFERY K. TAUBENBERGER‡F
[8]. Alhowail A, Chigurupati S, Elgharabawy R, Aldubayan M. Co-administration of imipramine and doxorubicin reduces the survival rate and body weight of mice. Eur Rev Med Pharmacol Sci. 2020 Dec;24(24):12978-12982. doi: 10.26355/eurrev_202012_24202. PMID: 33378049.
[9]. Sampedro-Carrillo EA. Sample Preparation and Fixation for Histology and Pathology. Methods Mol Biol. 2022;2422:33-45. doi: 10.1007/978-1-0716-1948-3_3. PMID: 34859397.
[10]. Ward JM, Uno H, Frith CH. Immunohistochemistry and morphology of reactive lesions in lymph nodes and spleen from rats and mice. Toxicol Pathol. 1993;21(2):199-205. doi: 10.1177/019262339302100212. PMID: 8210942.
[11]. Suhail M, Mahmood A, Mahmood R, Zahir S, Shafaat SS, Illyas S. Bone marrow infiltration by Non-Hodgkin lymphoma: An experience in a tertiary care centre. J Pak Med Assoc. 2023 Mar;73(3):558-561. doi: 10.47391/JPMA.6730. PMID: 36932759.
[12]. Chung YR, Kim E, Abdel-Wahab O. Femoral bone marrow aspiration in live mice. J Vis Exp. 2014 Jul 5;(89):51660.doi:10.3791/51660.PMID:25045847;PMCID:PMC4211899.
[13]. Iannitto E, Minardi V, Calvaruso G, Ammatuna E, Florena AM, Mulè A, Tripodo C, Quintini G, Abbadessa V. Deoxycoformycin (pentostatin) in the treatment of splenic marginal zone lymphoma (SMZL) with or without villous lymphocytes. Eur J Haematol. 2005 Aug;75(2):130-5. doi: 10.1111/j.1600-0609.2005.00426.x. PMID: 16000129.
Cite this article
Huang,Z.;Tang,Z.;Zhang,X. (2024). LNPs as delivery platform of Rituximab for SMZL. Theoretical and Natural Science,45,1-8.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Santos TSD, Tavares RS, Farias DLC. Splenic marginal zone lymphoma: a literature review of diagnostic and therapeutic challenges. Rev Bras Hematol Hemoter. 2017 Apr-Jun;39(2):146-154. doi: 10.1016/j.bjhh.2016.09.014. Epub 2016 Dec 22. PMID: 28577652; PMCID: PMC5457460.
[2]. Amhaz G, Bazarbachi A, El-Cheikh J. Immunotherapy in indolent Non-Hodgkin’s Lymphoma. Leuk Res Rep. 2022 May 18; 17:100325. doi: 10.1016/j.lrr.2022.100325. PMID: 35663281; PMCID: PMC9160834.
[3]. de Blaey, C. J., and Polderman, J. (1980). “Rationales in the design of rectal and vaginal delivery forms of drugs,” in Medicinal Chemistry, ed. E. J. Ariens (London: Academic Press), 237. doi: 10.1016/B978-0-12-060309-1.50011-2
[4]. Scioli Montoto S, Muraca G, Ruiz ME. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front Mol Biosci. 2020 Oct 30;7:587997. doi: 10.3389/fmolb.2020.587997. PMID: 33195435; PMCID: PMC7662460
[5]. Fredrickson TN, Lennert K, Chattopadhyay SK, Morse HC 3rd, Hartley JW. Splenic marginal zone lymphomas of mice. Am J Pathol. 1999 Mar;154(3):805-12. doi: 10.1016/S0002-9440(10)65327-8. PMID: 10079258; PMCID: PMC1866400.
[6]. Algarni A, Pilkington EH, Suys EJA, Al-Wassiti H, Pouton CW, Truong NP. In vivo delivery of plasmid DNA by lipid nanoparticles: the influence of ionizable cationic lipids on organ-selective gene expression. Biomater Sci. 2022 May 31;10(11):2940-2952. doi: 10.1039/d2bm00168c. PMID: 35475455.
[7]. [7] Characterization of a New Antigen Expressed by B and Myeloid Lineage Cells Identified by the Monoclonal Antibody LIP-6,by KEVIN L. HOLMES,*,2 LARRY M. LANTZ,* JOON S. LEE,* HENDRICK G. BEDIGIAN,† AND JEFFERY K. TAUBENBERGER‡F
[8]. Alhowail A, Chigurupati S, Elgharabawy R, Aldubayan M. Co-administration of imipramine and doxorubicin reduces the survival rate and body weight of mice. Eur Rev Med Pharmacol Sci. 2020 Dec;24(24):12978-12982. doi: 10.26355/eurrev_202012_24202. PMID: 33378049.
[9]. Sampedro-Carrillo EA. Sample Preparation and Fixation for Histology and Pathology. Methods Mol Biol. 2022;2422:33-45. doi: 10.1007/978-1-0716-1948-3_3. PMID: 34859397.
[10]. Ward JM, Uno H, Frith CH. Immunohistochemistry and morphology of reactive lesions in lymph nodes and spleen from rats and mice. Toxicol Pathol. 1993;21(2):199-205. doi: 10.1177/019262339302100212. PMID: 8210942.
[11]. Suhail M, Mahmood A, Mahmood R, Zahir S, Shafaat SS, Illyas S. Bone marrow infiltration by Non-Hodgkin lymphoma: An experience in a tertiary care centre. J Pak Med Assoc. 2023 Mar;73(3):558-561. doi: 10.47391/JPMA.6730. PMID: 36932759.
[12]. Chung YR, Kim E, Abdel-Wahab O. Femoral bone marrow aspiration in live mice. J Vis Exp. 2014 Jul 5;(89):51660.doi:10.3791/51660.PMID:25045847;PMCID:PMC4211899.
[13]. Iannitto E, Minardi V, Calvaruso G, Ammatuna E, Florena AM, Mulè A, Tripodo C, Quintini G, Abbadessa V. Deoxycoformycin (pentostatin) in the treatment of splenic marginal zone lymphoma (SMZL) with or without villous lymphocytes. Eur J Haematol. 2005 Aug;75(2):130-5. doi: 10.1111/j.1600-0609.2005.00426.x. PMID: 16000129.









