1. Introduction
In 2022, approximately 18.6 million people were affected by a chronic non-healing wound such as a diabetic foot ulcer, with 80% of the individuals with diabetes-related amputations being linked to a heightened mortality risk [1]. Most diabetic foot ulcers are initially without symptoms in the early stages of diabetes, primarily due to the intricating presence of underlying peripheral vascular disease. As the disease progresses to more advanced stages, signs of tissue damage become more apparent, often manifesting as chronic non-healing wounds [2]. The pathology of diabetic foot ulcers is complex due to prolonged high blood sugar levels and the related complications of diabetes. These complications encompass several factors: breakdown of the skin barrier leading to infections, heightened oxidative stress, nerve damage (neuropathy), issues with tiny blood vessels (microvascular complications), and ongoing inflammatory response [3]. Collectively, these factors create a challenging environment for proper wound healing, often resulting in the persistence of chronic diabetic wounds that demand specialized interventions for successful resolution.
Wound dressing materials are crucial in promoting wound healing and preventing infections. Traditional wound dressings, however, come with their limitations. Cotton wool and gauze, the two most common dressings, absorb a significant amount of the wound’s moisture to dry the wound’s surface. This will result in a reduction in the rate of healing and cause discomfort when removing the dressing. By contrast, wet dressings would accelerate the wound healing process, where several polymers were developed, including films, foams, and gels, to offer optimal condition to enhance microbial growth [4].
In this context, the biomaterial self-healing hydrogel holds a promising solution for effectively addressing these challenges in wound treatment. Hydrogels are soft materials distinguished by their three-dimensional network structure. The inherent fluidity of hydrogels before gelation facilitates the filling of irregular shapes, obviating the prerequisite for pre-shape forming processing. Thus, using hydrogels has been an idea in treating scaffolds in tissue engineering due to their similarity in water-retention abilities and network structure to those of the extracellular matrix [5]. On the other hand, hydrogels possessing the capability to autonomously restore their original set of properties in response to damage are referred to as “self-healing hydrogels.” ‘Self-healing’ is achieved by incorporating hydrocarbon side chains containing polar functional groups on a polymer network to facilitate hydrogen bonding between two distinct hydrogel pieces [6]. Yet, to achieve effective and durable recovery, the side chains need an appropriate length and flexibility, along with the polymer network suitably malleable for functional groups at the interface to interact unhindered despite any surface irregularities. Simultaneously, the side chains must also prevent excessive steric hindrance among the interacting functional groups and undesirable hydrophobic folding of the side chains. Consequently, creating mechanically robust hydrogels with inherent self-healing abilities increases their durability and reliability in various biomedical applications [7]. Besides, its barrier properties also provide a safer approach than traditional wound dressings.
This review systematically discussed the mechanisms and properties of self-healing hydrogels and their biomedical applications. First, self-healing mechanisms were thoroughly discussed, with two major properties that make self-healing hydrogels an effective biomaterial to cure diabetic foot ulcers discussed in detail. Challenges and future development directions were also illustrated and summarized to improve a broader use of self-healing hydrogels.
2. Mechanisms
Self-healing hydrogels employ various mechanisms to repair damage and regain their structures. These mechanisms are broadly divided into covalent (chemical cross-linking) and non-covalent bonds (physical cross-linking) [8]. Covalent bonds include oxime bonds, disulfide bonds, Diels-Alder reaction, boronate bonds, Acylhydrazone bonds, and Imine bonds. On the other hand, non-covalent bonds are made up of ionic bonds, hydrogen bonds, hydrophobic bonds and host-guest interactions.
2.1. Dynamic Covalent Bonding
Dynamic covalent bonding is a permanent bond that can break and re-form [9]. Imine bonds, also known as Schiff base, facilitate the creation of self-healing hydrogels through the cross-linking of amine groups with aldehyde or ketone groups within polymers under physiological conditions. The formation of Schiff base bonds performs greater reaction kinetics under moderate conditions, leading to rapid gelation and self-healing ability [10]. These attributes position Schiff base bonds as a compelling choice for advancing applications in tissue engineering. The disulfide exchange process occurs in the presence of nucleophilic thiolates, resulting in the creation of disulfide bonds in a neutral or alkaline environment [11], specifically at a basic pH. This exchange reaction involves the neighboring S-S bonds being disrupted and reformed via ionic intermediates. Combining thiolated polyethylene glycol and silver nitrate, a composite hydrogel was developed that withstands external mechanical forces and exhibits antibacterial properties during the repair of diabetic wounds. The Ag-S disulfide bond elevates its self-healing capability, making it a valid material for diabetic wound applications [12].
2.2. Non-covalent Bonding
Hydrophobic bonds involve the reversible relations among non-polar hydrophobic groups. When disrupted, the network of polymer chains with hydrophobic monomers can rapidly restore its structure. A method for leveraging hydrophobic bonding is “micellar polymerization,” which involves four essential components: hydrophobic units, hydrophilic units, surfactants, and electrolytes. To create self-healing hydrogels, hydrophobic units dissolved in an aqueous solution that contains surfactants and electrolytes copolymerize with hydrophilic units. Hydrogen bonding occurs between two polar groups, hydrogen atoms and strongly electronegative atoms like nitrogen (N), oxygen (O), or fluorine (F). While not as strong as covalent or ionic bonds, concerted hydrogen bonding interactions boost bond strength, aiding hydrogel formation. For instance, intermolecular hydrogen bonding can be observed through hydroxyl moieties in poly (vinyl alcohol) (PVA). Synthetic amino acid 3,4-Dihydroxyphenyl-L-alanine (DOPA), inspired by Mussel foot protein 3 (Mfp3), contains catechol groups capable of hydrogen bonding. Notably, interactions among catechol groups initiate and expedite the self-healing process [13]. Ionic bonds, resulting from the electrical attraction between ions with opposing charges, are another significant non-covalent mechanism in self-healing hydrogels. Hydrogels can be formed by combining monomers or polymers with opposite charges. The mobility of uncross-linked polymer chains and the migration of free ions are vital components of the self-healing mechanism, with the allowance of the 3D network’s ionic bonding to be dynamic and reversible [10].
3. Properties
To address the issues of bacterial infection, high reactive oxygen species (ROS) production, chronic inflammation, and impaired angiogenesis, researchers aim to develop multi-functional hydrogels that can meet the multiple requirements to heal diabetic wounds effectively. Self-healing hydrogels were introduced to maintain the structures during healing and are more suitable for use in the long term. Briefly, this section examines the research advances of self-healing hydrogels that can drastically increase the efficiency of healing diabetic wounds.
3.1. Self-healing Hydrogels to Prevent Bacterial Infection
To overcome wound infection in diabetic ulcers, one strategy is to use self-healing hydrogels with antibacterial properties to disinfect. Self-healing hydrogels with intrinsic antimicrobial properties or self-healing hydrogels with antibiotic or drug-like ingredients can reduce bacterial viability. Another strategy is to use self-healing hydrogels with antifouling properties to prevent the initial supplement of planktonic cells, thereby reducing biofilm formation.
3.1.1. Self-healing Hydrogels with Antimicrobial Properties. Chitosan and specific antimicrobial peptides with inherent antimicrobial properties can be employed to create hydrogels with antimicrobial. However, these hydrogels primarily operate through contact-based microbial elimination, necessitating proximity to the infection site, which can hinder effective eradication. To address this limitation, hydrogels are increasingly used as carriers for antimicrobial agents. The focus then shifts towards designing hydrogels that facilitate the controlled release of antimicrobial components, where the principal types of antimicrobial agents include antibiotics, metallic nanoparticles, and non-metallic nanoparticles. By stimulating this controlled release strategy within self-healing hydrogels, these agents can be effectively delivered to diabetic sites.
3.1.2. Antimicrobial Properties Employed to Prevent Fouling. Diabetic patients are more prone to chronic wounds with co-infections due to defective leukocyte chemotaxis and phagocytosis, leading to poor bacterial load clearance and the formation of refractory biofilms. Furthermore, twenty bacteria endotoxins contribute to tissue damage and further impair wound healing. Thus, hydrogel dressings that discourage bacterial adherence become paramount is developed. Among these solutions, amphoteric hydrogels are highly suitable for diabetic wound treatment, offering dense charged networks, ultra-low contamination properties, and excellent biocompatibility. Nonetheless, the practical application of amphoteric gels is limited due to their suboptimal mechanical properties [14]. To overcome this situation, researchers at Wenzhou University have introduced amphoteric poly (sulfobetaine methacrylate) (polySBMA) hydrogels with varying elasticity moduli. These hydrogels were applied to regenerate total excisional acute wounds in mice. The study’s outcomes demonstrated the substantial influence of crosslinkers on gel network growth. As predicted, the swollen transparent hydrogels exhibited reduced mechanical strength due to the disruption of hydrogen bonds and a decrease in polymer volume fraction. The introduction of crosslinkers significantly curtails gel network expansion, aligning with expectations [15].
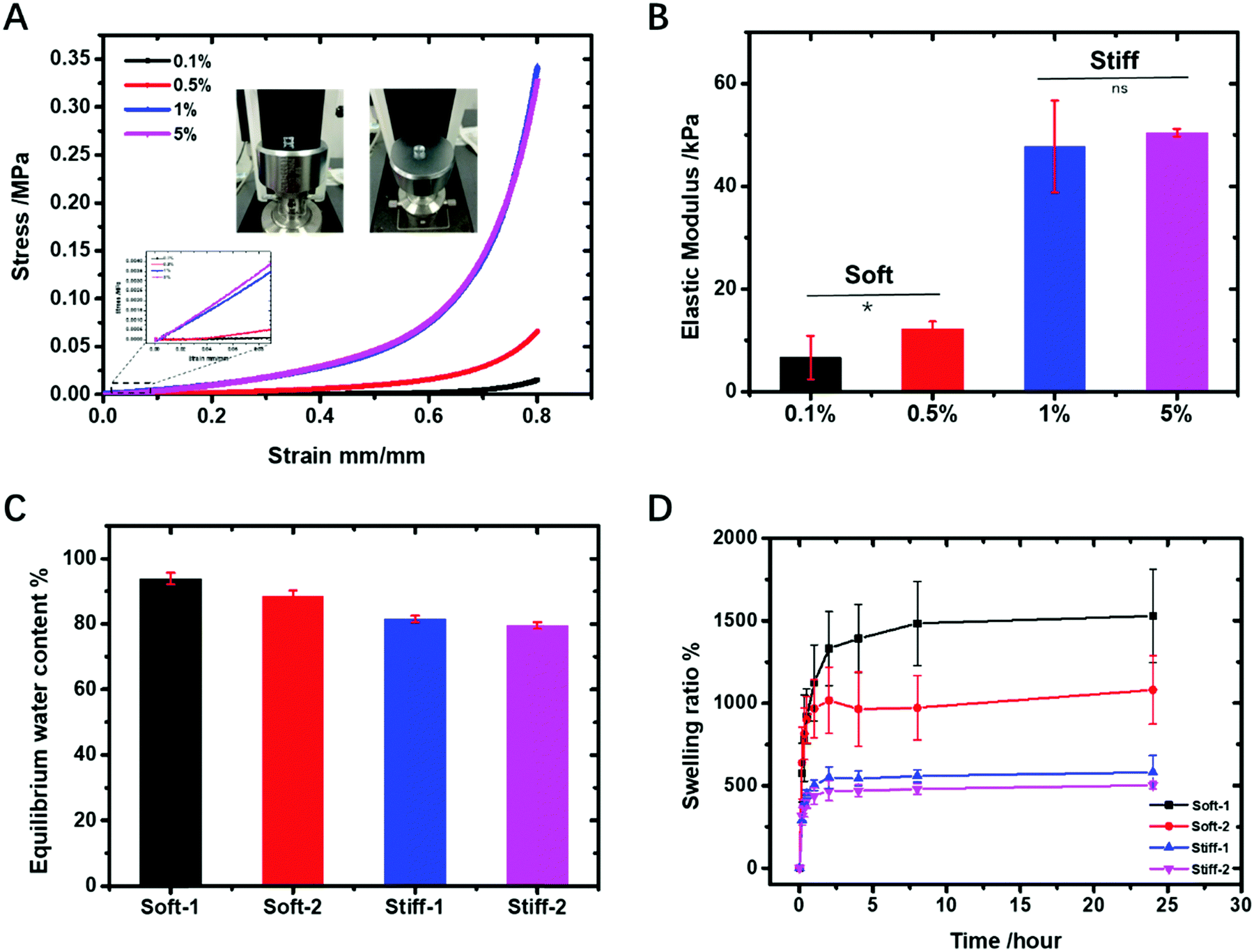
Figure 1. (A) Compressive stress–strain curves, (B) elastic modulus, (C) equilibrium water content (EWC), and (D) swelling kinetics of as-prepared polySBMA hydrogels [16].
3.2. Self-healing hydrogels with antioxidant properties
Oxidative stress, vascular impairment, pro-inflammatory cytokine secretion, and bacterial infections are all brought about by the accumulation of reactive oxygen species (ROS) in the wound area of a diabetic microenvironment. The accumulation of ROS and oxidative stress in a chronic inflammatory microenvironment not only produce an intense inflammatory response, making the wound enduring, but also leads to the differentiation of endogenous stem cells and the lack of macrophage polarisation, which can impede healing. Moreover, the poor microenvironment of diabetic wounds can cause endothelial dysfunction and inadequate angiogenesis, thus further delaying healing. As a result, a potential approach for treating diabetic wounds involves efficiently eliminating excessive ROS within localized wounds. Substantial research efforts have been dedicated to investigating the microenvironmental factors contributing to the buildup of ROS in diabetic wounds. Recently, active compounds such as anti-inflammatory agents, exosomes, antibiotics, and growth factors have emerged as possible options to counter chronic inflammation and expedite the wound healing process [17]. If the hydrogel incurs damage while engaging in the scavenging of ROS, resulting in structural disruption, it could affect its adsorption and scavenging capabilities. However, self-healing capability enables the hydrogel to rectify the damaged portion. This restoration ensures the hydrogel’s uninterrupted structural and functional attributes, securing its efficacy in effectively scavenging ROS.
3.3. Self-healing Hydrogel that Promotes Angiogenesis
The process of angiogenesis, which establishes a new vascular system to supply oxygen and other vital nutrients to tissues, plays a critical role in tissue regeneration. However, in diabetic wounds, angiogenesis can be impaired by hyperglycemia, persistent inflammation, and overproduction of ROS at the wound site. This impairment of angiogenesis can contribute to the persistence of non-healing wounds. Therefore, there is a pressing need for research focused on diabetic angiogenesis.
When a type of hydrogel is considered self-healing, its benefits become evident. Firstly, these hydrogels form a protective layer over the damaged vascular areas, promoting the regeneration and repair of blood vessels in diabetic individuals. Secondly, the self-healing properties of hydrogels can establish an environment conducive to the growth and attachment of vascular endothelial cells, thereby facilitating the process of new angiogenesis. Moreover, self-healing hydrogels can release bioactive substances, such as growth factors and cytokines, which further enhance the repair and regeneration of blood vessels.
Overall, the self-healing capability of the hydrogel offers an innovative treatment for diabetic patients. By promoting angiogenesis, these hydrogels hold the potential to significantly improve the vascular health of individuals with diabetes, ultimately contributing to enhanced wound healing and overall well-being.
3.4. Self-healing Hydrogels with Synergistic Effects
Compared to those with only one action, self-healing hydrogels with synergistic effects present a comprehensive approach to addressing multifaceted challenges in diabetic wound treatment. This includes infection, inflammation, and ischemia, thereby reducing therapeutic impediments and optimizing treatment outcomes through antimicrobial, anti-inflammatory, and pro-angiogenic mechanisms. Given the variability of diabetic wounds across patients, a personalized approach to treatment becomes imperative. Hydrogels with synergistic effects can be adapted to individual circumstances to tailor the selection of drugs and their release rates, thus enabling a customized treatment protocol that maximizes therapeutic efficacy [18]. Consequently, the development of self-healing hydrogels with synergistic attributes for diabetic wounds holds the potential to revolutionize diabetic wound care by providing more potent and effective treatment modalities. This not only aids in the acceleration of wound healing but also enhances the overall healing process for diabetic patients with improved outcomes.
4. Challenges and Future Perspectives
The quest for a comprehensive all-in-one wound treatment hydrogel that effectively combats biofilms while simultaneously mitigating the multifaceted impacts of diabetes is yet to be developed. Various single-function hydrogel dressings, ranging from antifouling to antibacterial and pro-healing, have demonstrated relative success. However, translating these successes to overall wound closure outcomes in vivo has proven challenging, often failing short compared to the natural wound healing process. This is primarily due to the inability of single-function hydrogel dressings to address the intricate interplay of factors contributing to nonhealing wounds. For instance, the simultaneous pursuit of pro-healing and antibacterial functionalities frequently yields worthless healing results, with slight gains in successfully treating the wound.
Secondly, the lipophilicity of hydrogel is a prime concern of self-healing hydrogels for drug delivery. Hydrophilic polymer hydrogels, characterized by hydrophilic domains and pores, compress [19]. When a hydrophobic drug is present, the drug’s loading and homogeneity within the gel matrix may be restricted [20]. Conversely, hydrophilic drugs may exhibit rapid release kinetics due to the hydrogel structure’s porous and highly hydrated nature, potentially leading to adverse patient side effects.
Stability within the in vivo environment during swelling is another critical challenge for self-healing hydrogels. Many self-healing hydrogels, particularly those with physical crosslinking, tend to disintegrate upon water exposure, making them unstable rapidly. While chemically crosslinked hydrogels can exhibit mechanical strength and stability in water, their self-healing capabilities often remain insignificant, proving inadequate for real-time biomedical engineering applications [21]. Striking a balance between mechanical toughness, self-healing capacity, and biocompatibility presents a significant trade-off, substantially limiting the scope of hydrogel applications in the biomedical arena. Furthermore, the challenges associated with self-healing hydrogels in treating diabetic wounds are manifold, from achieving comprehensive wound closure to addressing drug delivery concerns, stability, and the intricate relationship between mechanical properties and biocompatibility.
5. Conclusion
In conclusion, the paper discusses the substantial challenges of diabetes, which can result in severe injuries that take longer to recover from and heal. Managing diabetes through clinical methods presents its own set of difficulties. Often, this leads to the development of chronic diabetic wounds, perpetuating a cycle of health complications. Notably, Neurotherapy, a standard diabetic treatment, strips patients of their ability to sense pain, contributing to delayed wound detection. Insufficient wound care will further promote infection or inflammation. The body’s inability to mount a successful defense against pathogens adds another layer of complexity, increasing the risk of infection and impeding the natural healing process. To create a favorable environment for an efficient wound-healing process, self-healing hydrogels appear to be a potential approach to overcome the complexities in wound treatment. These cutting-edge polymers can autonomously mend damage acquired during usage, closely simulating the regenerative capabilities of natural tissues. Self-healing hydrogels are extremely important for treating wounds because of their unique qualities. Additionally, self-healing hydrogels offer the added benefit of encapsulating therapeutic molecules, enabling controlled and gradual drug release that accelerates wound healing. Recognizing the severe role of wound dressing materials in wound healing and infection prevention, this review focuses on the potential applications of self-healing hydrogels in diabetic wound treatment. We hope this review will support other researchers to improve self-healing hydrogels by employing different mechanisms and experimenting with them in various conditions.
Acknowledgements
All authors contributed equally to this work and should be considered co-first authors.
References
[1]. D. G. Armstrong, T.-W. Tan, A. J. M. Boulton, and S. A. Bus, ‘Diabetic Foot Ulcers’, JAMA, vol. 330, no. 1, p. 62, Jul. 2023, doi: 10.1001/jama.2023.10578.
[2]. J. Z. M. Lim, N. S. L. Ng, and C. Thomas, ‘Prevention and treatment of diabetic foot ulcers’, J R Soc Med, vol. 110, no. 3, pp. 104–109, Mar. 2017, doi: 10.1177/0141076816688346.
[3]. J. L. Burgess, W. A. Wyant, B. A. Abujamra, R. S. Kirsner, and I. Jozic, ‘Diabetic wound-healing science’, Medicina (Lithuania), vol. 57, no. 10. MDPI, Oct. 01, 2021. doi: 10.3390/medicina57101072.
[4]. E. Rezvani Ghomi, S. Khalili, S. Nouri Khorasani, R. Esmaeely Neisiany, and S. Ramakrishna, ‘Wound dressings: Current advances and future directions’, J Appl Polym Sci, vol. 136, no. 27, p. 47738, Jul. 2019, doi: 10.1002/app.47738.
[5]. D. L. Taylor and M. in het Panhuis, ‘Self‐Healing Hydrogels’, Advanced Materials, vol. 28, no. 41, pp. 9060–9093, Nov. 2016, doi: 10.1002/adma.201601613.
[6]. A. Phadke et al., ‘Rapid self-healing hydrogels’, Proceedings of the National Academy of Sciences, vol. 109, no. 12, pp. 4383–4388, Mar. 2012, doi: 10.1073/pnas.1201122109.
[7]. H. Rammal et al., ‘Advances in biomedical applications of self-healing hydrogels’, Mater Chem Front, vol. 5, no. 12, pp. 4368–4400, 2021, doi: 10.1039/D0QM01099E.
[8]. Q. Li, C. Liu, J. Wen, Y. Wu, Y. Shan, and J. Liao, ‘The design, mechanism and biomedical application of self-healing hydrogels’, Chinese Chemical Letters, vol. 28, no. 9, pp. 1857–1874, Sep. 2017, doi: 10.1016/j.cclet.2017.05.007.
[9]. G. Zhu, H. A. Houck, C. A. Spiegel, C. Selhuber‐Unkel, Y. Hou, and E. Blasco, ‘Introducing Dynamic Bonds in Light‐based 3D Printing’, Adv Funct Mater, Apr. 2023, doi: 10.1002/adfm.202300456.
[10]. A. Devi V. K., R. Shyam, A. Palaniappan, A. K. Jaiswal, T.-H. Oh, and A. J. Nathanael, ‘Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications’, Polymers (Basel), vol. 13, no. 21, p. 3782, Oct. 2021, doi: 10.3390/polym13213782.
[11]. W. Wang et al., ‘Injectable, Self-Healing Hydrogel with Tunable Optical, Mechanical, and Antimicrobial Properties’, Chemistry of Materials, vol. 31, no. 7, pp. 2366–2376, Apr. 2019, doi: 10.1021/acs.chemmater.8b04803.
[12]. H. Chen et al., ‘An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair’, NPG Asia Mater, vol. 11, no. 1, p. 3, Dec. 2019, doi: 10.1038/s41427-018-0103-9.
[13]. L. Quan, Y. Xin, X. Wu, and Q. Ao, ‘Mechanism of Self-Healing Hydrogels and Application in Tissue Engineering’, Polymers (Basel), vol. 14, no. 11, p. 2184, May 2022, doi: 10.3390/polym14112184.
[14]. S. Yu Zheng et al., ‘Molecularly Engineered Zwitterionic Hydrogels with High Toughness and Self-Healing Capacity for Soft Electronics Applications’, Chemistry of Materials, vol. 33, no. 21, pp. 8418–8429, Oct. 2021, doi: 10.1021/acs.chemmater.1c02781.
[15]. ‘Zwitterionic poly(sulfobetaine methacrylate)’.
[16]. H. He et al., ‘Zwitterionic poly(sulfobetaine methacrylate) hydrogels with optimal mechanical properties for improving wound healing in vivo’, J Mater Chem B, vol. 7, no. 10, pp. 1697–1707, 2019, doi: 10.1039/C8TB02590H.
[17]. G. Jia et al., ‘Green tea derivative-based hydrogel with ROS-scavenging property for accelerating diabetic wound healing’, Mater Des, vol. 225, Jan. 2023, doi: 10.1016/j.matdes.2022.111452.
[18]. K. Li, J. Wang, P. Li, and Y. Fan, ‘Ternary hydrogels with tunable mechanical and self-healing properties based on the synergistic effects of multiple dynamic bonds’, J Mater Chem B, vol. 8, no. 21, pp. 4660–4671, 2020, doi: 10.1039/C9TB02885D.
[19]. S. D. Sarkar, M. M. Uddin, C. K. Roy, M. J. Hossen, M. I. Sujan, and M. S. Azam, ‘Mechanically tough and highly stretchable poly(acrylic acid) hydrogel cross-linked by 2D graphene oxide’, RSC Adv, vol. 10, no. 18, pp. 10949–10958, Mar. 2020, doi: 10.1039/d0ra00678e.
[20]. T. R. Hoare and D. S. Kohane, ‘Hydrogels in drug delivery: Progress and challenges’, Polymer, vol. 49, no. 8. Elsevier BV, pp. 1993–2007, Apr. 15, 2008. doi: 10.1016/j.polymer.2008.01.027.
[21]. Md. M. H. Rumon et al., ‘Graphene oxide based crosslinker for simultaneous enhancement of mechanical toughness and self-healing capability of conventional hydrogels’, RSC Adv, vol. 12, no. 12, pp. 7453–7463, 2022, doi: 10.1039/D2RA00122E.
Cite this article
Chan,P.;Zhang,J.;Zhang,R. (2024). A review of research status of self-healing hydrogels for treating a diabetic wound. Theoretical and Natural Science,45,232-238.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. D. G. Armstrong, T.-W. Tan, A. J. M. Boulton, and S. A. Bus, ‘Diabetic Foot Ulcers’, JAMA, vol. 330, no. 1, p. 62, Jul. 2023, doi: 10.1001/jama.2023.10578.
[2]. J. Z. M. Lim, N. S. L. Ng, and C. Thomas, ‘Prevention and treatment of diabetic foot ulcers’, J R Soc Med, vol. 110, no. 3, pp. 104–109, Mar. 2017, doi: 10.1177/0141076816688346.
[3]. J. L. Burgess, W. A. Wyant, B. A. Abujamra, R. S. Kirsner, and I. Jozic, ‘Diabetic wound-healing science’, Medicina (Lithuania), vol. 57, no. 10. MDPI, Oct. 01, 2021. doi: 10.3390/medicina57101072.
[4]. E. Rezvani Ghomi, S. Khalili, S. Nouri Khorasani, R. Esmaeely Neisiany, and S. Ramakrishna, ‘Wound dressings: Current advances and future directions’, J Appl Polym Sci, vol. 136, no. 27, p. 47738, Jul. 2019, doi: 10.1002/app.47738.
[5]. D. L. Taylor and M. in het Panhuis, ‘Self‐Healing Hydrogels’, Advanced Materials, vol. 28, no. 41, pp. 9060–9093, Nov. 2016, doi: 10.1002/adma.201601613.
[6]. A. Phadke et al., ‘Rapid self-healing hydrogels’, Proceedings of the National Academy of Sciences, vol. 109, no. 12, pp. 4383–4388, Mar. 2012, doi: 10.1073/pnas.1201122109.
[7]. H. Rammal et al., ‘Advances in biomedical applications of self-healing hydrogels’, Mater Chem Front, vol. 5, no. 12, pp. 4368–4400, 2021, doi: 10.1039/D0QM01099E.
[8]. Q. Li, C. Liu, J. Wen, Y. Wu, Y. Shan, and J. Liao, ‘The design, mechanism and biomedical application of self-healing hydrogels’, Chinese Chemical Letters, vol. 28, no. 9, pp. 1857–1874, Sep. 2017, doi: 10.1016/j.cclet.2017.05.007.
[9]. G. Zhu, H. A. Houck, C. A. Spiegel, C. Selhuber‐Unkel, Y. Hou, and E. Blasco, ‘Introducing Dynamic Bonds in Light‐based 3D Printing’, Adv Funct Mater, Apr. 2023, doi: 10.1002/adfm.202300456.
[10]. A. Devi V. K., R. Shyam, A. Palaniappan, A. K. Jaiswal, T.-H. Oh, and A. J. Nathanael, ‘Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications’, Polymers (Basel), vol. 13, no. 21, p. 3782, Oct. 2021, doi: 10.3390/polym13213782.
[11]. W. Wang et al., ‘Injectable, Self-Healing Hydrogel with Tunable Optical, Mechanical, and Antimicrobial Properties’, Chemistry of Materials, vol. 31, no. 7, pp. 2366–2376, Apr. 2019, doi: 10.1021/acs.chemmater.8b04803.
[12]. H. Chen et al., ‘An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair’, NPG Asia Mater, vol. 11, no. 1, p. 3, Dec. 2019, doi: 10.1038/s41427-018-0103-9.
[13]. L. Quan, Y. Xin, X. Wu, and Q. Ao, ‘Mechanism of Self-Healing Hydrogels and Application in Tissue Engineering’, Polymers (Basel), vol. 14, no. 11, p. 2184, May 2022, doi: 10.3390/polym14112184.
[14]. S. Yu Zheng et al., ‘Molecularly Engineered Zwitterionic Hydrogels with High Toughness and Self-Healing Capacity for Soft Electronics Applications’, Chemistry of Materials, vol. 33, no. 21, pp. 8418–8429, Oct. 2021, doi: 10.1021/acs.chemmater.1c02781.
[15]. ‘Zwitterionic poly(sulfobetaine methacrylate)’.
[16]. H. He et al., ‘Zwitterionic poly(sulfobetaine methacrylate) hydrogels with optimal mechanical properties for improving wound healing in vivo’, J Mater Chem B, vol. 7, no. 10, pp. 1697–1707, 2019, doi: 10.1039/C8TB02590H.
[17]. G. Jia et al., ‘Green tea derivative-based hydrogel with ROS-scavenging property for accelerating diabetic wound healing’, Mater Des, vol. 225, Jan. 2023, doi: 10.1016/j.matdes.2022.111452.
[18]. K. Li, J. Wang, P. Li, and Y. Fan, ‘Ternary hydrogels with tunable mechanical and self-healing properties based on the synergistic effects of multiple dynamic bonds’, J Mater Chem B, vol. 8, no. 21, pp. 4660–4671, 2020, doi: 10.1039/C9TB02885D.
[19]. S. D. Sarkar, M. M. Uddin, C. K. Roy, M. J. Hossen, M. I. Sujan, and M. S. Azam, ‘Mechanically tough and highly stretchable poly(acrylic acid) hydrogel cross-linked by 2D graphene oxide’, RSC Adv, vol. 10, no. 18, pp. 10949–10958, Mar. 2020, doi: 10.1039/d0ra00678e.
[20]. T. R. Hoare and D. S. Kohane, ‘Hydrogels in drug delivery: Progress and challenges’, Polymer, vol. 49, no. 8. Elsevier BV, pp. 1993–2007, Apr. 15, 2008. doi: 10.1016/j.polymer.2008.01.027.
[21]. Md. M. H. Rumon et al., ‘Graphene oxide based crosslinker for simultaneous enhancement of mechanical toughness and self-healing capability of conventional hydrogels’, RSC Adv, vol. 12, no. 12, pp. 7453–7463, 2022, doi: 10.1039/D2RA00122E.









