1. Introduction
COVID-19 is a highly contagious disease caused by the SARS-CoV-2 virus infection which appeared starting in the winter of 2019. The outbreak started in Wuhan, China, in a wet market and has quickly spread worldwide, with over 219 million confirmed cases by the World Health Organization (WHO) in less than one year [1]. COVID-19 causes symptoms such as coughing, fatigue, difficulty breathing, and fever, and can feel like the flu or pneumonia. COVID-19 seems to have the most significant effect on older people, as the risk of death increases with age. There is also a higher risk for COVID-19 patients who have respiratory diseases such as Asthma, Hematologic Malignancies, Bronchiectasis, or Interstitial lung disease, as well as pregnant women, smokers, physically inactive patients, and severe obesity [2]. Treatments for COVID-19 do exist, such as Remdesivir, a nucleotide prodrug of an adenosine analog. Remdesivir was able to reduce viral load in the lungs of mice but was unable to improve the survival and healing of the tissue. This suggests that Remdesivir is best for when COVID-19 is still in its early stages [3]. Remdesivir containing only one antiviral agent raises concerns, as this issue could lead to the virus developing resistance [4].
Recent studies have shown, however, that Tocilizumab (TCZ), an immunosuppressive drug, has been dose-dependent effective in COVID-19 patients. COVID influences the immune system to release many cytokines, known as a “cytokine storm.” Tocilizumab has been used in other cases (such as Rheumatoid Arthritis) to work against cytokine storms, and it seemed to improve symptoms of COVID-19, with a 75% success rate. Chinese case reports of Tocilizumab’s effects on COVID-19 have been promising, showing a fast recovery after the administration of the drug [5].
This review discusses how COVID-19 works, the therapeutic roles of Tocilizumab on COVID-19, and the results from the clinical trials of Tocilizumab.
2. Mechanisms of COVID-19 and effects of Tocilizumab
2.1. Structure and mechanisms of SARS-CoV-2 infection
The SARS-CoV-2 is a single, positive-strand RNA virus with four major parts. Inside the virus, genetic material contains instructions for replication. A protein shell then protects the genetic material as it travels. The envelope allows the virus to merge with the cells that it infects. Finally, the spike-like S protein molecules emerge from the envelope to insert themselves into the throat cells’ Angiotensin-converting enzyme-2 (ACE-2) receptor. From there, the virus travels in a cell membrane sac to ribosomes. It uses its genetic information to create viral proteins that eventually form a new virus, allowing it to spread throughout the body. Viral S proteins left on the infected throat cell will eventually fuse with nearby cells, which causes a giant cell that lets the virus spread between cells [6]. A buildup of SARS-CoV-2 will then cause the alveoli in your lungs to become inflamed, which may lead to fluid buildup in the alveoli, which causes difficulty breathing.
The phenotypic traits of the SARS-CoV-2 infection span from entirely asymptomatic through mild to moderate outcomes (headache, fever, dizziness, cough, etc.) to more severe manifestations like pneumonia that can lead to lung failure, acute respiratory distress syndrome (ARDS), and ultimately death [7]. This coincides with the lungs’ main target organ of the SARS-Cov-2 virus. The causes of these outcomes are thought to be interactions between the virus and the patient’s immune system. Those who lack symptoms and those who have only mild to moderate manifestations can mount a proper specific immune response mediated by lymphocytes. This requires B-cells to differentiate effectively from antibody-producing plasma cells, which requires activation by T helper 1 (Th1) cells [6].
2.2. Cytokine storm
Moreover, both CD4+ and CD8+ T-cells need to produce as well as secrete interferon-gamma, which is an antiviral protein cytokine with a wide range of affecting the innate and adaptive immune systems [8]. The innate immune pathways continue to operate if the patient cannot clear the virus efficiently using the adaptive immune response. They can cause a hyperinflammatory state that coincides with the release of many cytokines, known generally as a “cytokine storm.” Such storms have been observed in the most severe cases of COVID-19 and are associated with a condition known as lymphopenia, which is when the patient has a lower-than-normal lymphocyte presence in the blood, resulting in an overall defective immune response. In addition, the cytokines are responsible for inflamed tissue damage and subsequent death [6]. These events are far more likely to occur in individuals who either have already deficient immune systems due to instances that include comorbidities (obesity, diabetes, cancer, etc.) and old age or to the presence of an excessively high viral load. Therefore, preventing the downstream outcomes of the cytokine storm was hypothesized to be one way to reduce symptoms and prevent death [9].
The cytokine storm has been found to include a sizable complex array of diverse cytokines, including interleukin-6 (IL-6). IL-6 is a pleiotropic cytokine that responds to damaged tissue and leads the production of Interleukin-27 while also helping the virus progress and favoring the persistence of infected cells [10]. The release of IL-6 in response to SARS-CoV-2 infection can exert several detrimental effects on the body. High levels of IL-6 can exacerbate inflammation, leading to severe respiratory symptoms and complications such as ARDS. This excessive inflammation can damage lung tissue and other organs, contributing to the severity and mortality of COVID-19 [11].
2.3. Targeting of Tocilizumab
Tocilizumab works by blocking the IL-6 receptor (IL-6R), thereby reducing the inflammatory response and helping to control the cytokine storm. It binds to both the soluble and membrane-bound forms of IL-6R, thereby preventing IL-6 from exerting its pro-inflammatory effects. This blockade prevents IL-6 from the pro-inflammatory effects, which include the activation of T-cells, the differentiation of B-cells, as well as the stimulation of acute-phase protein synthesis in the liver (Figure 1). The inhibition of these pathways helps reduce inflammation and immune system overactivity, mitigating the symptoms and complications associated with severe COVID-19. This action helps decrease lung inflammation and damage, potentially improving outcomes for severely ill COVID-19 patients [12].
In vitro, Tocilizumab has been shown to inhibit IL-6 signaling in human cell lines derived from lung and endothelial tissues. In vivo, Tocilizumab has been shown to protect against inflammation and tissue damage in mouse models of rheumatoid arthritis as well as cytokine release syndrome. These models simulate the hyperinflammatory state observed in severe COVID-19, indicating Tocilizumab’s potential to mitigate excessive inflammatory responses and prevent tissue damage [13].
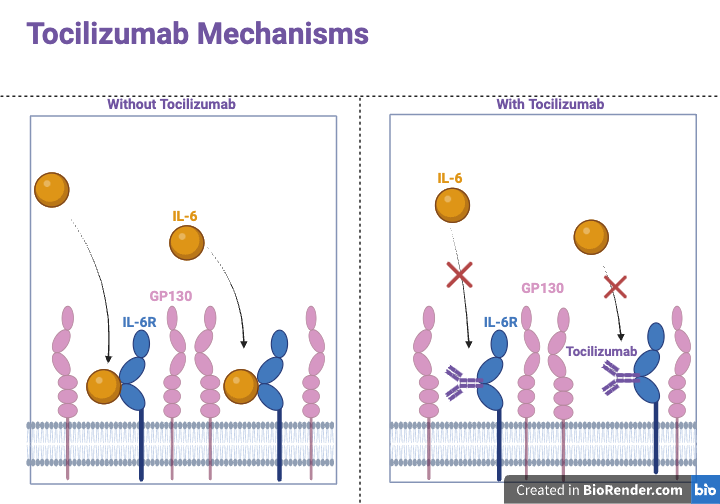
Figure 1. Mechanisms of Tocilizumab. In a normal scenario, Interleukin-6 binds to the Interleukin-6 receptor, forming a complex involving GP130. The IL-6/IL-6R complex triggers ligand-mediated oligomerization with GP130, causing GP130 homodimerization and subsequent formation of a hexameric complex, which is a crucial step in the IL-6 signaling pathway. L-6/GP130 signaling creates inflammation by inducing pro-inflammatory cytokines and chemokines, together with immune response and tissue regeneration. Tocilizumab prevents the binding of IL-6 to IL-6R, which prevents the signaling of IL-6 and reduces pro-inflammatory cytokines and chemokines. Abbreviations: interleukin-6, IL-6; IL-6R, IL-6 receptor; GP130, Glycoprotein 130. Figure credit: original.
3. Clinical trials
Clinical studies have demonstrated various benefits of Tocilizumab in COVID-19 treatment. For instance, patients receiving Tocilizumab had fewer days requiring respiratory and cardiovascular support and a lower hospital mortality rate than those receiving standard care [12]. However, not all results were positive, with others showing inconclusive benefits [14, 15].
The COVACTA trial is a multicenter, randomized, double-blind, placebo-controlled Phase III study evaluating the safety and effectiveness of Tocilizumab in hospitalized adult patients with severe COVID-19 pneumonia. This trial recruited 452 patients who were administered a single dose of Tocilizumab at 8 mg/kg, while the maximum dose was 800 mg. The trial aimed to assess whether Tocilizumab could improve clinical outcomes by mitigating the severe inflammatory response associated with COVID-19 [16].
The primary outcome of the trial, which was the improvement in status on Day 28, was not dramatically different between the Tocilizumab and placebo groups (Table 1). Patients treated with Tocilizumab had a shorter average time of getting discharged and a reduced likelihood of needing mechanical ventilation. However, no significant difference was found in overall mortality at day 28 between the two groups, which indicates that Tocilizumab does not significantly lower the mortality risk in severe COVID-19 cases [16].
The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial was to test the effects of Tocilizumab on hospitalized COVID-19 patients with hypoxia and systemic inflammation. This trial recruited 4116 patients who were administered 8 mg of body weight. The results show that Tocilizumab should be used in standard care, as there was a massive decrease in mortality, as well as an increase in discharge rate (Table 1). The trial recommended Tocilizumab to be in conjunction with standard care, including corticosteroids, for severe COVID-19 patients [17].
Research shows that Tocilizumab in patients with moderate to severe COVID-19 has resulted in the reduction of all-cause mortality, especially in those with severe illness and infection. In addition, treatment has been shown to lower the need for mechanical ventilation along with reduced intubation time as well as a reduction in overall hospital stay. It should be mentioned, however, that there are a number of randomized clinical trials that do not support this conclusion entirely. This is thought to be due to the fact that these trials recruited participants with only mild COVID-19 symptoms, while not necessarily those with severe disease. Therefore, it would appear that treatment with Tocilizumab is more effective in those who present with critical disease manifestation than those who present with only mild symptoms. This conclusion is supported by a published clinical trial where patients with severe COVID-19 pneumonia who were not intubated were randomly assigned to either a Tocilizumab treatment group or a placebo control. In the Tocilizumab group, 12.0% died by day 28, while the placebo-controlled group had a significantly higher mortality outcome of 19.3% [18]. In a second study done using 154 intubated patients, it was shown that outcomes in patients receiving Tocilizumab (n = 78) compared to controls resulted in lower mortality overall [19]. Therefore, the different degrees of illness due to COVID-19 infection appear to be relevant as to whether treatment with Tocilizumab would be beneficial, along with additional variables that include comorbidities, age, and sex. In addition, it should be cautioned that the application of Tocilizumab was thought to be related to an increased risk of low neutrophil counts, impaired liver function, and bleeding events.
Table 1. Comparison between COVACTA trial and RECOVERY trial
Studies | COVACTA trial | RECOVERY trial | Subjects | 452 patients | 4116 patients | Treatment | a single dose of Tocilizumab at 8 mg/kg, with a maximum dose of 800 mg | 8mg/kg of Tocilizumab | 28-day Mortality (Tocilizumab group vs placebo group) | 19.7% vs 19.4%, P=0.9400 | 31% vs 35%, P=0.0028 | [16] | [17] |
4. Conclusion
COVID-19 is caused by infection of SARS-CoV-2. Cytokine storm is one of the critical pathogenic processes during SARS-CoV-2 infection. IL-6 plays a vital role during the cytokine storm. Tocilizumab, as an IL-6 receptor agonist, was originally to treat rheumatoid arthritis. Tocilizumab has been shown to play a therapeutic role in reducing the mortality of patients with moderate to severe COVID-19 symptoms by targeting cytokine storms. Clinical trials, such as the COVACTA and RECOVERY trials, have shown a decrease in 28-day mortality, as well as improvement of other second outcomes. Future studies are required to understand how Tocilizumab affects cytokine storms, not only on COVID-19 but also on similar pneumonia by virus infections.
References
[1]. Henson JB and Muir AJ 2023 Clin. Liver Dis. 27 103-115
[2]. CDC, Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals (2024), Available online at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html.
[3]. Bartoli A, Gabrielli F, Alicandro T, Nascimbeni F and Andreone P 2021 Intern. Emerg. Med. 16 281-308
[4]. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines, National Institutes of Health (2024), Available online at: https://www.covid19treatmentguidelines.nih.gov/.
[5]. Dinarello CA 2000 Chest 118, 503-508
[6]. Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen MC, O’Mahony L, Gao Y, Nadeau K, and Akdis CA 2020 Allergy 75 1564-1581
[7]. Pelaia C, Tinello C, Vatrella A, De Sarro G, and Pelaia G 2020 Ther. Adv. Respir. Dis. 14, 1753466620933508
[8]. Peng Y, et al 2020 Nat. Immunol. 21 1336-1345
[9]. Procopio G, et al. 2020 Ther. Adv. Respir. Dis. 2020 14 1753466620963016
[10]. Shekhawat J, Gauba K, Gupta S, Purohit P, Mitra P, Garg M, Misra S, Sharma P, Banerjee M 2021 Indian. J. Clin. Biochem. 36 440-450
[11]. Du P, Geng J, Wang F, Chen X, Huang Z, Wang Y 2021 Int. J. Med. Sci. 18 1356-1362
[12]. University of Minnesota, Tocilizumab (2024), Available online at: https://covidebm.umn.edu/evidence-based-therapies/tocilizumab
[13]. Baylor College of Medicine, Trial results show potential benefits of tocilizumab for COVID-19 (2020), Available online at: https://www.bcm.edu/news/trial-results-show-potential-benefits-of-tocilizumab-for-covid-19
[14]. Abidi E, El Nekidy WS, Alefishat E, Rahman N, Petroianu GA, El-Lababidi R, Mallat J 2022 Front Pharmacol. 18 13:825749
[15]. Fu B, Xu X, Wei H 2020 J. Transl. Med. 18 164
[16]. Rosas IO, et al. 2022 EClinicalMedicine. 47 101409
[17]. RECOVERY Collaborative Group 2021 Lancet 397 1637-1645
[18]. Rosas IO, et al. 2021 N. Engl. J. Med. 384 1503-1516
[19]. Somers EC, et al. 2020 Clin. Infect. Dis. 73 e445-e454
Cite this article
Wang,Z. (2024). Therapeutic effects of Tocilizumab on COVID-19. Theoretical and Natural Science,47,65-69.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Environmental Geoscience and Earth Ecology
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Henson JB and Muir AJ 2023 Clin. Liver Dis. 27 103-115
[2]. CDC, Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals (2024), Available online at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html.
[3]. Bartoli A, Gabrielli F, Alicandro T, Nascimbeni F and Andreone P 2021 Intern. Emerg. Med. 16 281-308
[4]. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines, National Institutes of Health (2024), Available online at: https://www.covid19treatmentguidelines.nih.gov/.
[5]. Dinarello CA 2000 Chest 118, 503-508
[6]. Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen MC, O’Mahony L, Gao Y, Nadeau K, and Akdis CA 2020 Allergy 75 1564-1581
[7]. Pelaia C, Tinello C, Vatrella A, De Sarro G, and Pelaia G 2020 Ther. Adv. Respir. Dis. 14, 1753466620933508
[8]. Peng Y, et al 2020 Nat. Immunol. 21 1336-1345
[9]. Procopio G, et al. 2020 Ther. Adv. Respir. Dis. 2020 14 1753466620963016
[10]. Shekhawat J, Gauba K, Gupta S, Purohit P, Mitra P, Garg M, Misra S, Sharma P, Banerjee M 2021 Indian. J. Clin. Biochem. 36 440-450
[11]. Du P, Geng J, Wang F, Chen X, Huang Z, Wang Y 2021 Int. J. Med. Sci. 18 1356-1362
[12]. University of Minnesota, Tocilizumab (2024), Available online at: https://covidebm.umn.edu/evidence-based-therapies/tocilizumab
[13]. Baylor College of Medicine, Trial results show potential benefits of tocilizumab for COVID-19 (2020), Available online at: https://www.bcm.edu/news/trial-results-show-potential-benefits-of-tocilizumab-for-covid-19
[14]. Abidi E, El Nekidy WS, Alefishat E, Rahman N, Petroianu GA, El-Lababidi R, Mallat J 2022 Front Pharmacol. 18 13:825749
[15]. Fu B, Xu X, Wei H 2020 J. Transl. Med. 18 164
[16]. Rosas IO, et al. 2022 EClinicalMedicine. 47 101409
[17]. RECOVERY Collaborative Group 2021 Lancet 397 1637-1645
[18]. Rosas IO, et al. 2021 N. Engl. J. Med. 384 1503-1516
[19]. Somers EC, et al. 2020 Clin. Infect. Dis. 73 e445-e454









