1. Introduction
Breast cancer is the most frequently diagnosed cancer in women and ranks as the second leading cause of cancer deaths among women globally [1]. Most recently, in 2022, approximately 2.3 million women worldwide had a breast cancer diagnosis in 2022, which resulted in 670,000 deaths. After puberty, women of any age can develop breast cancer, while older women are more likely to suffer from breast cancer. Human development levels (HDI) have a substantial impact on breast cancer mortality and rates. Approximately, a 1 in 12 diagnoses and a 1 in 71 death rate for women living in high HDI nations, compared to a 1 in 27 diagnoses and a 1 in 48 death rate in low HDI countries. Breast cancer results from uncontrolled cell growth in the breast, forming tumors in the milk ducts or lobules. Early-stage breast cancer is not life-threatening and can be detected early, but invasive cancer can spread to nearby tissue and organs, potentially becoming fatal [2].
Photothermal therapy (PTT) has developed as a potential cancer treatment technique that uses near-infrared (NIR) light-absorbing compounds to selectively heat and kill tumor cells while preserving healthy tissue. This approach has various benefits over standard treatments, including excellent specificity, low invasiveness, and effective tumor ablation. Recent improvements have focused on generating imageable photothermal agents to improve therapy planning, as demonstrated by a new albumin-based nano-complex developed for multimodal imaging-guided PTT. This theragnostic technique uses Gd3+-chelated human serum albumin and NIR fluorophores to precisely monitor and treat metastatic lesions, such as sentinel lymph nodes (SLNs), using focused laser irradiation. The albumin-based nano-complex method, which combines photothermal ablation with surgical resection, considerably improves survival results in preclinical animals, underlining its clinical cancer treatment potential [3].
This review summarizes the current advancements in utilizing carbon nanomaterials for enhanced photothermal therapy in breast cancer treatment. It explores the mechanisms and benefits of PTT, the role of various nanoparticles including carbon nanomaterials, and reviews both in vitro and in vivo studies, highlighting their potential and ongoing clinical trials.
2. Nanoparticle technology in PTT
2.1. Mechanism of PTT
PTT operates on the principle of converting light energy, typically NIR wavelengths, into heat through light-absorbing agents known as photothermal agents (PTAs) (Figure 1). These agents often nanoparticles like gold nanorods or carbon nanotubes (CNTs) selectively accumulate in tumor tissues due to enhanced permeability and retention (EPR) effects, minimizing damage to healthy cells while maximizing thermal effects on cancerous cells [4]. When exposed to NIR light, PTAs absorb photons, causing localized heating that induces several mechanisms of cell death within tumors, including direct cellular membrane disruption, denaturation of tumoral DNA and inhibition of angiogenesis [5]. The selective thermal destruction of cancer cells is advantageous over traditional therapies due to its high specificity and minimal invasiveness, thereby reducing systemic side effects [3]. Recent advancements in PTT include the development of theragnostic nanocomplexes that integrate imaging capabilities with therapeutic functionality, enabling precise monitoring and treatment of metastatic lesions like sentinel SLNs [6]. By combining PTT with other modalities such as chemotherapy or photodynamic therapy (PDT), synergistic effects can be achieved, enhancing overall treatment efficacy and potentially overcoming drug resistance mechanisms [4, 5]. Thus, PTT represents a promising strategy in oncology, offering improved therapeutic outcomes and paving the way for personalized cancer treatment regimens [6].
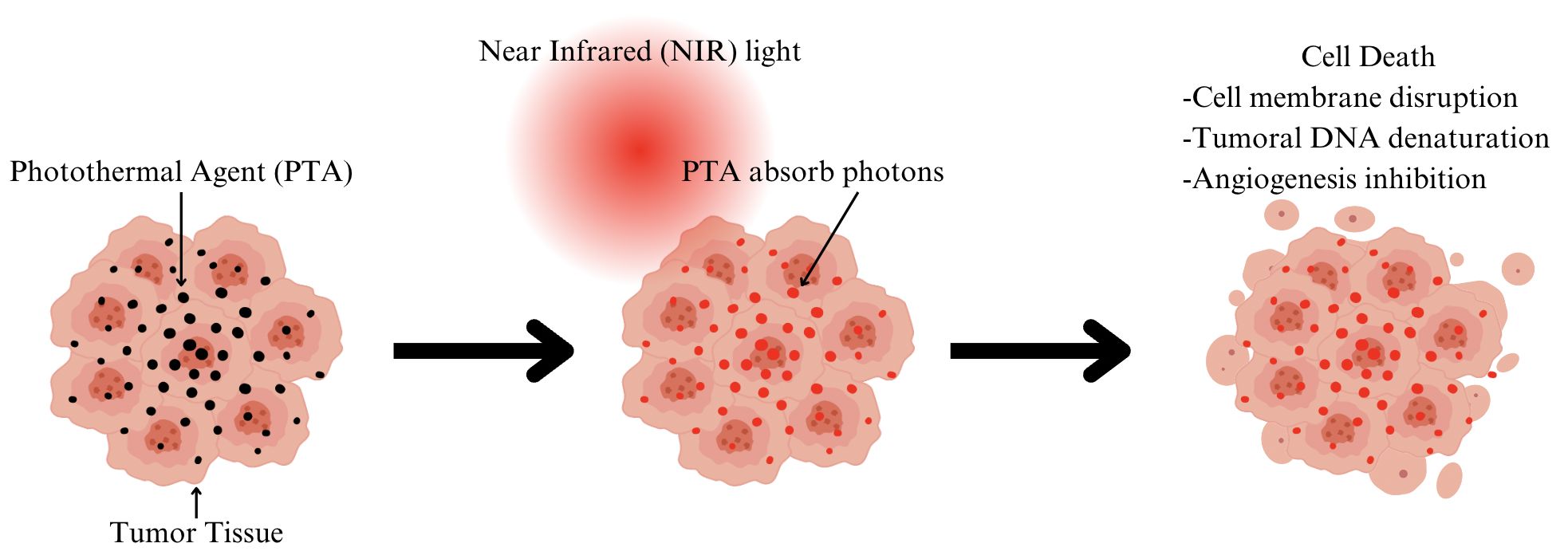
Figure 1. Mechanism of Photothermal Therapy (PTT). Figure created by Canva. Figure credit: original.
2.2. Nanoparticles in PTT
Nanoparticles have an important role in improving the efficacy of PTT in cancer treatment. PTT researchers take advantage of the unique optical and physical features of materials such as gold, iron oxides, silica, and carbon-based nanomaterials such as graphene to accomplish targeted tumor elimination. These nanoparticles are engineered to absorb certain wavelengths of light, often in the NIR region, allowing for deep tissue penetration while minimizing absorption by surrounding biological molecules. When exposed to NIR light, these nanoparticles undergo plasmon resonance, which effectively converts light energy into heat, resulting in localized hyperthermia inside the tumor microenvironment. This heat production is critical because it causes permanent cellular damage and promotes tumor regression by increasing the local temperature. Furthermore, the tiny size and surface chemistry of nanoparticles allow for their circulation in the bloodstream and accumulation at the tumor site via the increased permeability and retention (EPR) effect, maximizing therapeutic efficacy while minimizing systemic adverse effects. Overcoming challenges such as tumor heterogeneity and inefficient nanoparticle penetration is an ongoing research focus, prompting innovations in nanoparticle design, such as surface functionalization and size optimization, to improve their delivery and therapeutic impact across a wide range of tumor types [4].
3. Carbon Nanomaterials
Carbon nanomaterials have garnered significant attention in biomedical applications due to their unique physicochemical properties. These materials, including CNTs and graphene, exhibit high surface area, excellent electrical conductivity, and biocompatibility [7]. They hold promise in diverse fields such as drug delivery, biosensing, and cancer therapy, owing to their ability to interact with biological systems at the molecular level [8]. The versatile nature of carbon nanomaterials allows for tailored modifications to enhance their functionality and application-specific properties, making them valuable tools in modern biomedical research [9].
3.1. Types of Carbon Nanomaterials
CNTs represent one of the most studied forms of carbon nanomaterials [7]. These cylindrical structures exhibit extraordinary mechanical strength and electrical conductivity, ideal for applications ranging from nanoelectronics to biomedical devices [8]. Graphene, a two-dimensional carbon allotrope, has also gained prominence for its exceptional mechanical strength and high surface area [7]. Its single-atom thickness and unique electronic properties make graphene suitable for various biomedical applications, including tissue engineering scaffolds and biosensors [9]. Additionally, fullerenes, spherical carbon molecules like buckyballs, offer another class of carbon nanomaterials known for their antioxidant properties and potential in drug delivery systems [7]. Nanodiamonds are nanostructures with diamond-like crystallisation. Subsequently, nano-onions are made up of many layers of graphene [9]. These distinct types of carbon nanomaterials illustrate the breadth of their applications in biomedical research and highlight their ongoing exploration for innovative healthcare solutions [8].
3.2. Mechanism of Carbon Nanomaterial in PTT
Carbon nanomaterials, including CNTs and graphene oxide, offer promising prospects for PTT due to their efficient photothermal conversion and biocompatibility (Figure 2). They effectively absorb NIR light, allowing deep tissue penetration and selective heating of tumor tissues above 42 °C, which induces necrosis and apoptosis and enhances the permeability of tumor vasculature and cell membranes. This facilitates the accumulation of co-administered chemotherapy agents, thereby improving treatment outcomes. Additionally, carbon nanomaterial-mediated PTT can disrupt cellular homeostasis through DNA damage and protein denaturation within tumor cells, promoting cell death pathways [10].
Moreover, carbon nanomaterials exhibit the ability to modulate heat shock proteins (HSPs) like HSP70 in cancer cells, crucial for anti-apoptotic processes. By inhibiting HSP70 expression, these nanomaterials sensitize cancer cells to thermal stress induced by PTT, thereby reducing anti-apoptotic defenses and enhancing treatment efficacy. Furthermore, these materials can be engineered to target specific cellular pathways, such as the JAK/STAT signaling pathway, which regulates cell survival and apoptosis, thus augmenting the cytotoxic effects of PTT. The targeted approach synergizes effectively with other therapies like photodynamic therapy (PDT) or molecular inhibitors, offering a comprehensive strategy against cancer. The immune-modulatory properties of carbon nanomaterials further enhance their potential in combined therapies with PTT and immunotherapy, fostering a systemic immune response against primary tumors and residual/metastatic cancer cells [11]. This integration into advanced PTT strategies highlights their versatility and potential as next-generation therapeutics in oncology, addressing both tumor ablation and long-term cancer management to prevent disease recurrence [10,11].
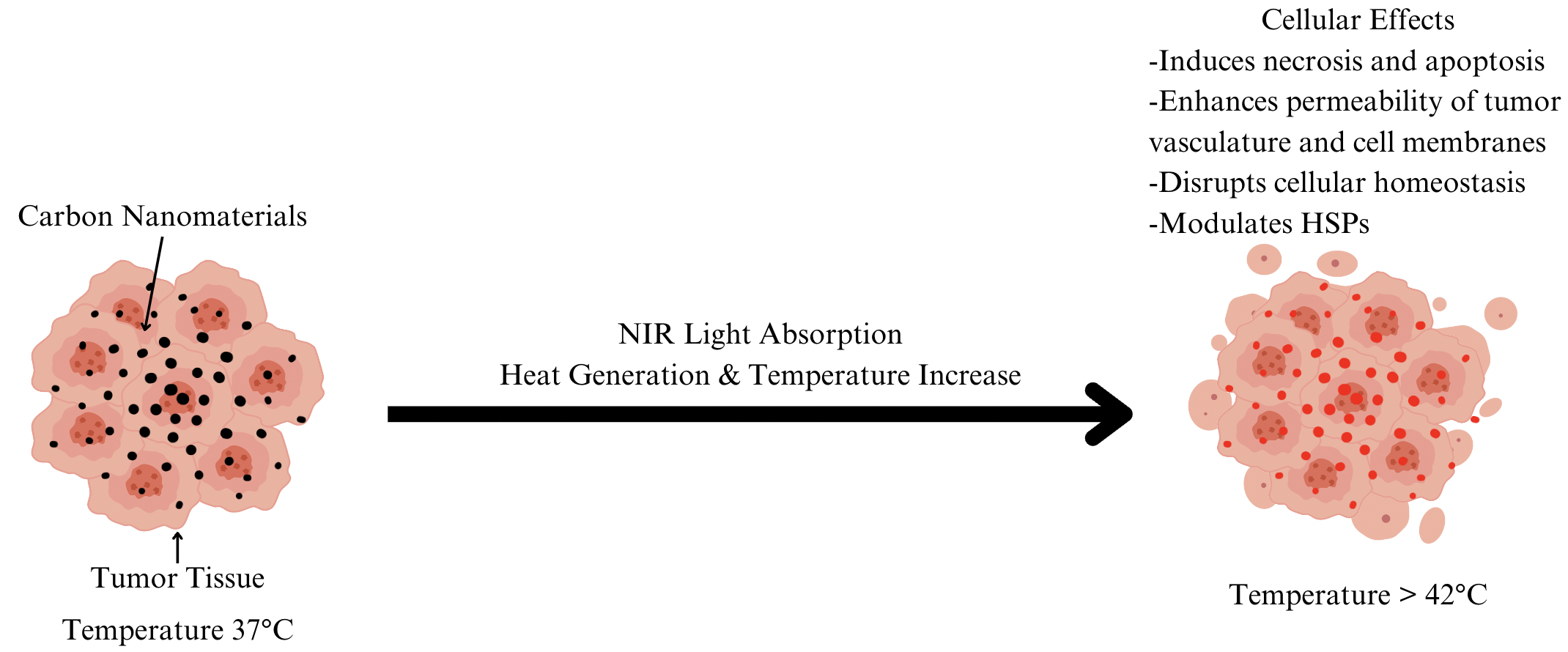
Figure 2. Mechanism of Carbon Nanomaterial-Mediated Photothermal Therapy (PTT) Effects. Figure created by using Canva. Figure credit: original.
4. In Vitro and In Vivo Carbon Nanomaterial for Breast Cancer
4.1. Studies on CNTs in Breast Cancer
In vitro studies have demonstrated the potential of CNTs in breast cancer research and treatment (Table 1). Oxidized graphene nanoribbons coated with folic acid and loaded with tamoxifen citrate, derived from multi-walled CNTs, showed effective drug loading and concentration-dependent death in breast cancer cells. The results showed preferential internalization into cancer cells, indicating a viable technique for the targeted delivery of therapeutic drugs such as tamoxifen to breast cancer cells. Furthermore, electrochemical immunosensors based on electrospun carbon nanofiber mats modified with Au nanoparticles, cysteamine, CNTs, and specific antibodies have shown promise for the precise and rapid detection of Her-2, a biomarker critical for breast cancer diagnosis and treatment decision-making [9].
In vivo, investigations into carbon nanotube-based therapies for breast cancer have demonstrated their potential to improve diagnostic and therapeutic approaches. Yang et al. developed an electronic nose utilizing carbon nanotube sensors that showed high sensitivity (86%) and specificity (97%) in distinguishing breast cancer through volatile metabolite analysis in breath samples [12]. This non-invasive diagnostic tool could aid in intraoperative decision-making and the identification of molecular subtypes, facilitating personalized treatment strategies for breast cancer patients. Furthermore, dopamine and mucin-1 functionalized electroactive CNTs have been utilized to construct electrochemical immunosensors capable of detecting MUC-1, with a high detection sensitivity in vitro. These findings suggest that carbon nanotube-based approaches hold promise for advancing both diagnostic and therapeutic aspects of breast cancer management in clinical settings [9].
4.2. Graphene in Breast Cancer
Several in vitro studies have proved the efficacy of graphene-based nanomaterials in treating breast cancer (Table 1). For example, when subjected to near-infrared (NIR) lasers, GO-SPION-MTX nanocomposites containing graphene oxide, superparamagnetic iron oxide nanoparticles, polyethylene glycol and methotrexate demonstrated significant cytotoxicity against folate receptor-positive breast cancer cells. The LBL Lipo-graph system, which consists of alternating layers of graphene oxide and poly(L-lysine) linked to graphene oxide coated on cationic liposomes containing doxorubicin, successfully kills MD-MB-231 cells following NIR irradiation. Furthermore, HA-GO-Met nanoparticles loaded with metformin and grafted with hyaluronic acid was discovered to cause apoptosis and inhibit the migration of triple-negative breast cancer cells by targeting the miR-10b/PTEN axis through NFκB-p65. These findings demonstrate the effectiveness of graphene-based nanomaterials in improving cell uptake, boosting drug accumulation, and inducing cancer cell death in vitro [9].
In vivo studies demonstrate the therapeutic potential of graphene-based nanoparticles in breast cancer therapy. HA-GO-Met nanoparticles have demonstrated considerable anticancer effectiveness in triple-negative breast cancer cells, both in vitro and in vivo, lowering tumour burden and eradicating tumor-borne toxicity in peripheral organs. The multifunctional CePO4/CS/GO scaffold, which includes graphene oxide nanoparticles, CePO4 nanobars and bioactive chitosan, shows promise in photothermal treatment for killing tumour cells, inducing macrophage polarisation and promoting bone and blood vessel development. This scaffold has the potential to treat breast cancer bone metastases by killing leftover tumour cells and promoting bone repair. These in vivo results suggest the viability of graphene-based techniques to enhance breast cancer treatment outcomes [9].
4.3. Fullerenes in Breast Cancer
In vitro research has shown that fullerenes have the potential to improve breast cancer therapy techniques. Molecular coupling experiments have demonstrated that letrozole, a medication used in breast cancer treatment, has more biological activity when paired with graphene and fullerenes in self-assembled structures than when used alone (Table 1). This synergistic method shows more efficacy in preventing breast cancer cell growth. Additionally, the application of fullerene-based arrays for targeted hyperthermia has been investigated in vitro, with the goal of optimizing therapeutic effects while minimizing injury to neighboring healthy tissues [9].
In vivo studies have found intriguing possibilities for fullerene-based treatments in breast cancer therapy (Table 1). Studies using fullerene-based arrays for targeted hyperthermia have shown controlled super-radiance processes with external electric fields, which might improve treatment accuracy while minimizing collateral harm to healthy tissues. Furthermore, molecular coupling experiments using letrozole, graphene, and fullerenes have demonstrated improved therapeutic efficacy in vivo, indicating a role for fullerene-based techniques in optimizing drug delivery and treatment results for breast cancer. These findings highlight fullerene-based techniques as a possible route for enhancing breast cancer treatment [9].
Table 1. Applications of Carbon Nanomaterials in Breast Cancer.
Method | Result | |
Carbon Nanotubes (CNTs) | • Effective drug loading and concentration-dependent death in breast cancer cells • Preferential internalization into cancer cells for targeted delivery of therapeutic drugs • Detection of Her-2 biomarker for breast cancer diagnosis • Distinguishing breast cancer through volatile metabolite analysis in breath samples | [9,13] |
Graphene | • Significant cytotoxicity against folate receptor-positive breast cancer cells • Killing MD-MB-231 cells following NIR irradiation • Inhibiting migration of triple-negative breast cancer cells • Treating breast cancer bone metastases | [9,14] |
Fullerenes | • Improving breast cancer therapy techniques when paired with graphene and letrozole • Optimizing therapeutic effects while minimizing injury to healthy tissues through targeted hyperthermia • Enhancing therapeutic efficacy in vivo | [9,15] |
5. Clinical Trials
Although laboratory studies indicate the therapeutic role of carbon nanoparticle-based diagnoses and therapeutic approaches in breast cancer cells, future clinical trials are required to verify the role. Currently, a prospective, multicentre study aims to apply nano-carbon to mark preoperative axillary lymph nodes in Breast Cancer Patients before neoadjuvant chemotherapy (ClinicalTrials.gov ID NCT05512468). This clinical trial is currently recruiting patients with invasive breast cancer, and to be treated with neoadjuvant chemotherapy.
6. Conclusion
Research has shown the potential of PTT in selectively targeting and destroying breast cancer cells by using NIR light-absorbing chemicals. Carbon nanomaterials, for example, CNTs and graphene, improve PTT because of their large surface area, good electrical conductivity, and biocompatibility. These materials may absorb NIR light, and cause localized heating, resulting in tumor cell necrosis and death. In vitro and in vivo research showed that carbon nanomaterials improve PTT results and medication distribution and reduce harm to healthy tissues in breast cancer, including CNTs, Graphene, and Fullerenes. The clinical trial to apply PPT to breast cancer is still recruiting patients. Future research should concentrate on improving carbon nanomaterial characteristics and performing clinical studies to confirm their therapeutic potential and incorporation into routine breast cancer treatment regimens.
References
[1]. Alkabban FM, Ferguson T 2022 National Available from: https://www.ncbi.nlm.nih.gov/books/NBK482286/
[2]. World Health Organization. Breast cancer (2024), Available: online at: https://www.who.int/news-room/fact-sheets/detail/breast-cancer
[3]. Blau R, Krivitsky A, Epshtein Y and Satchi-Fainaro R 2016 Drug Resist. Updat. 27 39-58
[4]. Alamdari SG, Amini M, Jalilzadeh N, Baradaran B, Mohammadzadeh R, Mokhtarzadeh A and Oroojalian F 2022 J. Control Release 349 269–303
[5]. Pinto A and Pocard M 2018 Pleura Peritoneum 3
[6]. Oliveira BSA, de Assis ACC, Souza NM, Ferreira LFR, Soriano RN, Bilal M, Iqbal HMN 2021 Life Sci. 279 119667
[7]. Speranza G 2021 Nanomaterials (Basel) 11 967.
[8]. Zaytseva O and Neumann G. 2016 Chemical and Biological Technologies in Agriculture 3
[9]. Orrantia-Borunda E, Acuña-Aguilar LE, Ramírez-Valdespino CA 2022 PubMed, Exon Publications. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK583824/
[10]. Han HS and Choi KY 2021 Biomedicines 9 305
[11]. Hou YJ, Yang XX, Liu RQ, Zhao D, Guo CX, Zhu AC, Wen MN, Liu Z, Qu GF and Meng HX 2020 Int. J. Nanomedicine 15 6827–6838
[12]. Yang HY, Wang YC, Peng HY and Huang CH 2021 Sci. Rep. 11 103
[13]. Abu Lila AS, et al. 2021 J. Drug Deliv. Sci. Technol. 63 102499
[14]. Ge YW, Liu XL, Yu DG, Zhu ZA, Ke QF, Mao YQ, Guo YP and Zhang JW 2021 J. Nanobiotechnology 19 11
[15]. Almuqrin AH, Al-Otaibi JS, Mary YS, Mary YS and Thomas R 2021 J Biomol Struct Dyn 39 5509–5515
Cite this article
Wei,L.X. (2024). Utilizing Carbon Nanomaterials for Enhanced Photothermal Therapy in Breast Cancer Treatment. Theoretical and Natural Science,50,42-47.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2024 Workshop: Workshop on Intelligent Medical Data Analysis for Precision Medicine
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Alkabban FM, Ferguson T 2022 National Available from: https://www.ncbi.nlm.nih.gov/books/NBK482286/
[2]. World Health Organization. Breast cancer (2024), Available: online at: https://www.who.int/news-room/fact-sheets/detail/breast-cancer
[3]. Blau R, Krivitsky A, Epshtein Y and Satchi-Fainaro R 2016 Drug Resist. Updat. 27 39-58
[4]. Alamdari SG, Amini M, Jalilzadeh N, Baradaran B, Mohammadzadeh R, Mokhtarzadeh A and Oroojalian F 2022 J. Control Release 349 269–303
[5]. Pinto A and Pocard M 2018 Pleura Peritoneum 3
[6]. Oliveira BSA, de Assis ACC, Souza NM, Ferreira LFR, Soriano RN, Bilal M, Iqbal HMN 2021 Life Sci. 279 119667
[7]. Speranza G 2021 Nanomaterials (Basel) 11 967.
[8]. Zaytseva O and Neumann G. 2016 Chemical and Biological Technologies in Agriculture 3
[9]. Orrantia-Borunda E, Acuña-Aguilar LE, Ramírez-Valdespino CA 2022 PubMed, Exon Publications. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK583824/
[10]. Han HS and Choi KY 2021 Biomedicines 9 305
[11]. Hou YJ, Yang XX, Liu RQ, Zhao D, Guo CX, Zhu AC, Wen MN, Liu Z, Qu GF and Meng HX 2020 Int. J. Nanomedicine 15 6827–6838
[12]. Yang HY, Wang YC, Peng HY and Huang CH 2021 Sci. Rep. 11 103
[13]. Abu Lila AS, et al. 2021 J. Drug Deliv. Sci. Technol. 63 102499
[14]. Ge YW, Liu XL, Yu DG, Zhu ZA, Ke QF, Mao YQ, Guo YP and Zhang JW 2021 J. Nanobiotechnology 19 11
[15]. Almuqrin AH, Al-Otaibi JS, Mary YS, Mary YS and Thomas R 2021 J Biomol Struct Dyn 39 5509–5515









