1. Introduction
Acute Myeloid Leukemia (AML), a malignancy marked by its clinical and genomic heterogeneity, diverges from other cancers through its relatively sparse genomic mutation landscape. Among the mutations identified in AML, alterations within the nucleophosmin (NPM1) gene, are notably prevalent at the kinase level [1].
The genesis and progression of AML are significantly influenced by mutated bone marrow cells of the myeloid lineage or progenitors. These cells, through a mechanism akin to expropriation, usurp the properties of normal hematopoietic stem cells, thereby initiating the leukemic transformation. This process involves the mutated cells co-opting the self-renewal and proliferative capabilities of hematopoietic stem cells, resulting in the unchecked expansion of leukemic cells. Consequently, the normal function of hematopoietic cells is suppressed, contributing to the clinical manifestations and complications characteristic of AML, such as anemia, bleeding, and susceptibility to infections [1].
In the contemporary landscape of AML therapeutics, allo-HSCT remains the singular modality with curative potential. Despite its therapeutic efficacy, the persistence of long-term adverse effects and the incidence of disease recurrence post allo-HSCT represent significant clinical hurdles. In light of these limitations, researchers have turned their investigative focus towards adoptive immunotherapies as a promising avenue for the management of hematological malignancies. Among these novel approaches, chimeric antigen receptor (CAR) T-cell therapy has gained substantial interest and recognition for its potential to address the unmet needs in AML treatment [2]. This review elaborates the current challenges and future prospects for CAR T cell therapy with AML.
2. Structure and classification of CAR
Comprising an extracellular antigen-binding domain, an outside of cells spacer, a transmembrane domain, and intracellular domains for T cell activation, CARs are synthetic proteins that, upon binding to their target antigen, elicit anti-tumor activity. A typical intracellular domain consists of a CD3ζ activation signal, both of these signals are activated upon antigen binding, enabling the T cell to effectively function as a "tumour killer" [3].
In 1989, the 1st-generation CARs were collaborative discovered by Zelig Eshhar et al., T cell lymphocytes were engineered by introducing a splicing variant of the variable regions of an antibody's heavy and light chains into T cells, along with the constant region of the T cell receptor (TCR), have been found to possess certain limitations in their functionality. These CARs, consisting of a singular intracellular domain primarily derived from the CD3ζ subunit, are capable of activating T cells. However, due to the simplicity of their signaling architecture, they are not adept at priming naive T cells or sustaining persistent T cell activity and cytokine production over time. This limitation is attributed to their insufficient signaling capacity, indicating that subsequent generations of CARs may require more complex signaling domains to achieve improved therapeutic outcomes in cancer immunotherapy [4].
In 2013, Ritchie et al. published the inaugural report of clinical trial that demonstrate the biological activity the 2nd-generation CARs were applied [5].
The 3rd generation of CARs is characterized by the incorporation of two intracellular co-stimulatory signaling domains. These additional domains are engineered to amplify the therapeutic response and targeting efficacy against malignancies [3].
The 4th generation of CARs, often designated as TRUCKs, builds upon the 2nd generation design by integrating additional features. These enhancements are specifically designed to optimize the therapeutic index of CAR-T cell therapy, as evidenced by reduced systemic toxicity in preclinical studies. The incorporation of cytokine expression within 4th generation CAR-T cells allows for the release of cytokines that can attract and activate additional immune system components, potentially improving the overall anti-tumor response while minimizing side effects [3].
The 5th generation of CARs, while preserving the foundational architecture of the 2nd generation, introduces a more complex signaling cascade. This advanced design allows for simultaneous activation of the TCR, CD28 domain, and cytokine signaling pathways in response to antigen engagement. The integration of these multiple signaling pathways is aimed at more accurately mimicking the physiological activation of T-cells, thereby enhancing their functionality and persistence within the tumor microenvironment. The concurrent activation of these pathways in 5th generation CARs is hypothesized to improve the specificity and potency of T-cell responses, potentially leading to more effective tumor eradication with reduced off-target effects, thus advancing the field of adoptive cell therapy [3]. The development of five generations of CAR is shown in figure 1.
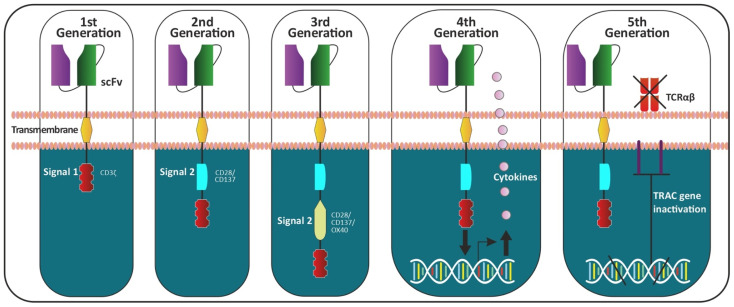
Figure 1. The development of five generations of CAR [5].
3. Current challenges in CAR-T therapy for AML
3.1. Heterogeneousness of AML
AML is divided into six specific groups: AML with recurrent genetic abnormalities, denoting instances marked by particular genetic alterations, AML with MRC, involving cases with features linked to myelodysplastic syndromes, t-MN, encompassing AML that develops subsequent to previous treatment with chemotherapy or radiation, AML, not otherwise specified (NOS), a catch-all category for cases that do not align with other defined subtypes, Myeloid sarcoma, characterized by solid tumors comprised of myeloid blasts, Myeloid proliferations related to Down syndrome (DS), a classification exclusive to individuals with Down syndrome who are diagnosed with AML. This systematic classification aids in the comprehensive understanding of AML's various forms, each bearing unique characteristics and clinical significance [6,7].
At present, on the basis of cytogenetic and molecular mutations, AML is categorized into heterogeneous groups. During the development of AML, the most commonly engaged genomics in the process which include transcription factors, epigenetic pathways modifiers and cell kinases etc. (figure 2) [6]. Mutations at the transcription factor level include RUNX1-RUNX1T1/(RUNX1-ETO), CEBPA and GATA-1. IDH1/2 and TET2 mutations, DNMT3A and Mixed lineage leukemia (MLL); 11q23 translocation, are mutations at the epigenetic pathways’ modifiers level. Mutations at the kinases level, which include FTL-3 mutations, nuclear pore protein-nucleophosmin.
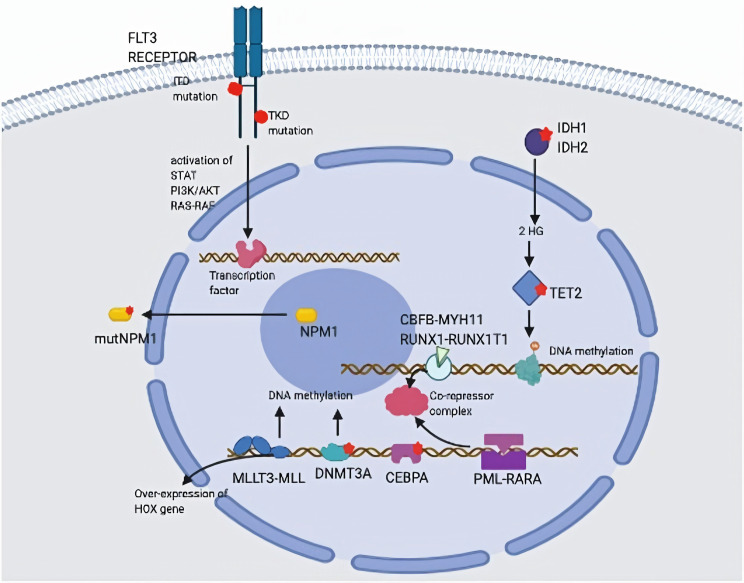
Figure 2. The heterogeneous of AML [6].
3.2. Toxicities
Despite the promising success rates of CAR-T therapy, it is accompanied by significant toxicities that pose considerable challenges [8,9]. In the toxic side effect of CAR-T therapy, CRS is the most common one, express as a type of systemic inflammatory response, occurred during the activating and expanding of CAR-T cell in patients’ body, it is considered have relation to the secretion of inflammatory cytokines during the interacting of target cells and immune effector cells, this phenomenon could lead to multiorgan dysfunction and destabilizes membrane stability [10].
ICANS is a serious neurological adverse event associated with the use of CAR-T cell therapy. Its pathogenesis involves inflammatory cytokine release, leading to neuroinflammation and potential damage to neuronal tissue [11]. Furthermore, Monocytes play a crucial role in the pathogenesis of ICANS by secreting IL-1 and IL-6, contributing to the activation of myeloid cells and the breakdown of the BBB. GM-CSF released during CAR-T cell therapy further exacerbates this process. These factors lead to the development of ICANS, necessitating careful management and the exploration of targeted therapies to reduce neurological toxicity [6].
3.3. Tumour microenvironment
The tumor microenvironment (TME) is a huge challenge for CAR-T cell therapy efficacy. It obstructs CAR-T cell trafficking, alters their metabolism. Strategies to enhance CAR-T cell function in the TME include co-administration of immunomodulatory agents and genetic modifications to resist TME-induced suppression, aiming to improve therapy outcomes [12].
The immunosuppressive tumour microenvironment (TME) is characterized by the preferential accumulation of Tregs, MDSCs, and TAMs, which collectively orchestrate a suppressive milieu that impedes immune responses against tumours [12]. Tregs, in particular, actively participates in inhibiting the cytotoxic activity of T cells through 4 four approaches roughly, respectively are Cytokine Secretion, Tregs secrete immunoregulatory cytokines which exert suppressive effects on cytotoxic T lymphocytes (CTLs), thereby curtailing their antitumor activities. IL-2 Consumption, by competitively utilizing IL-2, Tregs limit its availability for other immune cells, thus restraining T cell proliferation and function. CTLA-4-Mediated Suppression, the high expression of CTLA-4 on Tregs allows them to bind with higher affinity to CD80/CD86 on antigen-presenting cells (APCs) than CD28, effectively inhibiting T cell activation and blunting the immune response. Suppression of APCs, Tregs can also indirectly suppress APCs, including dendritic cells, through CTLA-4 interactions, which results in reduced presentation of tumour antigens to T cells and dampens the overall antitumor immune response.
Because of MDSCs have been shown a strong immunosuppressive capability which targeting effector T cells directly, so the consequence of immunosuppressive which mediate by MDSCs is serious. Tumour-associated macrophages (TAMs) are abundant in the TME and suppress T cell activity. They secrete cytokines and enzymes, which deplete amino acids, hindering T cell function. TAMs also aid in the recruitment of regulatory T cells (Tregs), enhancing the TME's immunosuppressive effects. Targeting TAMs is a promising strategy to improve cancer therapies [12].
4. CAR-T Cell Therapy in AML
Currently, anti-CD19 CAR-T cell therapies have been integrated into clinical practice for the treatment of B-lineage malignancies. However, the therapeutic landscape for AML presents a distinct set of challenges. Unlike lymphoid malignancies, the antigens targeted by ADCs, bispecific antibodies, and CAR-T cells in AML are often shared with normal HSPCs and healthy tissues. This overlap increases the risk of on-target, off-tumour toxicity, a significant concern that necessitates careful consideration in treatment planning. The primary aim is to eliminate chemorefractory (residual) AML cells, thereby reducing the risk of relapse, while simultaneously mitigating the potential for profound and prolonged cytopenia. This strategy aims to harness the robust antileukemic capabilities of CAR-T cell therapy, ensuring a reduction in residual disease burden while safeguarding hematopoietic function [13].
In a 2021 clinical trial, an innovative approach targeting autologous CD123-specific CAR-T cells was employed for patients with R/R AML and BPDCN. Prior to the administration of CAR-T cells, a lymphodepletion regimen was implemented, involving a three-day course of fludarabine (25-30 mg/m²) and cyclophosphamide (300 mg/m²) for three days. Patients who met the stringent safety and eligibility criteria and demonstrated ongoing CD123+ disease post-lymphodepletion were eligible for a single dose of CD123-targeted CAR-T cells. In cases where necessary, a second infusion was considered. In the most recent iteration of the trial, seven patients (six with AML) were administered CD123-CAR-T cells. The outcomes were promising, with one patient achieving a complete response (CR) and subsequently proceeding to a second allo-HSCT. Another patient, who had already attained CR prior to the trial, maintained this response post-therapy and successfully underwent a transplant. Notably, two patients showed significant reduction in blasts, including one who achieved a morphologic leukemia-free state (MLFS). Cytokine release syndrome (CRS) was observed in five patients. Of these, four experienced grade 1 symptoms, while one patient exhibited grade 2 symptoms. Importantly, all toxicities were reversible and effectively managed without the occurrence of treatment-related cytopenia [13].
5. Promising strategies to overcome challenges
5.1. Minimizing toxicity
Based on Boolean logic, researchers proposed the multi-antigen-targeting strategy [3]. These receptors are ingeniously designed to distinguish between two distinct antigens, a feature that empowers T cells to differentiate between target tumour cells and healthy cells. A strategic approach involves the implementation of two independent receptors on the T cell surface, each with a distinct role in activation and co-stimulation. One receptor is equipped with an endodomain derived from CD3ζ, crucial for providing the necessary signals for T cell survival, proliferation, and function. When human T cells are engineered with this dual chimeric receptor system, they exhibit potent cytotoxic activity against tumour cells and achieve an efficient reduction in tumour burden. Through this dual-targeting approach, T cells are activated and co-stimulated to effectively eradicate cancer cells while minimizing damage to healthy tissues. This study not only demonstrates the efficacy of dual chimeric receptors in cancer immunotherapy but also contributes to the development of the "tumour sensing" theory. This theory posits that engineered T cells can effectively sense and respond to the presence of tumours by recognizing multiple tumour-associated antigens, thereby enhancing their specificity and reducing off-target effects [3]. In comparison to the single antigen, tumour which have both antigens will be selectively eliminated, this method will help preventing the off-tumour side effects. Fundamentally, dual-receptor CAR-T cells are meticulously engineered to be activated and armed only upon the simultaneous engagement of two specific antigens, embodying the principle of an 'AND-gate'. This sophisticated design ensures that the CAR-T cells remain dormant and inactive until they encounter cells expressing both targeted antigens, thereby significantly enhancing specificity and reducing the risk of on-target, off-tumor toxicity. Intricate experimental strategies have been developed to target dual antigens using synNotch receptors in conjunction with CARs (dual CAR-T cells). The synNotch receptor, upon binding to its cognate ligand, triggers a cascade, effectively enabling a synergistic and selective activation mechanism. This approach ensures that the CAR-T cells are not only activated in the presence of both antigens but also that the activation process is amplified when both antigens are bound, further enhancing the selectivity and efficacy of the therapy. The apply of dual-targeting CAR-T cell therapy aims to mitigate the issue of on-target, off-tumor toxicity, which arises when CAR-T cells mistakenly target. The synNotch-enhanced dual CAR-T cell approach represents a novel strategy that could enhance the specificity and effectiveness of CAR-T cell therapies. This approach involves engineering T-cells to express two different CARs, each recognizing a distinct tumor antigen, along with a synNotch receptor that can be activated by a tumor-associated antigen. This dual-targeting approach has the potential to improve the therapeutic index by reducing the likelihood of off-target toxicity while enhancing the ability to eliminate tumor cells, even in cases where the tumor antigen is also expressed on normal cells [3].
5.2. Extensible CARs
Because of the high heterogeneity of antigen expression observed in AML cells, it is evident that combinatorial targeting strategies to enhance the efficacy of therapeutic interventions. A modular Ab-based system was applied in the design of a flexible and programmable adaptor, RevCAR platform [10,11]. Reversible Chimeric Antigen Receptors (RevCARs) employ a distinct mechanism, where they do not engage tumour antigens directly. Instead, their functionality is orchestrated through interactions with bispecific target modules, referred to as RevTMs. This dual-targeting capability enables the RevTM to act as a molecular bridge between RevCAR-expressing T cells and tumor cells, facilitating T cell activation against the tumour. The modular design of the RevTM acts as a regulatory mechanism. By adjusting the dose of RevTMs administered, the activity of the RevCAR T cells can be effectively turned on or off, providing a level of safety and control that is not typically associated with traditional CAR-T cell therapies. This feature is particularly advantageous in managing potential toxicities or in cases where the therapy needs to be modulated based on the clinical response [3].
5.3. Application of CAR-NK Cells
For several decades, CAR-T cell therapy has been established as a viable treatment option for various hematological malignancies. However, the persistent challenge of managing the toxic side effects. Consequently, an increasing number of researchers are turning into the explore of the potential of genetically modified NK cells as an alternative CAR immune effector cell option. These cells have the capacity to traverse HLA barriers without the adverse effects previously observed [9]. In the comparation with the CAR-T cell, CAR-NK cells are less toxic, so it has a low pathogenicity for CRS and ICANS, CAR-NK cell also has a lower risk of off-target effects, enhanced immunosuppressive effect which caused by AML cells etc [9].
6. Conclusion
Acute Myeloid Leukemia (AML), a malignancy marked by its clinical and genomic heterogeneity, diverges from other cancers through its relatively sparse genomic mutation landscape, it will express resistant to treatments over time, so it’s in an urgent need to discover a type of effective treatment modalities with low side effects in order to achieve deep and sustainable remissions. More and more immunotherapies will be applied to the treatment AML.
References
[1]. Zarka J, Short NJ, Kanagal-Shamanna R, Issa GC. Nucleophosmin 1 Mutations in Acute Myeloid Leukemia. Genes (Basel). 2020 Jun 12;11(6):649.
[2]. Xiao Zhiwen, Yu Min, Li Fei. Research and application progress of CAR-T cell therapy in acute myeloid leukemia[J].Chinese Journal of Clinical Oncology,2022,49(20):1052-1055.
[3]. Shao Ruonan, Xin Honglei, Shi Xiaofeng. Target selection for CAR-T therapy for acute myeloid leukemia[J].Chinese Journal of Experimental Hematology,2024,32(03):965-969.
[4]. Peng Zhengkang, Huang Yuting, Ren Rui, et al.Research progress in the field of CAR-T cell therapy for tumors[J].Pharmaceutical Biotechnology,2024,31(03):325-330.
[5]. Vishwasrao P, Li G, Boucher JC, Smith DL, Hui SK. Emerging CAR T Cell Strategies for the Treatment of AML [J]. Cancers (Basel). 2022,14(5):1241.
[6]. Rahul E, Goel H, Chopra A, Ranjan A, Gupta AK, Meena JP, Bakhshi S, Misra A, Hussain S, Viswanathan GK, Rath GK, Tanwar P. An updated account on molecular heterogeneity of acute leukemia [J]. Am J Blood Res. 2021,15;11(1):22-43.
[7]. Hwang SM. Classification of acute myeloid leukemia. Blood Res. 2020 Jul 31;55(S1):S1-S4.
[8]. Li Feng, Yang Feifei, Xu Yanli. Recent research progress on immune heterogeneity in leukemia microenvironment[J]. Chinese Journal of Experimental Hematology,2023,31(05):1569-1573.
[9]. Zhang Li, Qin Chunjie. Research progress on targeted therapy for acute myeloid leukemia[J].Jilin Medical Journal,2024,45(03):714-717.
[10]. Li Chunmin, Li Yu, Yuan Zhongtao, et al. The control effect of dasatinib on CD123-targeting CAR-T in the treatment of AML and adverse reactions: a case report and literature review[J].Journal of Army Medical University,2024,46(04):347-351.
[11]. Chen Mengmeng, Fu Huali, Kan Slowly, et al. Research progress on CAR-T therapy based on tumor microenvironment[J].Chemistry of Life,2024,44(01):95-101.
[12]. Zhou Qian, Cui Lijuan. Progress in targeted therapy for acute myeloid leukemia[J]. Ningxia Medical Journal,2024,46(04):360-364.
[13]. Jiang Huihui, Shen Zhenming, Wang Chunjie, et al.Research status and prospect of CAR-T cells[J].Systems Medicine,2019,4(12):183-185.
Cite this article
Song,B. (2024). Emerging Role of CAR-T Therapy in Acute Myeloid Leukemia. Theoretical and Natural Science,50,48-53.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2024 Workshop: Workshop on Intelligent Medical Data Analysis for Precision Medicine
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Zarka J, Short NJ, Kanagal-Shamanna R, Issa GC. Nucleophosmin 1 Mutations in Acute Myeloid Leukemia. Genes (Basel). 2020 Jun 12;11(6):649.
[2]. Xiao Zhiwen, Yu Min, Li Fei. Research and application progress of CAR-T cell therapy in acute myeloid leukemia[J].Chinese Journal of Clinical Oncology,2022,49(20):1052-1055.
[3]. Shao Ruonan, Xin Honglei, Shi Xiaofeng. Target selection for CAR-T therapy for acute myeloid leukemia[J].Chinese Journal of Experimental Hematology,2024,32(03):965-969.
[4]. Peng Zhengkang, Huang Yuting, Ren Rui, et al.Research progress in the field of CAR-T cell therapy for tumors[J].Pharmaceutical Biotechnology,2024,31(03):325-330.
[5]. Vishwasrao P, Li G, Boucher JC, Smith DL, Hui SK. Emerging CAR T Cell Strategies for the Treatment of AML [J]. Cancers (Basel). 2022,14(5):1241.
[6]. Rahul E, Goel H, Chopra A, Ranjan A, Gupta AK, Meena JP, Bakhshi S, Misra A, Hussain S, Viswanathan GK, Rath GK, Tanwar P. An updated account on molecular heterogeneity of acute leukemia [J]. Am J Blood Res. 2021,15;11(1):22-43.
[7]. Hwang SM. Classification of acute myeloid leukemia. Blood Res. 2020 Jul 31;55(S1):S1-S4.
[8]. Li Feng, Yang Feifei, Xu Yanli. Recent research progress on immune heterogeneity in leukemia microenvironment[J]. Chinese Journal of Experimental Hematology,2023,31(05):1569-1573.
[9]. Zhang Li, Qin Chunjie. Research progress on targeted therapy for acute myeloid leukemia[J].Jilin Medical Journal,2024,45(03):714-717.
[10]. Li Chunmin, Li Yu, Yuan Zhongtao, et al. The control effect of dasatinib on CD123-targeting CAR-T in the treatment of AML and adverse reactions: a case report and literature review[J].Journal of Army Medical University,2024,46(04):347-351.
[11]. Chen Mengmeng, Fu Huali, Kan Slowly, et al. Research progress on CAR-T therapy based on tumor microenvironment[J].Chemistry of Life,2024,44(01):95-101.
[12]. Zhou Qian, Cui Lijuan. Progress in targeted therapy for acute myeloid leukemia[J]. Ningxia Medical Journal,2024,46(04):360-364.
[13]. Jiang Huihui, Shen Zhenming, Wang Chunjie, et al.Research status and prospect of CAR-T cells[J].Systems Medicine,2019,4(12):183-185.









