1. Introduction
Cancer, as one of the major causes of mortality globally, has a subsequential financial impact on healthcare systems across the world. By 2040, these numbers are projected to increase to 29.9 million new diagnoses and 15.3 million deaths [1]. Among various cancer treatments, immunotherapies stand out as a revolutionary approach, utilizing the patient’s own immune system to identify as well as target cancer cells. Immunotherapies include monoclonal antibodies, checkpoint inhibitors, cytokines, CAR T-cell therapy, and cancer vaccines [2]. Similar to normal vaccines that work against infectious diseases, the cancer vaccine as a type of immunotherapy mainly targets tumor-specific antigens (TSAs) and tumor-associated antigens (TAAs) to elicit antitumor immunity [2]. TSAs are proteins expressed uniquely among cancer cells, whereas TAAs are presented in both tumor and normal cells, but typically in a smaller amount in healthy cells [3]. Cancer vaccines could have a transformative impact on cancer treatment due to their abilities to prevent cancer relapse, attack leftover cancer cells after other treatments ended, stop a tumor from growing or metastasizing, etc. [3].
Virus-based cancer vaccines can leverage both innate and adaptive immune responses for effective and long-lasting antitumor immunity [4]. Virus-based cancer vaccines can be further divided into a few categories: inactivated, live attenuated, and subunit viral vaccines that target oncogenic viruses, oncolytic viral vaccines that selectively attack tumor cells, and viral vector vaccines that mainly use a modified virus as a vector to deliver the antigens into the body [5]. An example of the first FDA-approved oncolytic virus-based cancer vaccine, Talimogene laherparepvec (T-VEC), remains the most striking one that is used to treat advanced melanoma that has metastasized or is inoperable [6].
This review summarizes the mechanisms of the three different forms of virus-based cancer vaccines, clinical applications and mechanism of T-VEC and other virus-based cancer vaccines that are currently undergoing clinical trials.
2. Mechanism of virus-based cancer vaccines
2.1. Inactivated, live attenuated, and subunit vaccines
Inactivated, live attenuated, and subunit cancer vaccines are typically used against viruses that can lead to cancer. Approximately 10-12% of total cancer diagnoses are likely associated with virus infections, and it is estimated that there would be a 19% decrease in cancer cases in developing countries and a 3.8% decrease in developed countries if virus-associated cancers are prevented [7]. Understanding specific viruses that are responsible for certain cancers is crucial for developing and applying virus-based cancer vaccines. Cancerogenic viruses include human papillomavirus (HPV) for cervical cancer, hepatitis B and hepatitis C for liver cancer, Epstein-Barr virus (EBV) for nasopharyngeal carcinoma, human herpesvirus 8 (HHV-8) for Kaposi’s sarcoma, Merkel cell polyomavirus (MCV) for Merkel Cell Carcinoma, etc. [7].
Inactivated cancer vaccines are made from viruses that are killed or inactivated thus they are no longer disease causing [7]. After vaccine administration, the immune system can recognize the virus even without an active infection, and the antigen-presenting cells (APCs) such as the dendritic cells can then process the viral particles and present viral antigens on their cell surface which subsequently results in T-cell and B-cell activation, production of antibodies, and formation of memory cell which can enable the immune system to respond to the virus more timely and effectively when exposed to these cancer-causing virus again [7]. The maintenance time of immunity is usually short and multiple doses are generally required for inactivated virus vaccines [8]. Live attenuated virus vaccines, on the other hand, usually contain weakened viruses that are obtained by reverse genetics or adaption. They were developed to obtain a higher immunogenicity than inactivated virus vaccines by mimicking live pathogen infections [8]. With the development of new technologies, subunit cancer vaccines aim to stimulate immune response without using the whole virus, instead, it uses carefully selected antigen-specific protein or peptides from the virus to induce stronger and more specific immune response as they are designed to improve purity, safety, and stability [8]. Subunit vaccines against cancer target specific TSAs or TAAs, introduce these antigens to the immune system, thus training them to recognize and attack tumor cells to limit tumor growth and progression [8].
2.2. Oncolytic virus vaccines
Oncolytic virus vaccines generate anti-tumor effects by directing lysing cancer cells and stimulating host’s antitumor immunity [9]. They are therapeutic cancer vaccines that only infect and kill cancer cells through the use of native or genetically modified viruses, and the oncolysis ability of the virus is dependent on various factors, for example, viral replication ability in tumor cells, efficiency at targeting cell receptors, and the antiviral mechanism of host cell. [9].
Following administration, antiviral pathways can be triggered in healthy cells via local interferon (IFN) release and Toll-like receptors (TLRs), which can identify pathogen-associated molecular patterns (PAMPs). Subsequently, a cascade involving TNF (Tumor Necrosis Factors)-associated factor 3 (TRAF3), interferon-related factors IRF2 and IRF7, and retinoic acid-inducible gene 1 (RIG-1) is initiated, leading to antiviral response [9]. Additionally, the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway is involved as well to coordinates the antiviral response, and this results in the activation of protein kinase R (PKR), which promotes virus clearing by inhibiting protein synthesis and promoting rapid cell death [9]. Cancer cells, on the other hand, often have defects in antiviral pathways such as impaired IFN signalling pathways, and limited activity of virus particle detection by TLRs and RIG-1 [9]. After oncolytic virus infection, cancer cells generate Reactive Oxygen Species (ROS) and cytokines. Then cancer cells further enhance the activation of immune cells [4]. Consequently, tumor cells exhibit a diminished capacity to detect and respond to viral infections, rendering them more vulnerable to viral infection and replication compared to healthy cells. Therefore, oncolytic cancer vaccines can kill cancer cells directly through viral replication and lysis [9].
Furthermore, the induction of systemic antitumor immunity is crucial for tumor eradication in the body, at both injected and uninjected sites. Following lysis of tumor cells, TAAs are released by the tumor cells, which can promote adaptive immune response and mediate tumor regression at distant sites in the body [4]. Additionally, the death of cancer cells leads to the release of viral PAMPs and cellular danger-associated molecular pattern signals (DAMPs) such as heat shock proteins and high mobility group box 1 (HMGB1) [9]. Moreover, cytokines including type I IFNs, TNF, IFN, and interleukin (IL)-12 are also released. These factors facilitate the maturation of dendritic cells and other APCs, followed by stimulating antigen-specific CD4+ and CD8+ T cell responses. Upon activation, CD8+ T cells proliferate and differentiate to cytotoxic effector cells that can migrate to distant tumor locations in the body, where they can recognize and eliminate tumor cells upon antigen recognition [9]. Therefore, oncolytic virus cancer vaccines work by directly destroying cancer cells at the site of injection and by inducing the host’s anti-tumor immunity to attack the tumor at distant sites [9].
2.3. Viral vector vaccines
Viral vector cancer vaccines are vaccines that use a viral vector to deliver tumor antigens into the body and cause a series of anti-tumor immune response. Most viruses have immunogenic properties by nature and could be made to express tumor antigen transgenes thus inducing a robust anti-tumor immune response [10]. Viral vector cancer vaccines are engineered to express TSAs by inserting antigen-encoding genes into the viral genome, and these recombinant viruses can infect professional APCs, such as the dendritic cells, and make them express the viral transgene [10]. The APCs can then process and present tumor antigens on their cell surfaces through major histocompatibility complex (MHC) molecules and subsequently activate CD8+ cytotoxic T cells that recognize and eliminate cancer cells that express the same antigens. Through enhancing the tumor antigen presentation to the immune system, a higher frequency and stronger binding affinity of cytotoxic T cells can be obtained, thus leading a targeted immune response against the cancer [10]. Compared with other traditional antigen administration methods, viral vector cancer vaccines generate a stronger immune response due to the pro-inflammatory environment created by viral protein expression and their ability to increase both the frequency and avidity of cytotoxic T cells [10]. Furthermore, memory T cells are also generated to achieve long-term recognition and immunity against the tumor antigens, which is proven beneficial in the prevention of cancer recurrence [10].
There are several viruses that are commonly used as vectors for viral vector cancer vaccines, such as adenoviruses, poxviruses, and alphaviruses [11]. Adenoviruses are non-enveloped viruses with double-stranded DNA, with a genome size ranges from 25 to 48kpb. They are often engineered in cancer vaccines due to their ability to infect a wide ranges of cell types, carry large genetic payloads, and induce strong immune responses [11]. Poxviruses are double-stranded viruses with linear DNA. Among them, the vaccinia virus, with a size of approximately 190 kbp, stands out due to its substantial capacity for foreign gene insertion. It is particularly notable for its role in eradicating smallpox [10]. Benefits of using poxviruses as vectors in cancer vaccines include their broad host range, stable recombinant formation, accuracy in replicating, and proficient post-translational processing of inserted genes [10]. Alphaviruses are positive single-stranded RNA viruses that allows for increased antigen expression via rapid replication within host cells, which is crucial for stimulating a strong immune response against tumor cells [11]. The replicon system is one of the key technologies that are often used in alphavirus-based cancer vaccines. The replicon RNA can replicate within the host cells without producing infectious viral particles, thus avoiding the production of neutralizing antibodies towards the vector, and allowing for multiple injections [10]. Different viral vectors are used in cancer vaccines due to their unique properties and advantages, such as the specific cell types they can infect, efficiency in delivering genetic materials, capacity to induce immune response, and the level of safety for the individual. Table 1 highlights the components and features of the five different types of virus-based cancer vaccines.
Table 1. Summary of various types of virus-based cancer vaccines.
Type of vaccine | Components | Features | Reference(s) |
Inactivated vaccine | Viruses that are killed or inactivated | - Short immune maintenance - require multiple doses - lower immunogenicity | [7] |
Live attenuated vaccine | Weakened viruses obtained from reverse genetics or adaption | - mimic live pathogen infections - higher immunogenicity | [8] |
Subunit vaccine | Whole viruses with antigen-specific protein or peptides | - higher immunogenicity - induce more specific immune response - improved purify, safety, and stability | [8] |
Oncolytic virus vaccine | Genetically modified viruses that selectively target cancer cells | - triggers antiviral pathways in healthy cells so they stay unharmed - viral replication and lysis in cancer cells - induce local and distal immune responses | [9] |
Viral vector vaccine | Recombinant viruses (such as adenoviruses, poxviruses, alphaviruses) that encode tumor antigens in their genome | - infect antigen-presenting cells (APCs) and make them express viral transgene - high efficiency in delivering genetic materials - strong immune response - high levels of safety | [10,11] |
3. Clinical Applications
3.1. T-VEC
FDA approved T-VEC as a virus-based therapeutic cancer vaccine for the treatment of melanoma that is unresectable, metastasized, or recurrent after initial surgery in 2015 [6]. It is an oncolytic virus cancer vaccine that mediates both local and systemic anti-tumor immunity [6]. T-VEC uses a genetically modificated type I herpes simplex virus that has functional deletions of neurovirulence factors ICP34.5 and ICP47 which reduce the viral pathogenicity and ensure target selectivity, and an additional insertion of the GM-CSF gene [12]. While infecting both healthy and cancer cells, this non-pathogenic virus selectively replicates and secrete granulocyte-macrophage colony-stimulating factor (GM-CSF) in cancer cells till the they lyses, and subsequently releases more viruses, tumor antigens, and GM-CSF [12]. GM-CSF attracts more APCs, specifically dendritic cells, to site and enhances their antigen presentation ability, which then expresses tumor antigens to T cells and prime them to initiate a local tumoricidal effect [12]. Moreover, these primed T cells can circulate to different parts of the body, attack the cancer cells, and trigger distant immune response. Therefore, efficacies have been observed at injection site as well as distant metastasis in other organs [12]. Figure 1 illustrates the dual mechanisms of T-VEC, showcasing how it selectively infects and lyses cancer cells while also stimulating a robust immune response.
The T-VEC vaccine can be injected directly into metastatic melanoma nodules, and the following doses can be administered after three weeks, and once every two weeks after the second dose till no treatable lesions remain [12]. It has shown effectiveness in treating advanced melanoma. In a phase I study, T-VEC demonstrated expected biological activities and a mild and tolerable toxicity profile, mainly fatigue, chills, pyrexia, and nausea. Researchers also conducted a phase II study with 50 patients with advanced melanoma and achieved a 26% Overall Response Rate (ORR), observing durable responses at both injection and non-injection sites. In a phase III study conducted with 436 patients, T-VEC confirmed higher durable response rate (19.3%) and Overall Survival (OS; 23.3 months) comparing to patients who were treated with GM-CSF therapy (durable response rate 1.4%, OS 18.9 months) [12]. Another phase II clinical trial (NCT02211131) illustrated that T-VEC has a superior Relapse-Free Survival (RFS; 22.3%) and OS (77.3%) at 5 years comparing to surgery alone (RFS 15.2% and OS 62.7%) [13]. These findings demonstrate that injections of oncolytic T-VEC cancer vaccines directly into the tumor sites can produce a significant and lasting systemic effect [13]. Additionally, combination of T-VEC and other therapies are being investigated and tested as some of the early findings demonstrated encouraging results. Studies found that combining T-VEC with immune checkpoint inhibitors like pembrolizumab (anti-PD-1) or ipilimumab (anti-CTLA-4) resulted in increased tumor-specific CD8+ T cells and greater systemic efficiency [12].
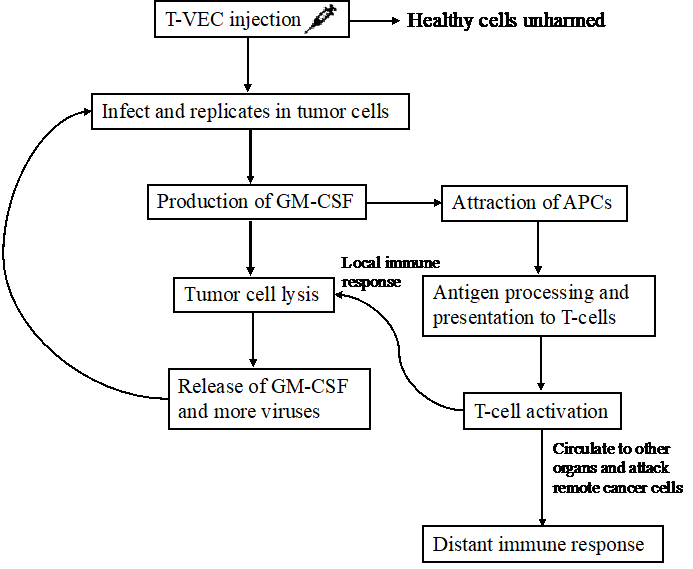
Figure 1. The mechanism of Talimogene laherparepvec (T-VEC). The T-VEC vaccine is injected directly at the tumor site; the modified virus then selectively replicates in tumor cells only followed by leaving the healthy cells unharmed. Post-infection, the granulocyte-macrophage colony-stimulating factor (GM-CSF) gene encoded T-VEC promotes the secretion of GM-CSF, which can attract more antigen-presenting cells (APCs), for example, dendritic cells, to the site of injection. The replication and production of GM-CSF subsequently leads to tumor cell lysis, resulting in the release of GM-CSF and more viruses, which can then attract more APCs and infect more tumor cells. APCs process and present tumor antigens to T-cells and activate them to attack tumor cells both locally by secreting cytotoxic contents and distally by circulating to other organs and triggering distant immune responses that eliminate remote tumor cells. Figure credit: original.
3.2. Other virus-based vaccines
Several virus-based cancer vaccines are under clinical trials to further investigate their efficacy and safety profiles.
Pexa-Vec, also known as JX-594, targets advanced hepatocellular carcinoma as an oncolytic vaccinia virus vaccine. It is designed to selectively replicate and destroy cancer, causing tumor cell lysis, disruption of tumor vascularization, and initiation of anti-tumor immunity [14]. In a global phase III randomized open-label clinical trial study (NCT02562755), 456 participates were recruited and were given multiple intratumoral injections of Pexa-Vec every two weeks at a dosage of 109 pfu [14]. This phase III trial aim to confirm the efficacy and safety of Pexa-Vec in a larger population, although still pending results, earlier phase clinical trials (NCT00554372) have demonstrated its ability to induce anti-tumor activity and improve OS in patients with advanced hepatocellular carcinoma with manageable side effects mainly consist of flu-like symptoms [15].
PROSTVAC-VF is another promising therapeutic virus-based cancer vaccine designed to treat prostate cancer, it is a viral vector vaccine that are engineered to express prostate-specific antigen (PSA) which is a protein commonly found on prostate cancer cells and make it a target for immune attack [16]. In a randomized, controlled, and blinded phase II study (NCT00078585), 125 patients with minimally symptomatic castration-resistant metastasized prostate cancer (mCRPC) were recruited. They were divided into two groups: one received PROSTVAC-VF plus GM-CSF, while the other received injection of control empty vectors and saline [16]. While larger scale phase III studies are ongoing and pending results, results from this phase II study showed an 8.5-month increase in median OS (25.1 for PROSTVAC vs. 16.1 months for control) and a 44% decrease in lethal rate, demonstrating a survival benefit for men with mCRPC [16].
In addition to Pexa-Vec and PROSTVAC, there are numerous other virus-based cancer vaccines in ongoing clinical trials for the treatment of different cancers, highlighting the ongoing efforts to expand and improve cancer immunotherapy.
4. Conclusion
Virus-based cancer vaccines represent a novel approach to cancer treatment by leveraging the natural properties of different viruses to stimulate the anti-tumor immunity of the body. In this review, the mechanisms and clinical applications of various types of virus-based cancer vaccines are summarized, with a focus on T-VEC, Pexa-Vec, and PROSTVAC-VF. The modes of actions vary depending on the types of vaccines. Inactivated, live attenuated, and subunit vaccines use killed or weakened viruses, or viral components to induce immune response against cancer, while oncolytic virus vaccines use genetically modified viruses to selectively attack tumor cells and stimulate systemic immunity, and viral vector cancer vaccines use designed viruses as carriers to deliver desired genetic materials which can initiate anti-tumor immunity. Although T-VEC is the only virus-based therapeutic cancer vaccine that is approved by the FDA to treat advanced melanoma, Pexa-Vec, PROSTVAC-VF, and several other virus-based cancer vaccines are currently in clinical trials to further evaluate their efficacy and safety in treating various types of cancers. Current studies have shown encouraging results for these cancer vaccines, such as improved OS, RFS and ORR, as well as reduced cancer recurrence and mortality rates. With their abilities in selective cell targeting and specific tumor antigen delivery, these virus-based cancer vaccines possess the potential to revolutionize cancer immunotherapy, particularly given the rapid advancements in technology. In future prospects, more research and clinical trials are necessary to further enhance the immune response elicited by virus-based cancer vaccines, improve their stability and safety for immunocompromised patients, and investigate combination therapies to optimize treatment outcomes.
References
[1]. NIH National Cancer Institute, Cancer Statistics (2024), Available online at: https://www.cancer.gov/about-cancer/understanding/statistics.
[2]. Zhang Y and Zhang Z 2020 Cell. Mol. Immunol. 17 807–821
[3]. Lin MJ, Svensson-Arvelund J, Lubitz GS, Marabelle A, Melero I, Brown BD and Brody JD 2022 Nat. Cancer 3 911–926
[4]. Liu J, Fu M, Wang M, Wan D, Wei Y and Wei XJ 2022 Hematol. Oncol. 15 28
[5]. Kaczmarek M, Poznańska J, Fechner F, Michalska N, Paszkowska S, Napierała A, Mackiewicz 2023 A. Cells 12 2159
[6]. Dhanyamraju PK, Patel TN J 2022 Biomed. Res. 36 77–97
[7]. Tashiro H, Brenner MK 2017 Cell Res. 27 59–73
[8]. Hou Y, Chen M, Bian Y, Zheng X, Tong R, Sun X 2023 Acta Pharm. Sin. B 13 3321–3338
[9]. Kaufman HL, Kohlhapp FJ, Zloza A 2015 Nat. Rev. Drug Discov. 14 642–662
[10]. Larocca C, Schlom J 2011 Cancer J. 17 359–371
[11]. Xie L, Han Y, Liu Y, Zhou Y, Yu J, von Brunn A, Lei J. 2023 MedComm – Oncol. 2 e55
[12]. Ferrucci PF, Pala L, Conforti F, Cocorocchio E 2021 Cancers (Basel) 13 1383
[13]. Dummer R, Gyorki D, Hyngstrom J, Berger A, Conry R, Demidov L, Ning L, Lawrence T, Faries M, Ross M 2022 Annals of Oncol. 33 S1406
[14]. Abou-Alfa GK, et al. 2016 JCO 34 TPS4146–TPS4146
[15]. Heo J, et al. 2013 Nat. Med. 19 329–336
[16]. Kantoff, P. W, et al. 2010 J. Clin. Oncol. 28 1099–1105
Cite this article
Zhang,W. (2024). Mechanisms and Clinical Applications of Virus-Based Cancer Vaccines. Theoretical and Natural Science,50,54-60.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2024 Workshop: Workshop on Intelligent Medical Data Analysis for Precision Medicine
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. NIH National Cancer Institute, Cancer Statistics (2024), Available online at: https://www.cancer.gov/about-cancer/understanding/statistics.
[2]. Zhang Y and Zhang Z 2020 Cell. Mol. Immunol. 17 807–821
[3]. Lin MJ, Svensson-Arvelund J, Lubitz GS, Marabelle A, Melero I, Brown BD and Brody JD 2022 Nat. Cancer 3 911–926
[4]. Liu J, Fu M, Wang M, Wan D, Wei Y and Wei XJ 2022 Hematol. Oncol. 15 28
[5]. Kaczmarek M, Poznańska J, Fechner F, Michalska N, Paszkowska S, Napierała A, Mackiewicz 2023 A. Cells 12 2159
[6]. Dhanyamraju PK, Patel TN J 2022 Biomed. Res. 36 77–97
[7]. Tashiro H, Brenner MK 2017 Cell Res. 27 59–73
[8]. Hou Y, Chen M, Bian Y, Zheng X, Tong R, Sun X 2023 Acta Pharm. Sin. B 13 3321–3338
[9]. Kaufman HL, Kohlhapp FJ, Zloza A 2015 Nat. Rev. Drug Discov. 14 642–662
[10]. Larocca C, Schlom J 2011 Cancer J. 17 359–371
[11]. Xie L, Han Y, Liu Y, Zhou Y, Yu J, von Brunn A, Lei J. 2023 MedComm – Oncol. 2 e55
[12]. Ferrucci PF, Pala L, Conforti F, Cocorocchio E 2021 Cancers (Basel) 13 1383
[13]. Dummer R, Gyorki D, Hyngstrom J, Berger A, Conry R, Demidov L, Ning L, Lawrence T, Faries M, Ross M 2022 Annals of Oncol. 33 S1406
[14]. Abou-Alfa GK, et al. 2016 JCO 34 TPS4146–TPS4146
[15]. Heo J, et al. 2013 Nat. Med. 19 329–336
[16]. Kantoff, P. W, et al. 2010 J. Clin. Oncol. 28 1099–1105









