1. Introduction
A class of disorders known as cancer is distinguished by aberrant cell division and invasion potential. Cancer continues to be a condition that is highly contested in the medical profession and claims the lives of millions of people annually. The most recent Global Cancer Statistics report estimates that there are around 9.7 million cancer-related deaths and close to 20 million new cases of cancer globally. While traditional treatments still produce therapeutic results in the clinic, such as surgery, radiotherapy, and chemotherapy. However, there is a constant search for treatments with fewer side effects and better therapeutic effects. Cancer immunotherapy first appeared in the 1890s when Dr william.B.Coley gave streptococcus as a therapeutic vaccine to sarcoma patients [1]. Unlike conventional medicine, immunotherapies aim to enhance or restore the immune system's ability to recognize and eliminate cancerous cells. Compared with traditional cancer therapies such as chemotherapy and radiotherapy, immunotherapy causes less damage to normal tissues, resulting in fewer adverse consequences. Depending on how it works and how it is applied, immunotherapy may be categorized into a number of different areas.
2. Cancer immunity cycle
The cancer immune cycle is a set of sequential processes that must be started and allowed for continuous, iterative amplification in order for an anti-tumor immune response to effectively destroy cancer cells [1]. dendritic cells capture and process new antigens produced and released by cancer cells. Antigens on MCHI and MCHII are captured and presented to T cells by dendritic cells. Consequently, effector T-cell responses specific to cancer cells are activated and initiated. T cells enter the tumor by migration. Additionally, the homologous antigen attached to MHCI interacts with the TCR receptor after passing through it. The anti-tumor immune response is implicated when ligand proteins of negative signaling receptors produced by tumor cells in the tumor tissue's microenvironment bind to negative regulatory receptor proteins upon the outside of killer T cells [2]. Through the release of perforin and granzyme or the ligation of FasL, cytotoxic T lymphocytes destroy tumor cells. Furthermore, by secreting cytokines like IFN-γ and TNF-α, which cooperate to assault cancer cells, T cells can further stimulate other immune cells [3]. In cancer patients, the cancer immune cycle is not functioning at full capacity. T cells may not be able to colonize tumors or may be prevented from infiltrating tumors, tumor microenvironmental factors may suppress the production of effector cells, tumor antigens may not be detectable, DCs and T cells may perceive antigens as internal rather than external, leading them to generate regulatory T cell responses instead of effector responses (figure 1).
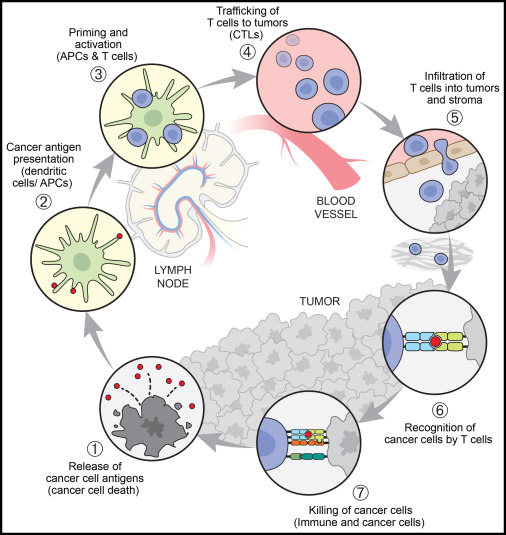
Figure 1. The cancer-immunity cycle [4].
3. Application of Immunotherapy
3.1. Oncolytic virus therapies
Oncolytic virus therapies are designed to enhance systemic anti-tumour immunity by stimulating a pro-inflammatory environment. In 2015, the FDA approved the first over the counter (OV) medication for treating patients with metastatic melanoma (T-VEC). Imlygic, another name for the genetically engineered herpes simplex virus, is the medication. Oncolytic virus, as small particles that are allowed to replicate within host cells and induce host inflammation, are mainly classified as DNA viruses and RNA viruses [4-6]. Talimogene laherparepvec (T-VEC) has been progressively approved for the treatment of melanoma in the United States and Australia around 2015 [5].Treatment for melanoma, head and neck cancer, and several other malignancies has demonstrated the effectiveness of lentiviral therapy.
3.2. Cancer vaccines
T cell-mediated anti-tumor immune responses are triggered by antigens unique to tumors in cancer vaccines. vaccinations against cancer may be divided into three groups based on their kind and structure: vaccinations containing cells, vaccines containing proteins or peptides, and vaccines containing nucleic acids that can carry DNA, RNA, or viruses. These vaccines can prevent the development of cancer (such as the cervical cancer vaccine) or treat existing cancers. The development of therapeutic cancer vaccines faces many challenges, but a number of vaccines are already progressing in clinical trials. In the bid to prevent liver cancers attributed to the hepatitis B virus (HBV) and HPV-related infections, the U.S. Food and Drug Administration (FDA) has approved licenses for two preventative vaccinations against the HBV virus [1].
3.3. Cytokines therapies
Low molecular weight, soluble proteins called cytokines facilitate intercellular communication [7]. As some specific cells of the immune system secrete proteins that can act on other cells (bind to receptors on the membrane surface) thereby activating downstream signaling pathways. Cytokine therapy uses cytokines such as interferon (IFN) and interleukin (IL) to regulate and enhance the immune response. Cytokines both directly cause apoptosis and affect the host immunological response to cancer cells. Although cytokine-based immunotherapy has shown great promise, to far, Only licenses to use IFN-α and IL-2 for the treatment of cancer have been issued by the US Food and Drug Administration. Cytokine therapy has significant side effects and needs to be used under strict monitoring. They are now usually replaced by immune checkpoint inhibitors. In 2022, the US Food and Drug Administration (FDA) certified a biologics license application (BLA) for the in-situ treatment of non-muscle invasive bladder cancer (NMIBC) through the IL-15 agonist N-803 (AnktivaTM) [8,9].
3.4. Adoptive cell transfer
The first clinical investigations of cell transfer treatment date back to the development of recombinant human cytokines, such as the in vitro production of highly active, highly-affinity tumor-reactive T cell cultures derived from internal tumors-infiltrating lymphocytes or genetically modified cells to express novel tumor antigen recognition skills by use of antigen receptor genes. The first clinical investigations of cell transfer treatment date back to the development of recombinant human cytokines, such as the in vitro production of highly active, highly-affinity tumor-reactive T cell cultures derived from internal tumors-infiltrating lymphocytes or genetically modified cells to express novel tumor antigen recognition capacities by use of antigen receptor genes [9]. Adoptive cell transfer (ACT) involves the infusion back into the body of a patient's own immune cells (e.g., T-cells) that have been expanded and activated outside of the body, thereby enhancing the anti-cancer immune response. ACT shows unlimited potential in the treatment of cancers such as melanoma. The future holds promise in improving durable response rates and expanding the population of possible treatments. it is typically used in conjunction with other therapies. Tumors can suppress t-cell activity in a variety of ways, and studies have engineered cells to overcome this problem. Clinical trials that have assessed the efficacy of CAR-t cells in treating solid tumors have targeted tumor-induced t-cell suppression and focused on certain surface metrics. CAR-T cells have demonstrated considerable potential not only for the management of hematologic cancers but also in the treatment of solid tumors that have been clinically evaluated for their effectiveness. However, due to the high price there is still huge room for development in the future.
3.5. Immune checkpoint inhibitor
Immune checkpoint inhibitor is a signalling pathway that maintains the body's autoimmune tolerance, regulates the magnitude of the immune response, and generates strong immune suppression [10]. Tumor cells activate the PD-1/PD-L1 pathway, a defense mechanism of the adaptive immune system designed to respond to endogenous immunological anti-tumor activity. Durvalumab is a PD-L1-targeting antibody which is a human antibody. According to the results of a Phase III clinical trial involving 713 patients (NCT02125461), patients in the durvalumab-treated group had significantly longer progression-free survival. It then received its first FDA approval in February 2018 for use against unresectable stage III NSCLC.CTLA-4 inhibitors, such as ipilimumab and tremelimumab, increase the anti-tumor immune response by suppressing CTLA-4 from attaching to CD80/CD86, removing T cell inhibition, and promoting T cell activation and spread [11]. Immune checkpoint inhibitor-treated individuals had immune-related side events rather often. These drugs have shown excellent efficacy in a variety of cancers (e.g., melanoma, lung cancer, etc.) and have become an important tool in cancer immunotherapy.
4. Conclusion
Overall, regardless of the type of immunotherapy, cancer immunotherapy still has a lot of room for exploration, which is why it has become one of the hot medical topics in recent years. Cancer immunotherapy still has many drawbacks and shortcomings that cannot be ignored, such as being effective against only a very small number of cancers, still having side effect problems and being expensive, to name a few. In the future, we are optimistic about combining immunotherapy with other therapies to make immunotherapy more widely available, with fewer side effects and less drug resistance. The cancer immune cycle is a complex and dynamic process, and each component is critical to the success of immunotherapy. The development of new therapeutic strategies through in-depth research and optimisation of these links can bring more hope to cancer patients. In the future, cancer immunotherapy is expected to be combined with other treatments (e.g. surgery, radiotherapy, chemotherapy) to form an integrated treatment strategy, improving efficacy and reducing side effects. Continuing to promote basic research and clinical trials to discover new anti-cancer targets and immunoregulatory mechanisms will help to continuously innovate and optimise cancer immunotherapy and make greater contributions to the global cancer prevention and treatment cause.
References
[1]. Von Rueden SK, Fan TM. Cancer-Immunity Cycle and Therapeutic Interventions- Opportunities for Including Pet Dogs With Cancer. Front Oncol. 2021 Nov 19;11:773420.
[2]. Fu Y, Tang R, Zhao X. Engineering cytokines for cancer immunotherapy: a systematic review. Front Immunol. 2023 Jul 6;14:1218082.
[3]. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020 Aug;17(8):807-821.
[4]. Mellman I, Chen DS, Powles T, Turley SJ. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity. 2023 Oct 10;56(10):2188-2205.
[5]. Igarashi Y, Sasada T. Cancer Vaccines: Toward the Next Breakthrough in Cancer Immunotherapy. J Immunol Res. 2020 Nov 17;2020:5825401.
[6]. Abbott M, Ustoyev Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin Oncol Nurs. 2019 Oct;35(5):150923.
[7]. Ma R, Li Z, Chiocca EA, Caligiuri MA, Yu J. The emerging field of oncolytic virus-based cancer immunotherapy. Trends Cancer. 2023 Feb;9(2):122-139.
[8]. Zhang li.(2020).A critical review of 200 cases of adverse reactions associated with immune checkpoint inhibitors. Peking: Tsinghua University Press
[9]. Fu Y, Tang R, Zhao X. Engineering cytokines for cancer immunotherapy: a systematic review. Front Immunol. 2023 Jul 6;14:1218082.
[10]. Tajiri K, Aonuma K, Sekine I. Immune checkpoint inhibitor-related myocarditis. Jpn J Clin Oncol. 2018 Jan 1;48(1):7-12.
[11]. Cheng Yuwei, Yang Feng, Zhang Yunchang. Research progress of phototherapy combined with immune checkpoint inhibitors in the treatment of tumors. Chinese Journal of Cancer Biotherapy.2024 Jul 31;06:626-631.
Cite this article
Li,Y. (2024). Current development and trends of cancer immunotherapy. Theoretical and Natural Science,54,95-99.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2024 Workshop: Workshop on Intelligent Medical Data Analysis for Precision Medicine
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Von Rueden SK, Fan TM. Cancer-Immunity Cycle and Therapeutic Interventions- Opportunities for Including Pet Dogs With Cancer. Front Oncol. 2021 Nov 19;11:773420.
[2]. Fu Y, Tang R, Zhao X. Engineering cytokines for cancer immunotherapy: a systematic review. Front Immunol. 2023 Jul 6;14:1218082.
[3]. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020 Aug;17(8):807-821.
[4]. Mellman I, Chen DS, Powles T, Turley SJ. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity. 2023 Oct 10;56(10):2188-2205.
[5]. Igarashi Y, Sasada T. Cancer Vaccines: Toward the Next Breakthrough in Cancer Immunotherapy. J Immunol Res. 2020 Nov 17;2020:5825401.
[6]. Abbott M, Ustoyev Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin Oncol Nurs. 2019 Oct;35(5):150923.
[7]. Ma R, Li Z, Chiocca EA, Caligiuri MA, Yu J. The emerging field of oncolytic virus-based cancer immunotherapy. Trends Cancer. 2023 Feb;9(2):122-139.
[8]. Zhang li.(2020).A critical review of 200 cases of adverse reactions associated with immune checkpoint inhibitors. Peking: Tsinghua University Press
[9]. Fu Y, Tang R, Zhao X. Engineering cytokines for cancer immunotherapy: a systematic review. Front Immunol. 2023 Jul 6;14:1218082.
[10]. Tajiri K, Aonuma K, Sekine I. Immune checkpoint inhibitor-related myocarditis. Jpn J Clin Oncol. 2018 Jan 1;48(1):7-12.
[11]. Cheng Yuwei, Yang Feng, Zhang Yunchang. Research progress of phototherapy combined with immune checkpoint inhibitors in the treatment of tumors. Chinese Journal of Cancer Biotherapy.2024 Jul 31;06:626-631.









