1. Introduction
Global warming has emerged as a giant difficulty in latest years, generally due to over-dependence on finite fossil gas supplies, which generate greenhouse gases which include CO2, CH4 and O3 [1]. To cope with this difficulty, more than one international location has applied matching techniques and proposed the notions of Carbon Peak and Carbon Neutrality [2]. To meet those targets, there's a urgent call for for improvements in novel power sources, with the maximum auspicious alternatives being hydrogen, sun, and secondary batteries [3].
Compared with sun power and secondary batteries, hydrogen power famous great benefits, in particular in gas mobileular programs [4,5]. High power density and portability are of the primary benefits [6]. Therefore, the fuel cells are regularly seen as the generation with the finest ability to replace traditional inner combustion engines, therefore substantially lowering dependence on fossil fuels [7]. The hydrogen fuel cell is regularly composed of a cathode, anode and electrolyte [8], which converts hydrogen and oxygen into energy via a chemical response [9]. The essential response includes the mixture of hydrogen and oxygen to generate water and power. An oxygen reduction response (ORR) happens on the cathode in this process, a hydrogen oxidation response (HOR) taking region on the anode [10]. Furthermore, the handiest chemical reactant of fuel cells is water, doing no damage to the environment [11]. Fuel cells are quieter than inner combustion engines, making them brilliant for low-noise programs [12].
Hydrogen fuel cells are utilized in numerous ordinary scenarios, which include green electric powered vehicles, planes, in addition to making sure consistent electricity deliver to important centers like hospitals [13]. Nevertheless, the extensive industrial use of the hydrogen fuel cells is currently restrained due to the use of luxurious metal platinum because of the ORR catalysts [14]. Therefore, numerous research have targeted on growing the ORR catalysts with low cost, high activity and stability. The maximum promising and famous techniques encompass alloying platinum with different 3d transmission metals (Ni, Fe, Cu, etc.), the usage of non-metal catalysts, and monatomic catalysts.
Regarding the Pt-M alloys, Chang et al. optimised pressure and ligand results to fine-music the size, shape and morphology of bimetallic nanocatalysts to decorate the activity and stability [15]. It is additionally stated that the primary instance of organising the nanoscale section shape is given with the aid of using nanoscale alloying bimetallic catalysts with thermally managed section segregation and core-shell evolution [16]. The electrocatalytic activity of an oxygen reduction reaction is correlated for the primary time, and this locating has extensive implications for the layout and nano-engineering of stepped-forward catalysts with extraordinary bimetallic or polymetallic nanostructures. In phrases of non-metal catalysts, Danilov et al. set up a thermochemical method for the manufacture of layered graphite nitride composites from urea and melamine [17], and Marinoiu et al. synthesised graphene iodide with excessive ORR electrochemical traits thru electrophilic substitution [18]. Moreover, a large quantity of research were carried out on unmarried atom ORR catalysts. By heavily doping zeolitic imidazolite frameworks with noble metals (PM), Liu et al. evolved a sequential coordination method to synthesise highly metal-loaded Ir, Rh, Pd, and Pt unmarried atom catalysts (SAC) [19]. With the usage of porous carbon (C-MOF-5) comprised of MOF-5, Xie et al. have been capable of correctly synthesise iron unmarried atom catalysts (Fe SAC) with lots of handy iron sites (Fe SAC-MOF-5) [20].
In conclusion, this observation will start with examining the hydrogen fuel cell technology with opportunity options, accompanied by using an in-depth exam of fuel cell-running principles. This paper additionally aims to check the substances designed as ORR catalysts and the corresponding techniques for activity and stability enhancement. Finally, the packages and potential of ORR catalysts use, as well as problems and destiny views, may be explored in brief.
2. Overview of Fuel Cell Technology and Importance of Oxygen Reduction Reaction (ORR)
2.1. Advantages and disadvantages of fuel cells
Table 1. Comparison of the advantages and disadvantages of various types of batteries [21-23]
Advantages | Disadvantages | |
Secondary Battery | 1. Rechargeable 2. Technically mature | 1. Long charging time 2. Lower capacity 3. Environmental pollution |
Solar Cell | 1. Renewable energy 2. Environmentally friendly | 1. Efficiency affected by weather and light 2. Larger installation cost and area |
Fuel Cell | 1. High energy efficiency 2. High energy density 3. Low emission 4. High capacity 5. Portable | 1. High cost 2. Complex fuel storage and delivery |
Compared to other types of techniques, it is evident that the fuel cells possess notable benefits, the shortcomings of which are most easily overcome, as shown in Table 1. Nevertheless, it is challenging to overcome the disadvantages of solar cells and secondary batteries because of their extreme sensitivity to weather and relatively low energy conversion efficiency. Also, their energy densities are much lower than that of fuel cells. Therefore, fuel cells are considered the most promising one.
2.2. Definition, construction and running principle of fuel cell
2.2.1. Definition and construction
The fuel cell is an electrochemical equipment that transforms a system's chemical power into electric power [24]. An opportunity attitude on the fuel cell is to explain it in a manner wherein the hydrogen undergoes combustion in a straightforward reaction [25].
2H2 + O2 → 2H2O
When oxygen and hydrogen molecules collide, a process takes place at a molecular level. Oxidation of the hydrogen molecules releases heat and produces water [26].
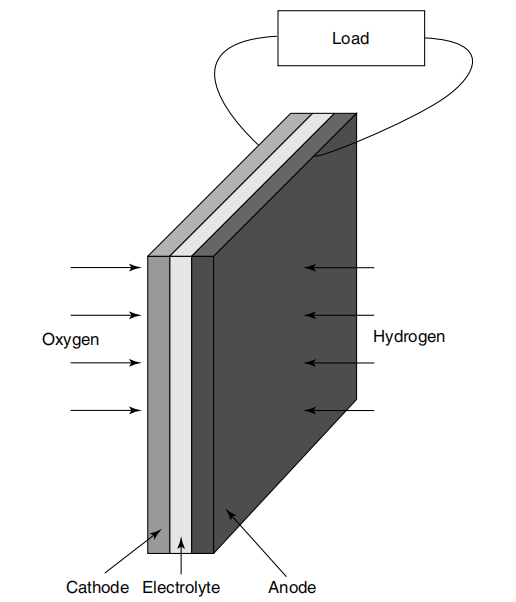
Figure 1. Basic structure of fuel cell [26]
The fundamental element of a fuel cell consists of electrodes, electrolytes and catalysts. The electrode is split into an anode and a cathode. As the number one constituent of a fuel cell, the electrolyte is liable for moving charged particles (ions) from one electrode to any other, even as stopping electrons from passing without delay through the indoors of the gas cell [27]. Typically, a porous carbon cloth lined with a catalyst (together with platinum) serves as the anode and cathode. The fuel is broken down into electrons and ions on the anode through oxidation. A reduction reaction occurs at the cathode, recombining the ions with electrons and interacting with oxygen to provide water or different byproducts [28].
2.2.2. Working principle
Different chemical environments (alkaline and acidic) result in two different reaction pathways, mostly due to the unique routes of the electrode processes.
In an acidic environment, the primary components of its operational mechanism encompass:
Anode (fuel side) reaction: Fuel (usually hydrogen) is decomposed into protons and electrons in the presence of anode catalysts.
H2→2H++2e-
Protons passing thru the proton change membrane: The protons produced migrate thru the proton change membrane (PEM) in the direction of the cathode.
Cathode (oxygen side) response: At the cathode, oxygen, protons and electrons integrate to shape water [29].
1/2O2+2H++2e-→H2O
In alkaline conditions, the operation works as follows: Anode (fuel side) reaction: In an alkaline medium, hydrogen reacts with hydroxide ions to produce water and release electrons [30].
H2+2OH-→2H2O+2e-
At the anode, electrons flow through an external circuit to the cathode. At the cathode, oxygen, water, and electrons combine to form hydroxide ions [31]. The reaction is shown below:
O2+2H2O+4e-→4OH-
The way these two types of fuel cells operate shows how they adapt to different chemical environments and reveals differences in their reaction processes. Acidic fuel cells create energy by moving protons, while alkaline fuel cells depend on hydroxide ions [32]. These differences influence factors like catalyst selection, membrane material choices, and the durability and overall efficiency of the fuel cell [33].
2.3. Fuel Cell Classification
Fuel cells are classified into various types depending on the electrolyte they use. These include alkaline fuel cells (AFC), which operate in an alkaline medium [34]; solid oxide fuel cells (SOFC), known for their high-temperature operation [35]; phosphoric acid fuel cells (PAFC), which use phosphoric acid as the electrolyte [36]; molten carbonate fuel cells (MCFC), suitable for high-temperature applications [37]; and proton exchange membrane fuel cells (PEMFC), which are commonly used in vehicles due to their efficiency [38].
Table 2. Comparison of various types of fuel cells [25]
PEMFC | MCFC | PAFC | SOFC | AFC | |
Electrolyte materials | exchange membrane | Lithium Carbonate, Sodium Carbonate, Carbonic Acid | phosphates | e.g. stabilised zirconia | Potassium hydroxide aqueous solution |
mobile ion | H+ | CO32- | H+ | O2- | OH- |
catalysts | platinum | / | platinum | / | Non-precious metals |
anodic reaction | H2→2H++2e- | H2+CO32-→H2O+CO2+2e- | H2→2H++2e- | H2+O2-→H2O+2e- | H2 → 2H+ + 2e- (often involves O2 and CO2) |
cathodic reaction | 1/2O2+2H++2e-→H2O | 1/2O2+CO2+2e-→CO32- | 1/2O2+2H++2e-→H2O | 1/2O2+2e-→O2- | 1/2O2 + 2H+ + 2e- → H2O |
Operating temperature (°C) | 60-80 | 650-700 | 190-220 | 800-1000 | 60-250 |
Reaction product | H2O | H2O, CO2 | H2O | H2O | H2O |
As can be seen from table 2, Proton Exchange Membrane Fuel Cells (PEMFCs) are known for their operation at lower temperatures, typically between 60°C and 80°C. This makes them particularly suitable for use in transportation and portable devices, where quick response times are crucial. On the other hand, Molten Carbonate Fuel Cells (MCFCs) operate at much higher temperatures, ranging from 650°C to 700°C, which makes them ideal for generating electricity on a large scale, especially when natural gas is the primary fuel source. Furthermore, Phosphoric acid fuel cells (PAFCs), which utilize phosphoric acid as the electrolyte, operate at intermediate temperatures (190°C to 220°C) and are often favored for commercial power generation. Solid Oxide Fuel Cells (SOFC) make use of stabilized zirconia, providing the best running temperatures (800°C to 1000°C), are appropriate for desk-bound installations and are extraordinarily green. Alkaline Fuel Cells (AFC), which make use of potassium hydroxide answers and feature a wide variety of running temperatures (60°C to 250°C), are commonly utilized in space and army equipment, even though interest desires to be paid to their sensitivity to CO2. Choosing the proper kind of fuel cell calls for attention to precise application situations and overall performance requirements.
2.4. The role of ORR in fuel cells and the importance of studying ORR catalysts
In fuel cell systems, the performance and kinetics of the oxygen reduction response (ORR) are vital to the general cell performance [39]. The ORR is the key electrochemical technique that takes location on the cathode, in which oxygen molecules are decreased to water. The technique is particularly sluggish and consequently appears as a bottleneck of the response fee of the fuel cell [40]. This sluggish response fee no longer reduces the performance of the electricity conversion; additionally, it will increase the warmth of the system [41]. To cope with this challenge, catalysts with high activity are regularly required to boost the ORR, amongst which noble metals, such as platinum, are broadly used because of their great catalytic properties [42].
However, the high cost of platinum and different noble precious catalysts substantially will increase the production cost of gas cells and limit their market competitiveness [43]. Therefore, it's crucial to broaden new non-precious metal catalysts or more cost-effective platinum-based catalysts. These new catalysts intend to lessen expenses without sacrificing catalytic activity, making gas molecular technology extra economically attractive.
In addition, the stability and sturdiness of the catalysts additionally entice the worldwide interest in research [44]. The commercialisation and sensible software of the fuel cells require catalysts that could maintain a green overall performance for the duration of long-time operation without corrosion and degradation [45]. The development of more stable catalysts with higher activity can ensure that fuel cell systems work reliably and efficiently under various operating conditions, thus extending their service life and reducing maintenance costs.
3. Classification, characterization and synthesis of ORR catalysts
ORR catalysts inside the fuel cells may be grouped into precious metal catalysts, non-precious metal catalysts, and novel catalysts [46,47]. Compared to the precious metal catalysts that have been efficaciously commercialised, the non-precious metal and novel catalysts have awesome blessings in cost and sustainability but lack durability and stability [48]. Therefore, a lot of research has been performed to broaden diverse ORR catalysts.
3.1. Comparison of the performance of precious and non-precious metal catalysts
3.1.1. Non-precious metal catalysts
Non-precious metal catalysts inside the fuel cells are commonly composed of carbon-primarily based totally compounds (e.g. graphene), metal oxides (e.g. Co3O4), and metal nitrides (e.g. TiN). The choice of those substances is primarily based totally on their effective features for catalytic reactions, in addition to their fee-effectiveness, which allows their extensive programs inside the fuel cells [49].
One of the number one strategies for creating non-precious metal catalysts is the sol-gel technique, which entails the usage of a chemical approach to create a gel through a sol-gel procedure, and then the catalyst is acquired with the aid of using warmth treatment [50]. The function of nanoscale structures, along with nanoparticles, nanotubes, or nanosheets, can also enhance catalytic activity by using growing floor regions and including extra energetic sites [51].
Further exploration has shown that doping and surface modification are effective strategies for developing non-precious metal catalysts. Doping involves altering the electrical properties and boosting catalytic performance by incorporating elements like nitrogen, boron, or phosphorus into carbon-based materials [52]. On the other hand, surface functionalization focuses on improving how the catalyst interacts with reactants by adding specific functional groups, such as acidic or basic groups, to its surface [53].
3.1.2. Precious metal catalysts
In fuel cells, precious metal catalysts are often based on platinum and its alloys, such as Pt-Co, Pt-Ni, and Pt-Fe. The addition of transition metals (TMs) enhances the structural stability of these alloys, improving their effectiveness, especially at higher temperatures and in corrosive environments [54]. The durability of fuel cells largely depends on this enhanced stability. Moreover, Pt-TM alloys demonstrate better catalytic activity in oxygen reduction reactions (ORR) due to modifications in the surface electronic structure caused by the presence of TMs [55]. These alloys can form different shapes, such as spherical, cubic, rod-like, or polyhedral, each influencing the surface characteristics and the arrangement of catalytically active sites [56]. Additionally, the size of the particles plays a crucial role in catalytic activity. As the particles become smaller, the surface area per unit mass increases, providing more active sites [57]. However, smaller particles may face challenges, such as reduced stability and a tendency to clump together during operation.
The efficiency of platinum-based catalysts in oxygen reduction reactions can be greatly improved by altering their surface electronic properties, particularly through the addition of transition metals via alloying [58]. A key factor in this improvement is the d-band centre, which indicates the average energy level of the d-electronic bands. By adjusting this level, the electron density and the electronic state of platinum are modified, influencing how the catalyst interacts with reactants [59]. These modifications can enhance the catalyst's ability to bind and activate reactant molecules [60]. Furthermore, the Fermi level, which denotes the highest energy level that electrons can occupy, is critical in determining the catalyst’s electron affinity or repulsion [61]. Changes in the Fermi level caused by alloying can directly impact the speed and direction of the catalytic reaction [62]. By carefully adjusting these electronic properties, significant gains in both the efficiency and selectivity of the catalyst can be achieved.
Deposition precipitation is an important method used to synthesize precious metal catalysts, involving techniques like physical or chemical vapour phase deposition, impregnation reduction, and electrochemical deposition [63,64]. Recently, the application of thin films or molecular layers has gained attention for protecting catalyst surfaces from toxicity and corrosion while also improving selectivity [65].
Moreover, applying surface coatings, such as carbon, nitrides, and oxides, to Pt-TM alloy particles enhances the durability of the catalyst and prevents the metal particles from corroding and oxidising [66]. Additionally, it enhances the catalyst's potential to resist toxicity. Nevertheless, precious metallic catalysts do have a few constraints. Platinum and different uncommon metals are of excessive price because of their confined supplies [67].
3.2. Novel catalysts (e.g. monatomic catalysts)
Monatomic catalysts have exhibited notable advantages in fuel cells. The catalytically active centres in these structures are made up of individual metal atoms that are spread out over carriers like graphene and nitrogen-doped carbon.
3.2.1. Main features and advantages
Monatomic catalysts exhibit extremely high catalytic efficiency due to their highly dispersed catalytically active sites. For instance, Pt monoatomic catalysts exhibit excellent catalytic activity in oxygen reduction reaction. Specifically, a ternary monoatomic catalyst consisting of Pt, Fe, and Co achieved a half-wave potential of 0.845 V in ORR, which was significantly higher than that of commercial 20 wt% Pt/C (0.835 V) [68].
Furthermore, single-atom catalysts provide a notable benefit in terms of their exceptional utilisation of atoms. Every atom participates in the catalytic process, so the metal atoms are extensively employed [69]. Conventional catalysts have metal atoms that are not entirely used because of their placement within the catalyst or restricted involvement in the reaction. On the contrary, monoatomic catalysts make the most of each atom, leading to better catalytic performance and lower costs.
The effectiveness of these catalysts depends on several factors, including the nature of the metal atoms, the type of carrier used, and the interactions between them. The combination of different metals and carriers can greatly influence the catalytic properties. Studies have found that strong interactions—particularly electronic ones—between the metal and the carrier can change the electronic structure of the metal atoms, enhancing both catalytic activity and selectivity [70]. Understanding these mechanisms is key to improving the design and function of single-atom catalysts.
3.2.2. Challenges
Making single-atom catalysts (SACs) is not easy. It usually involves methods like atomic layer deposition to spread metal atoms evenly on the surface. For example, the process often includes heating to high temperatures, between 700°C and 1100°C, but this can cause metal atoms to group into bigger particles, which reduces the catalyst's effectiveness. To prevent this, researchers have tried various methods like keeping the atoms in place with other atoms or using defects in the material to hold them better [71].
Additionally, keeping metal atoms from clumping together is a big challenge. Techniques like physically separating the atoms or using chemical bonds can help. For instance, adding different atoms (like N, P, and S) can anchor the metal atoms, improving both their performance and stability. Another approach is to use porous materials to trap the metal atoms and stop them from clumping during heating [72]. These methods are essential for creating stable and efficient SACs for real-world uses.
4. Practical applications and challenges
Catalysts play a crucial role in many real-world uses of fuel cell systems, including vehicles, backup power, distributed power generation, and portable electronics. In vehicles, proton exchange membrane fuel cells (PEMFCs) are often used as the main power source [73]. Enhancing the oxygen reduction catalysts in PEMFCs can greatly increase both the efficiency of the reaction and the overall energy conversion. This improvement could lead to longer driving ranges and lower hydrogen fuel consumption. Notably, advanced catalytic technologies have been incorporated into fuel cell vehicles like Toyota’s Mirai and Hyundai’s Nexo, enhancing their energy efficiency and overall performance [74]. Additionally, stationary fuel cell systems utilize various catalysts to ensure a reliable and consistent energy supply, particularly for backup power and distributed generation applications [75]. These catalysts enhance the effectiveness and longevity of the systems, rendering them a more appealing choice in both commercial and residential settings. These systems can offer continuous power supply to data centres, hospitals, and distant regions [76]. Micro fuel cells utilise catalysts to generate electricity for mobile phones, laptops, and other portable electronic devices [77]. This not only prolongs the devices' lifespan but also offers the benefits of being eco-friendly and having fast recharge times.
The implementation of fuel cells in practical settings encounters various technical and economic obstacles, such as the exorbitant expense of catalysts (particularly precious metal catalysts like platinum), concerns regarding durability and stability, the complexity of manufacturing and design, insufficient hydrogen fuelling infrastructure, and limited market acceptance [78]. Precious metal catalysts are expensive, and while non-precious metal and innovative catalysts can lower prices, their manufacturing methods still require optimisation. Enhancing the thermal and chemical resistance of fuel cells is necessary to ensure their stability at extreme temperatures and corrosive surroundings. The production process depends on the use of specialized materials and precise micromachining, which adds to the overall complexity. Additionally, the infrastructure needed for hydrogen fuel—covering production, storage, and transportation—is not yet fully developed, creating obstacles to broader market adoption [79]. To address these challenges, advancements in technology and supportive regulations will be crucial.
5. Conclusion
This article examines how hydrogen fuel cells could play a key role in reducing global warming and aiding the transition to cleaner energy by emphasizing their high energy density, portability, and environmental benefits. To begin with, it explains how fuel cells work and then compares hydrogen fuel cells with other energy sources, such as solar power and batteries, by pointing out both their advantages and drawbacks. Furthermore, the discussion highlights the potential for fuel cells to eventually replace traditional internal combustion engines. In addition to this, the article reviews different types of oxygen reduction reaction (ORR) catalysts used in hydrogen fuel cells, including alloy, non-precious metal, and single-atom catalysts, while also suggesting ways to improve their activity and stability. Overall, these insights provide important theoretical and technological guidance for the future commercial use of hydrogen fuel cells.
Fuel cell technology has a lot of potential in transportation, portable devices, and backup power. But for it to be more common, we need to lower the cost of precious metal catalysts. It's also important to quickly develop cheaper options like non-precious metal and alloy catalysts. Making these catalysts stable and durable is key, which will help them work well over a long time. Beyond the catalysts, advancements in hydrogen production and storage technologies, along with the creation of a reliable infrastructure for hydrogen fuel production, storage, and transportation, are vital. Government support and market incentives will also play essential roles in driving the development and adoption of fuel cell technology. As technology advances, fuel cells are anticipated to have a greater impact on the global energy system and contribute to the achievement of sustainable energy objectives.
References
[1]. Goldthau, A. & Tagliapietra, S. (2022) Energy crisis: five questions that must be answered in 2023. Nature (London). [Online] 612 (7941), 627–630.
[2]. Zheng, Y. et al. (2023) Progress and prospects of international carbon peaking and carbon neutral research –based on bibliometric analysis (1991–2022). Frontiers in energy research. [Online] 11.
[3]. Qazi, A. et al. (2019) Towards Sustainable Energy: A Systematic Review of Renewable Energy Sources, Technologies, and Public Opinions. IEEE access. [Online] 763837–63851.
[4]. Tarascon, J.-M. & Armand, M. (2001) Issues and challenges facing rechargeable lithium batteries. Nature (London). [Online] 414 (6861), 359–367.
[5]. Staffell, I. et al. (2019) The role of hydrogen and fuel cells in the global energy system.
[6]. Barbir, F. (2005) PEM electrolysis for production of hydrogen from renewable energy sources. Solar energy. [Online] 78 (5), 661–669.
[7]. Liu, X. et al. (2019) Comparison of well-to-wheels energy use and emissions of a hydrogen fuel cell electric vehicle relative to a conventional gasoline-powered internal combustion engine vehicle. International journal of hydrogen energy. 45 (1), .
[8]. Anon (2014) Samsung Electronics Co., Ltd.; Patent Issued for Solid-State Fuel Cell Including Anode and Cathode Chemical Electrolyte Protection Layers and a Hydrogen Ion Conductive Solid Oxide Dense Film. Atlanta: NewsRx.
[9]. Roffia, S. et al. (1988) The interconversion of electrical and chemical energy: The electrolysis of water and the hydrogen oxygen fuel cell. Journal of chemical education. [Online] 65 (3), 272–273.
[10]. Jervis, Rhodri. (2015) Development of novel alloy electrocatalysts for the hydrogen oxidation reaction in alkaline media and their application to low temperature fuel cells / Rhodri Jervis. Thesis (Ph.D.)--University College London, 2015.
[11]. Curran, K. M. (2010) Life cycle assessment of GHG emission impacts when hydrogen is produced to fuel cell quality for Canadian light duty vehicles. ProQuest Dissertations Publishing.
[12]. Karlstrom, M. (2005) Local environmental benefits of fuel cell buses—a case study. Journal of cleaner production. [Online] 13 (7), 679–685.
[13]. Eberle, U. & von Helmolt, R. (2010) ‘Fuel Cell Electric Vehicles, Battery Electric Vehicles, and Their Impact on Energy Storage Technologies: An Overview’, in Electric and Hybrid Vehicles. [Online]. Elsevier. pp. 1–1.
[14]. Debe, M. K. (2012) Electrocatalyst approaches and challenges for automotive fuel cells. Nature (London). [Online] 486 (7401), 43–51.
[15]. Chang, F. et al. (2017) Platinum-nickel nanowire catalysts with composition-tunable alloying and faceting for the oxygen reduction reaction. Journal of materials chemistry. A, Materials for energy and sustainability. [Online] 5 (24), 12557–12568.
[16]. Wanjala, B. N. et al. (2010) Nanoscale Alloying, Phase-Segregation, and Core−Shell Evolution of Gold−Platinum Nanoparticles and Their Electrocatalytic Effect on Oxygen Reduction Reaction. Chemistry of materials. [Online] 22 (14), 4282–4294.
[17]. Danilov, M. O. et al. (2021) Carbon Nitride is a Non-Metallic Catalyst for Oxygen Electrodes for Fuel Cells. ECS Transactions. [Online] 105 (1), 87–96.
[18]. Marinoiu, A. et al. (2017) Doped graphene as non-metallic catalyst for fuel cells. Medžiagotyra. [Online] 23 (2), 108–113.
[19]. Liu, Q. et al. (2020) Sequential Synthesis and Active‐Site Coordination Principle of Precious Metal Single‐Atom Catalysts for Oxygen Reduction Reaction and PEM Fuel Cells. Advanced energy materials. [Online] 10 (20), .
[20]. Xie, X. et al. (2022) Fe Single‐Atom Catalysts on MOF-5 Derived Carbon for Efficient Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. Advanced energy materials. [Online] 12 (3), .
[21]. Lee, Y.-H. et al. (2013) Wearable Textile Battery Rechargeable by Solar Energy. Nano letters. [Online] 13 (11), 5753–5761.
[22]. Zakaria, Z. et al. (2021) Fuel cells as an advanced alternative energy source for the residential sector applications in Malaysia. International journal of energy research. [Online] 45 (4), 5032–5057.
[23]. Bresser, D. et al. (2013) Recent progress and remaining challenges in sulfur-based lithium secondary batteries - a review. Chemical communications (Cambridge, England). [Online] 49 (9), 1545–1562.
[24]. Reitz, W. (2007) Handbook of Fuel Cells: Fundamentals, Technology, and Applications, (Volume 1) W. Vielstich, A. Lamm, and H. A. Gasteiger (editors): A Review of: ‘John Wiley and Sons, Ltd., 111 River St., Hoboken, NJ 07030, 2003, vol. 1, Fundamentals and Survey of Systems, 444+ pages, ISBN 0-471-49926-9.’ Materials and Manufacturing Processes 22 (6) p.788–788.
[25]. Larminie James & Dicks Andrew (2003) Fuel Cell Systems Explained (2nd Edition). John Wiley & Sons.
[26]. O’Hayre, R. et al. (2016) Fuel Cell Fundamentals (3rd Edition). Third edition. Newark: John Wiley & Sons.
[27]. Nea u, tefan et al. (2021) Recent progress in electrocatalysts and electrodes for portable fuel cells. Journal of materials chemistry. A, Materials for energy and sustainability. [Online] 9 (32), 1765–17128.
[28]. Oezaslan, M. et al. (2013) Pt-Based Core–Shell Catalyst Architectures for Oxygen Fuel Cell Electrodes. The journal of physical chemistry letters. [Online] 4 (19), 3273–3291.
[29]. Wang, Y. et al. (2020) Fundamentals, materials, and machine learning of polymer electrolyte membrane fuel cell technology. Energy and AI. [Online] 1100014-.
[30]. Li, Q. et al. (2019) The Comparability of Pt to Pt‐Ru in Catalyzing the Hydrogen Oxidation Reaction for Alkaline Polymer Electrolyte Fuel Cells Operated at 80 °C. Angewandte Chemie. [Online] 131 (5), 1456–1460.
[31]. Merle, G. et al. (2011) Anion exchange membranes for alkaline fuel cells: A review. Journal of membrane science. [Online] 377 (1), 1–35.
[32]. Barbir, F. (2012) PEM Fuel Cells - Theory and Practice. Second edition. San Diego: Elsevier.
[33]. Offer, G. J. (2009) ‘PEM fuel cell electrocatalysts and catalyst layers: Fundamentals and applications’. Platinum metals review. [Online] 53 (4), 219–220.
[34]. Thomas, J. et al. (eds.) (2024) Alkaline anion exchange membranes for fuel cells: from tailored materials to novel applications / edited by Jince Thomas, Alex Schechter, Flavio Grynszpan, Bejoy Francis, Sabu Thomas. [Online]. Weinheim: Wiley-VCH.
[35]. Millichamp, J. S. (2013) Development of a novel high temperature crystal microbalance in-situ sensor for the study of electrode processes in solid oxide fuel cells / Jason Shaun Millichamp. Thesis (Ph.D.)--University College London, 2013.
[36]. Zervas, P. L. et al. (2008) Development of a novel computational tool for optimizing the operation of fuel cells systems: Application for phosphoric acid fuel cells. Journal of power sources. [Online] 185 (1), 345–355.
[37]. Milewski, J. et al. (2018) Temperature influence on six layers samaria doped ceria matrix impregnated by lithium/potassium electrolyte for Molten Carbonate Fuel Cells. International journal of hydrogen energy. [Online] 43 (1), 474–482.
[38]. Soumeur, M. A. et al. (2020) Comparative study of energy management strategies for hybrid proton exchange membrane fuel cell four wheel drive electric vehicle. Journal of power sources. [Online] 462228167-.
[39]. Zhang, J. (2008) PEM fuel cell electrocatalysts and catalyst layers : fundamentals and applications / Jiujun Zhang, editor. London: Springer.
[40]. Upadhyay, S. N. et al. (2023) Illuminating the Role of Mo Defective 2D Monolayer MoTe2 toward Highly Efficient Electrocatalytic O2 Reduction Reaction. Langmuir. [Online] 39 (49), 17700–17712.
[41]. Yang, R. et al. (2007) Dependence of the activity of sputtered Co-C-N oxygen reduction electrocatalysts on heat-treatment temperature. Journal of the Electrochemical Society. [Online] 154 (9), B893–B901.
[42]. Loichet Torres, P. A. et al. (2023) ORR Activity and Voltage-Cycling Stability of a Carbon-Supported PtxY Alloy Catalyst Evaluated in a PEM Fuel Cell. Journal of the Electrochemical Society. [Online] 170 (12), .
[43]. Jeremiasse, A. W. et al. (2011) Performance of metal alloys as hydrogen evolution reaction catalysts in a microbial electrolysis cell. International journal of hydrogen energy. [Online] 36 (17), 10482–10489.[45] Guo, P. et al. (2009) Cu/ZnO/Al2O3 water-gas shift catalysts for practical fuel cell applications: the performance in shut-down/start-up operation. International journal of hydrogen energy. [Online] 34 (5), 2361–2368.
[44]. Zhang, Z. (2019) Durable Fuel Cell Electrocatalysts for Energy Conversion. ProQuest Dissertations & Theses.
[45]. Guo, P. et al. (2009) Cu/ZnO/Al2O3 water-gas shift catalysts for practical fuel cell applications: the performance in shut-down/start-up operation. International journal of hydrogen energy. [Online] 34 (5), 2361–2368.
[46]. Berger, D. J. (1999) Fuel Cells and Precious-Metal Catalysts. Science (American Association for the Advancement of Science). [Online] 286 (5437), 49–49.
[47]. Chen, Z., Higgins, D., Yu, A., Zhang, L. and Zhang, J. (2011). A review on non-precious metal electrocatalysts for PEM fuel cells. Energy & Environmental Science, 4(9), p.3167. doi:https://doi.org/10.1039/c0ee00558d.
[48]. Cui, J. et al. (2021) Recent advances in non-precious metal electrocatalysts for oxygen reduction in acidic media and PEMFCs: an activity, stability and mechanism study. Green chemistry : an international journal and green chemistry resource : GC. [Online] 23 (18), 6898–6925.
[49]. Yu, H. et al. (2023) Research Progress on Porous Carbon-Based Non-Precious Metal Electrocatalysts. Materials. [Online] 16 (8), 3283-.
[50]. Mohamed, H. F. M. et al. (2018) A promising fuel cell catalyst using non-precious metal oxide. IOP Conference Series: Materials Science and Engineering. [Online] 464 (1), 12002-.
[51]. Stamenkovic, V. R. et al. (2007) Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nature materials. [Online] 6 (3), 241–247.
[52]. Fretz, S. J. (2018) Adding Utility to Carbon Materials: Introducing Dopants Using Highly Soluble Metal Salts and Functionalizing Surfaces via Bromomethylation. ProQuest Dissertations & Theses.
[53]. Fang, Z. et al. (2020) The Effect of Carbon Support Surface Functionalization on PEM Fuel Cell Performance, Durability, and Ionomer Coverage in the Catalyst Layer. Journal of the Electrochemical Society. [Online] 167 (6), 64506-.
[54]. Lei, J.-T. et al. (2023) Hollow sea urchin-like microspheres of the dual transition metal oxides NiO/Co3O4 immobilized on rGO enhance lithium-ion battery cycling and rate performance. Journal of alloys and compounds. [Online] 969172376-.
[55]. CHIWATA, M. et al. (2016) Oxygen Reduction Reaction Activity of Carbon-Supported Pt-Fe, Pt-Co, and Pt-Ni Alloys with Stabilized Pt-Skin Layers. Denki kagaku oyobi kōgyō butsuri kagaku. [Online] 84 (3), 133–137.
[56]. Ren, X. et al. (2020) Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustainable energy & fuels. [Online] 4 (1), 15–3.
[57]. Gummalla, M. et al. (2015) Effect of particle size and operating conditions on Pt3Co PEMFC cathode catalyst durability. Catalysts. [Online] 5 (2), 926–948.
[58]. Janssen, M. M. P. & Moolhuysen, J. (1976) Platinum-tin catalysts for methanol fuel cells prepared by a novel immersion technique, by electrocodeposition and by alloying. Electrochimica acta. [Online] 21 (11), 861–868.
[59]. Hong, J. et al. (2023) First-principles study on the d-band center of Pt alloyed with 3d transition metals. Journal of the Korean Physical Society. [Online] 83 (12), 964–969.
[60]. Zhou, X. et al. (2023) Individually-atomic governing d—π orbital interactions via Cu-promoted optimization of Fe-d band centers for high-efficiency zinc-air battery. Nano research. [Online] 16 (4), 4634–4642.
[61]. Kim, C. E. et al. (2015) Effect of gold subsurface layer on the surface activity and segregation in Pt/Au/Pt3M (where M = 3 d transition metals) alloy catalyst from first-principles. The Journal of chemical physics. [Online] 142 (3), 034707–034707.
[62]. Xie, Z. et al. (2021) Identification of electronic descriptors for catalytic activity of transition-metal and non-metal doped MoS2. Physical chemistry chemical physics : PCCP. [Online] 23 (28), 15101–15106.
[63]. Anon (2020) Patent Issued for Preparation Method Of Carbon-Supported Metal Oxide And/Or Alloy Nanoparticles Catalyst Using Physical Vapour Deposition. NewsRX LLC.
[64]. Shi, L. et al. (2022) A new reduction method based on simultaneous TiAlC support etching and metal deposition to prepare Pt catalysts for aqueous-phase selective hydrogenation of furfural to furfuryl alcohol. New journal of chemistry. [Online] 46 (31), 14958–14966.
[65]. MacIsaac, C. et al. (2018) Atomic and Molecular Layer Deposition of Hybrid Mo–Thiolate Thin Films with Enhanced Catalytic Activity. Advanced functional materials. [Online] 28 (26),
[66]. Yamamoto, A. & Tsubakino, H. (2003) A New Technique for Surface Modification in Magnesium Alloys by Applying Magnesium Oxide Coating. Materials science forum. [Online] 419–422 (II), 903–908.
[67]. Zhang, Y. et al. (2023) Research Progress of Transition Metal Anode Catalysts for Direct Borohydride Fuel Cells. Journal of nanoparticle research : an interdisciplinary forum for nanoscale science and technology. [Online] 25 (12), 240-.
[68]. Zhang, R. et al. (2023) Pt-Fe-Co Ternary Metal Single Atom Catalyst for toward High Efficiency Alkaline Oxygen Reduction Reaction. Energies (Basel). [Online] 16 (9), 3684-.
[69]. Luo, J. et al. (2023) Recent progress in high-loading single-atom catalysts and their applications. Industrial Chemistry & Materials. [Online] 1 (4), 486–500.
[70]. Chen, J. et al. (2023) Metal-support interactions for heterogeneous catalysis: mechanisms, characterization techniques and applications. Journal of materials chemistry. A, Materials for energy and sustainability. [Online] 11 (16), 854–8572.
[71]. Fang, J. et al. (2023) The synthesis of single-atom catalysts for heterogeneous catalysis. Chemical communications (Cambridge, England). [Online] 59 (2), 2854–2868.
[72]. Gong, X., Song, P., Han, C., Xiao, Y., Mei, X. and Xu, W. (2023). Heterogeneous single-atom catalysts for energy process: recent progress, applications and challenges. Energy Materials. doi:https://doi.org/10.20517/energymater.2022.82.
[73]. Xi, F. et al. (2024) A 4750 hours’ durability investigation of the PEMFC stack based on fuel cell hybrid vehicles conditions. International journal of green energy. [Online] 21 (8), 1874–1885.
[74]. Williams, B. (2022). Toyota Mirai vs Hyundai Nexo: Hydrogen cars compared - HFN. [online] www.hydrogenfuelnews.com. Available at: https://www.hydrogenfuelnews.com/mirai-vs-nexo-hydrogen-cars/8551076/.
[75]. Trachte, U. & Fumey, B. (2013) ‘Applications – Stationary | Uninterruptible power supply and backup power with fuel cells and batteries’, in Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. [Online]. Elsevier Inc. p.
[76]. FuelCellsWorks (2023). Ireland, The European Data Center ‘Hub,’ Addresses Its Power Shortage With Fuel Cells With SK Ecoplant Supplying Fuel Cells To A New Data Center In The Country - FuelCellsWorks. [online] Available at: https://fuelcellsworks.com/news/ireland-the-european-data-center-hub-addresses-its-power-shortage-with-fuel-cells-with-sk-ecoplant-supplying-fuel-cells-to-a-new-data-center-in-the-country/ [Accessed 24 Jul. 2024].
[77]. taylor-research.yale.edu. (n.d.). Fuel Cells | Transformative Materials & Devices. [online] Available at: https://taylor-research.yale.edu/research/fuel-cells [Accessed 27 Jul. 2024].
[78]. Lin, Z. et al. (2013) Hydrogen vehicles: Impacts of DOE technical targets on market acceptance and societal benefits. International journal of hydrogen energy. [Online] 38 (19), 7973–7985.
[79]. Keshri, S. et al. (2024) State-of-the-art review on hydrogen’s production, storage, and potential as a future transportation fuel. Environmental science and pollution research international. [Online]
Cite this article
Hu,Q. (2024). An evaluate of ORR catalysts in fuel cell: Current Advances and Applications. Theoretical and Natural Science,53,19-30.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Applied Physics and Mathematical Modeling
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Goldthau, A. & Tagliapietra, S. (2022) Energy crisis: five questions that must be answered in 2023. Nature (London). [Online] 612 (7941), 627–630.
[2]. Zheng, Y. et al. (2023) Progress and prospects of international carbon peaking and carbon neutral research –based on bibliometric analysis (1991–2022). Frontiers in energy research. [Online] 11.
[3]. Qazi, A. et al. (2019) Towards Sustainable Energy: A Systematic Review of Renewable Energy Sources, Technologies, and Public Opinions. IEEE access. [Online] 763837–63851.
[4]. Tarascon, J.-M. & Armand, M. (2001) Issues and challenges facing rechargeable lithium batteries. Nature (London). [Online] 414 (6861), 359–367.
[5]. Staffell, I. et al. (2019) The role of hydrogen and fuel cells in the global energy system.
[6]. Barbir, F. (2005) PEM electrolysis for production of hydrogen from renewable energy sources. Solar energy. [Online] 78 (5), 661–669.
[7]. Liu, X. et al. (2019) Comparison of well-to-wheels energy use and emissions of a hydrogen fuel cell electric vehicle relative to a conventional gasoline-powered internal combustion engine vehicle. International journal of hydrogen energy. 45 (1), .
[8]. Anon (2014) Samsung Electronics Co., Ltd.; Patent Issued for Solid-State Fuel Cell Including Anode and Cathode Chemical Electrolyte Protection Layers and a Hydrogen Ion Conductive Solid Oxide Dense Film. Atlanta: NewsRx.
[9]. Roffia, S. et al. (1988) The interconversion of electrical and chemical energy: The electrolysis of water and the hydrogen oxygen fuel cell. Journal of chemical education. [Online] 65 (3), 272–273.
[10]. Jervis, Rhodri. (2015) Development of novel alloy electrocatalysts for the hydrogen oxidation reaction in alkaline media and their application to low temperature fuel cells / Rhodri Jervis. Thesis (Ph.D.)--University College London, 2015.
[11]. Curran, K. M. (2010) Life cycle assessment of GHG emission impacts when hydrogen is produced to fuel cell quality for Canadian light duty vehicles. ProQuest Dissertations Publishing.
[12]. Karlstrom, M. (2005) Local environmental benefits of fuel cell buses—a case study. Journal of cleaner production. [Online] 13 (7), 679–685.
[13]. Eberle, U. & von Helmolt, R. (2010) ‘Fuel Cell Electric Vehicles, Battery Electric Vehicles, and Their Impact on Energy Storage Technologies: An Overview’, in Electric and Hybrid Vehicles. [Online]. Elsevier. pp. 1–1.
[14]. Debe, M. K. (2012) Electrocatalyst approaches and challenges for automotive fuel cells. Nature (London). [Online] 486 (7401), 43–51.
[15]. Chang, F. et al. (2017) Platinum-nickel nanowire catalysts with composition-tunable alloying and faceting for the oxygen reduction reaction. Journal of materials chemistry. A, Materials for energy and sustainability. [Online] 5 (24), 12557–12568.
[16]. Wanjala, B. N. et al. (2010) Nanoscale Alloying, Phase-Segregation, and Core−Shell Evolution of Gold−Platinum Nanoparticles and Their Electrocatalytic Effect on Oxygen Reduction Reaction. Chemistry of materials. [Online] 22 (14), 4282–4294.
[17]. Danilov, M. O. et al. (2021) Carbon Nitride is a Non-Metallic Catalyst for Oxygen Electrodes for Fuel Cells. ECS Transactions. [Online] 105 (1), 87–96.
[18]. Marinoiu, A. et al. (2017) Doped graphene as non-metallic catalyst for fuel cells. Medžiagotyra. [Online] 23 (2), 108–113.
[19]. Liu, Q. et al. (2020) Sequential Synthesis and Active‐Site Coordination Principle of Precious Metal Single‐Atom Catalysts for Oxygen Reduction Reaction and PEM Fuel Cells. Advanced energy materials. [Online] 10 (20), .
[20]. Xie, X. et al. (2022) Fe Single‐Atom Catalysts on MOF-5 Derived Carbon for Efficient Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. Advanced energy materials. [Online] 12 (3), .
[21]. Lee, Y.-H. et al. (2013) Wearable Textile Battery Rechargeable by Solar Energy. Nano letters. [Online] 13 (11), 5753–5761.
[22]. Zakaria, Z. et al. (2021) Fuel cells as an advanced alternative energy source for the residential sector applications in Malaysia. International journal of energy research. [Online] 45 (4), 5032–5057.
[23]. Bresser, D. et al. (2013) Recent progress and remaining challenges in sulfur-based lithium secondary batteries - a review. Chemical communications (Cambridge, England). [Online] 49 (9), 1545–1562.
[24]. Reitz, W. (2007) Handbook of Fuel Cells: Fundamentals, Technology, and Applications, (Volume 1) W. Vielstich, A. Lamm, and H. A. Gasteiger (editors): A Review of: ‘John Wiley and Sons, Ltd., 111 River St., Hoboken, NJ 07030, 2003, vol. 1, Fundamentals and Survey of Systems, 444+ pages, ISBN 0-471-49926-9.’ Materials and Manufacturing Processes 22 (6) p.788–788.
[25]. Larminie James & Dicks Andrew (2003) Fuel Cell Systems Explained (2nd Edition). John Wiley & Sons.
[26]. O’Hayre, R. et al. (2016) Fuel Cell Fundamentals (3rd Edition). Third edition. Newark: John Wiley & Sons.
[27]. Nea u, tefan et al. (2021) Recent progress in electrocatalysts and electrodes for portable fuel cells. Journal of materials chemistry. A, Materials for energy and sustainability. [Online] 9 (32), 1765–17128.
[28]. Oezaslan, M. et al. (2013) Pt-Based Core–Shell Catalyst Architectures for Oxygen Fuel Cell Electrodes. The journal of physical chemistry letters. [Online] 4 (19), 3273–3291.
[29]. Wang, Y. et al. (2020) Fundamentals, materials, and machine learning of polymer electrolyte membrane fuel cell technology. Energy and AI. [Online] 1100014-.
[30]. Li, Q. et al. (2019) The Comparability of Pt to Pt‐Ru in Catalyzing the Hydrogen Oxidation Reaction for Alkaline Polymer Electrolyte Fuel Cells Operated at 80 °C. Angewandte Chemie. [Online] 131 (5), 1456–1460.
[31]. Merle, G. et al. (2011) Anion exchange membranes for alkaline fuel cells: A review. Journal of membrane science. [Online] 377 (1), 1–35.
[32]. Barbir, F. (2012) PEM Fuel Cells - Theory and Practice. Second edition. San Diego: Elsevier.
[33]. Offer, G. J. (2009) ‘PEM fuel cell electrocatalysts and catalyst layers: Fundamentals and applications’. Platinum metals review. [Online] 53 (4), 219–220.
[34]. Thomas, J. et al. (eds.) (2024) Alkaline anion exchange membranes for fuel cells: from tailored materials to novel applications / edited by Jince Thomas, Alex Schechter, Flavio Grynszpan, Bejoy Francis, Sabu Thomas. [Online]. Weinheim: Wiley-VCH.
[35]. Millichamp, J. S. (2013) Development of a novel high temperature crystal microbalance in-situ sensor for the study of electrode processes in solid oxide fuel cells / Jason Shaun Millichamp. Thesis (Ph.D.)--University College London, 2013.
[36]. Zervas, P. L. et al. (2008) Development of a novel computational tool for optimizing the operation of fuel cells systems: Application for phosphoric acid fuel cells. Journal of power sources. [Online] 185 (1), 345–355.
[37]. Milewski, J. et al. (2018) Temperature influence on six layers samaria doped ceria matrix impregnated by lithium/potassium electrolyte for Molten Carbonate Fuel Cells. International journal of hydrogen energy. [Online] 43 (1), 474–482.
[38]. Soumeur, M. A. et al. (2020) Comparative study of energy management strategies for hybrid proton exchange membrane fuel cell four wheel drive electric vehicle. Journal of power sources. [Online] 462228167-.
[39]. Zhang, J. (2008) PEM fuel cell electrocatalysts and catalyst layers : fundamentals and applications / Jiujun Zhang, editor. London: Springer.
[40]. Upadhyay, S. N. et al. (2023) Illuminating the Role of Mo Defective 2D Monolayer MoTe2 toward Highly Efficient Electrocatalytic O2 Reduction Reaction. Langmuir. [Online] 39 (49), 17700–17712.
[41]. Yang, R. et al. (2007) Dependence of the activity of sputtered Co-C-N oxygen reduction electrocatalysts on heat-treatment temperature. Journal of the Electrochemical Society. [Online] 154 (9), B893–B901.
[42]. Loichet Torres, P. A. et al. (2023) ORR Activity and Voltage-Cycling Stability of a Carbon-Supported PtxY Alloy Catalyst Evaluated in a PEM Fuel Cell. Journal of the Electrochemical Society. [Online] 170 (12), .
[43]. Jeremiasse, A. W. et al. (2011) Performance of metal alloys as hydrogen evolution reaction catalysts in a microbial electrolysis cell. International journal of hydrogen energy. [Online] 36 (17), 10482–10489.[45] Guo, P. et al. (2009) Cu/ZnO/Al2O3 water-gas shift catalysts for practical fuel cell applications: the performance in shut-down/start-up operation. International journal of hydrogen energy. [Online] 34 (5), 2361–2368.
[44]. Zhang, Z. (2019) Durable Fuel Cell Electrocatalysts for Energy Conversion. ProQuest Dissertations & Theses.
[45]. Guo, P. et al. (2009) Cu/ZnO/Al2O3 water-gas shift catalysts for practical fuel cell applications: the performance in shut-down/start-up operation. International journal of hydrogen energy. [Online] 34 (5), 2361–2368.
[46]. Berger, D. J. (1999) Fuel Cells and Precious-Metal Catalysts. Science (American Association for the Advancement of Science). [Online] 286 (5437), 49–49.
[47]. Chen, Z., Higgins, D., Yu, A., Zhang, L. and Zhang, J. (2011). A review on non-precious metal electrocatalysts for PEM fuel cells. Energy & Environmental Science, 4(9), p.3167. doi:https://doi.org/10.1039/c0ee00558d.
[48]. Cui, J. et al. (2021) Recent advances in non-precious metal electrocatalysts for oxygen reduction in acidic media and PEMFCs: an activity, stability and mechanism study. Green chemistry : an international journal and green chemistry resource : GC. [Online] 23 (18), 6898–6925.
[49]. Yu, H. et al. (2023) Research Progress on Porous Carbon-Based Non-Precious Metal Electrocatalysts. Materials. [Online] 16 (8), 3283-.
[50]. Mohamed, H. F. M. et al. (2018) A promising fuel cell catalyst using non-precious metal oxide. IOP Conference Series: Materials Science and Engineering. [Online] 464 (1), 12002-.
[51]. Stamenkovic, V. R. et al. (2007) Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nature materials. [Online] 6 (3), 241–247.
[52]. Fretz, S. J. (2018) Adding Utility to Carbon Materials: Introducing Dopants Using Highly Soluble Metal Salts and Functionalizing Surfaces via Bromomethylation. ProQuest Dissertations & Theses.
[53]. Fang, Z. et al. (2020) The Effect of Carbon Support Surface Functionalization on PEM Fuel Cell Performance, Durability, and Ionomer Coverage in the Catalyst Layer. Journal of the Electrochemical Society. [Online] 167 (6), 64506-.
[54]. Lei, J.-T. et al. (2023) Hollow sea urchin-like microspheres of the dual transition metal oxides NiO/Co3O4 immobilized on rGO enhance lithium-ion battery cycling and rate performance. Journal of alloys and compounds. [Online] 969172376-.
[55]. CHIWATA, M. et al. (2016) Oxygen Reduction Reaction Activity of Carbon-Supported Pt-Fe, Pt-Co, and Pt-Ni Alloys with Stabilized Pt-Skin Layers. Denki kagaku oyobi kōgyō butsuri kagaku. [Online] 84 (3), 133–137.
[56]. Ren, X. et al. (2020) Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustainable energy & fuels. [Online] 4 (1), 15–3.
[57]. Gummalla, M. et al. (2015) Effect of particle size and operating conditions on Pt3Co PEMFC cathode catalyst durability. Catalysts. [Online] 5 (2), 926–948.
[58]. Janssen, M. M. P. & Moolhuysen, J. (1976) Platinum-tin catalysts for methanol fuel cells prepared by a novel immersion technique, by electrocodeposition and by alloying. Electrochimica acta. [Online] 21 (11), 861–868.
[59]. Hong, J. et al. (2023) First-principles study on the d-band center of Pt alloyed with 3d transition metals. Journal of the Korean Physical Society. [Online] 83 (12), 964–969.
[60]. Zhou, X. et al. (2023) Individually-atomic governing d—π orbital interactions via Cu-promoted optimization of Fe-d band centers for high-efficiency zinc-air battery. Nano research. [Online] 16 (4), 4634–4642.
[61]. Kim, C. E. et al. (2015) Effect of gold subsurface layer on the surface activity and segregation in Pt/Au/Pt3M (where M = 3 d transition metals) alloy catalyst from first-principles. The Journal of chemical physics. [Online] 142 (3), 034707–034707.
[62]. Xie, Z. et al. (2021) Identification of electronic descriptors for catalytic activity of transition-metal and non-metal doped MoS2. Physical chemistry chemical physics : PCCP. [Online] 23 (28), 15101–15106.
[63]. Anon (2020) Patent Issued for Preparation Method Of Carbon-Supported Metal Oxide And/Or Alloy Nanoparticles Catalyst Using Physical Vapour Deposition. NewsRX LLC.
[64]. Shi, L. et al. (2022) A new reduction method based on simultaneous TiAlC support etching and metal deposition to prepare Pt catalysts for aqueous-phase selective hydrogenation of furfural to furfuryl alcohol. New journal of chemistry. [Online] 46 (31), 14958–14966.
[65]. MacIsaac, C. et al. (2018) Atomic and Molecular Layer Deposition of Hybrid Mo–Thiolate Thin Films with Enhanced Catalytic Activity. Advanced functional materials. [Online] 28 (26),
[66]. Yamamoto, A. & Tsubakino, H. (2003) A New Technique for Surface Modification in Magnesium Alloys by Applying Magnesium Oxide Coating. Materials science forum. [Online] 419–422 (II), 903–908.
[67]. Zhang, Y. et al. (2023) Research Progress of Transition Metal Anode Catalysts for Direct Borohydride Fuel Cells. Journal of nanoparticle research : an interdisciplinary forum for nanoscale science and technology. [Online] 25 (12), 240-.
[68]. Zhang, R. et al. (2023) Pt-Fe-Co Ternary Metal Single Atom Catalyst for toward High Efficiency Alkaline Oxygen Reduction Reaction. Energies (Basel). [Online] 16 (9), 3684-.
[69]. Luo, J. et al. (2023) Recent progress in high-loading single-atom catalysts and their applications. Industrial Chemistry & Materials. [Online] 1 (4), 486–500.
[70]. Chen, J. et al. (2023) Metal-support interactions for heterogeneous catalysis: mechanisms, characterization techniques and applications. Journal of materials chemistry. A, Materials for energy and sustainability. [Online] 11 (16), 854–8572.
[71]. Fang, J. et al. (2023) The synthesis of single-atom catalysts for heterogeneous catalysis. Chemical communications (Cambridge, England). [Online] 59 (2), 2854–2868.
[72]. Gong, X., Song, P., Han, C., Xiao, Y., Mei, X. and Xu, W. (2023). Heterogeneous single-atom catalysts for energy process: recent progress, applications and challenges. Energy Materials. doi:https://doi.org/10.20517/energymater.2022.82.
[73]. Xi, F. et al. (2024) A 4750 hours’ durability investigation of the PEMFC stack based on fuel cell hybrid vehicles conditions. International journal of green energy. [Online] 21 (8), 1874–1885.
[74]. Williams, B. (2022). Toyota Mirai vs Hyundai Nexo: Hydrogen cars compared - HFN. [online] www.hydrogenfuelnews.com. Available at: https://www.hydrogenfuelnews.com/mirai-vs-nexo-hydrogen-cars/8551076/.
[75]. Trachte, U. & Fumey, B. (2013) ‘Applications – Stationary | Uninterruptible power supply and backup power with fuel cells and batteries’, in Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. [Online]. Elsevier Inc. p.
[76]. FuelCellsWorks (2023). Ireland, The European Data Center ‘Hub,’ Addresses Its Power Shortage With Fuel Cells With SK Ecoplant Supplying Fuel Cells To A New Data Center In The Country - FuelCellsWorks. [online] Available at: https://fuelcellsworks.com/news/ireland-the-european-data-center-hub-addresses-its-power-shortage-with-fuel-cells-with-sk-ecoplant-supplying-fuel-cells-to-a-new-data-center-in-the-country/ [Accessed 24 Jul. 2024].
[77]. taylor-research.yale.edu. (n.d.). Fuel Cells | Transformative Materials & Devices. [online] Available at: https://taylor-research.yale.edu/research/fuel-cells [Accessed 27 Jul. 2024].
[78]. Lin, Z. et al. (2013) Hydrogen vehicles: Impacts of DOE technical targets on market acceptance and societal benefits. International journal of hydrogen energy. [Online] 38 (19), 7973–7985.
[79]. Keshri, S. et al. (2024) State-of-the-art review on hydrogen’s production, storage, and potential as a future transportation fuel. Environmental science and pollution research international. [Online]









