1. Introduction
Photodynamic therapy (PDT) is a non-invasive therapy for the treatment of cancer as well as many types of non-tumor diseases. Its molecular mechanism is mainly based on three elements: photosensitizers (PS), light of appropriate wavelength and oxygen dissolved in the cells [1], and it has become the fourth most important method of tumor treatment after radiotherapy, chemotherapy and surgery. In clinical practice, PDT has been reported to be used alone and in combination with other therapies[2], such as in the clinical treatment of superficial skin cancer [3], esophageal cancer [4], lung cancer [5] and other oncological diseases and some non-oncological diseases. In addition, a large body of research evidence suggests that PDT has the potential to treat a variety of cancers, such as breast cancer [6], bile duct cancer [7], and pancreatic cancer [7].
1.1. Mechanisms of action of PDT against tumors
The most important mechanism of PDT action on tumors is reactive oxygen species (ROS) killing. After entering the cell, PS absorbs photons and transforms from the ground state to an unstable electron-excited singlet by light irradiation of a certain wavelength, and then progresses to a more stable and longer-lasting electron-excited triplet state by intersystem crossover through the spin-switching of electrons in high-energy orbitals. PDT is usually classified into type I and type II based on the photochemical reaction process of reactive oxygen species. Type I PDT refers to the process in which PS in the excited triplet state reacts directly with triple oxygen (3O2) or biological substrates (such as cell membrane) to produce superoxide anion (O2−) and hydroxyl radical (OH ̇) respectively [8]. However, type II PDT is the process of energy exchange between PS and molecular oxygen to produce highly active singlet oxygen (1O2) [9]. Type I and type II reactions can occur simultaneously, and the generated ROS can cause oxidative damage to a variety of tumor cells. Although the ratio of the two reactions occurring depends on the substrate, PS, oxygen concentration, and the binding affinity of the photosensitizer to the substrate, in contrast, the type II reaction always dominates during PDT.
In addition, PDT can also achieve tumor killing effect by inducing tumor vascular damage and stimulating anti-tumor immune response.
2. Photosensitizers
2.1. Porphyrins
Porphyrins are essential to the biological activity of all organisms as major components of hemoglobin and myoglobin. It contains 18 π-electron aromatic macrocycles that exhibit characteristic absorption spectra with strong π-π* leaps in the visible region. Due to its strong 1O2 generation efficiency and excellent fluorescence properties, porphyrin is uniquely suited for PDT.
2.1.1. First-generation photosensitizers. First-generation photosensitizers are mainly mixtures of hematoporphyrin derivatives (HpD).In 1993, Dougherty et al. were the first to extract photosensitizing Photofrin from HpD by purification and chemical modification [10], which is now FDA-approved for the treatment of esophageal and endobronchial cancers in photodynamic therapy, and was the first photosensitizer to receive approval for clinical treatment. Despite the widespread use and efficacy of first-generation photosensitizers in PDT, they still have the drawbacks of low chemical purity and high skin phototoxicity.
2.1.2. Second-generation photosensitizers. Compared with first-generation photosensitizers, second-generation photosensitizers have higher chemical purity, higher yield of ROS formation, better targeting, and better penetration into deep tissues, overcoming certain first-generation photosensitizers’ shortcomings [11].
Tookad Palladium-barbiturate (Tookad) is a novel compound derived from the photosynthetic pigment BChla, the bacterial equivalent of plant chlorophyll. Using a 763 light source with a light penetration depth of 4 mm, monotherapy with Tookad-based PDT produced a 69% cure rate in subcutaneous tumors within 90 days of treating established bone metastases and orthotopic prostate models. An in situ model of the disease produced similar results [12].
Lebulan 5-aminolevulinic acid (5-ALA) is an intermediate product of porphyrin organism metabolism and can be involved in the biosynthesis of hemoglobin in humans. However, it is not photosensitive and needs to react with an enzyme to produce protoporphyrin IX (PpⅨ) in vivo before it can play a role.
Npe6 (Laserphyrin, Talaporfin) is a hydrophilic chlorine from chlorophyll a, which can be rapidly cleared from circulation. Npe6 is currently approved in Japan for the treatment of early and advanced lung cancer, esophageal cancer, and intracranial brain tumors [13]. However, there is also evidence that glioblastoma cells (GBM) passing through after the use of Npe6-PDT and short-term relapse may induce a more malignant phenotype in GBM [14].
Foscan tetrahydroxyphenyl chloride (mTHPC, Temoporfin) is a single, pure chlorine derivative with excellent photophysical properties and higher single-site oxygen equivalents [15]. In a study of Foscan-mediated PDT in early-stage oral squamous cell carcinoma (SCC), 85% of protocol-eligible patients achieved a complete tumor response. Patient survival rates were 89% and 75% at one and two years, respectively [16].
Phthalocyanine Phthalocyanine (Pc) exhibits a strong absorption band at 670-770 nm of the spectrum, produces high levels of ROS, and has a long-lived triplet state [17]. The photosensitivity of phthalocyanine can be enhanced when appropriate atoms such as zinc, aluminum, or silicon are present in the center. Wang et al [18] constructed a nanoscale photosensitizer (NanoPcM) by self-assembly of morphine-substituted silicon phthalocyanine (PcM) and albumin. NanoPcM-based PDT was effective in inhibiting tumor growth, reducing spontaneous lung metastasis.
2.1.3. Third-generation and novel photosensitizers. Due to the poor solubility and low bioavailability of some second-generation photosensitizers in water, the development of third-generation photosensitizers, in which the drug delivery system works in conjunction with second-generation photosensitizers, has become a major research direction [19]. Lu et al [20] developed a composite nanosystem of deep penetration and pH-responsiveness to synergistically enhance the efficacy of combined photothermal and photodynamic therapies against hypoxic tumors. R. Liang and his team [21] constructed a composite nanosystem consisting of polyvinyl pyrrolidone micelles in a carrier consisting of a photosensitizer (zinc phthalo dendrimer) and a targeting agent (folic acid). They demonstrated that zinc phthalate in the monomeric state contained in the polymer micelles could increase the efficiency of singlet oxygen generation and thus the PDT efficiency.
2.2. Non-porphyrins
The development of nonporphyrin photosensitizers for oncology applications has lagged far behind porphyrin-based PDT. Currently, cationic photosensitizers such as chalcogenopyrylium dyes, benzothiazine derivatives, and a number of thickened cyclic quinones are the main focus of research.
Methylene blue Methylene blue is a thiazine dye widely used in several medical disciplines to produce singlet oxygen and destroy nucleic acids in a nuclease-like manner. Since methylene blue suffers from poor localization to tumors and is easily reduced by cellular enzymes in vivo, nanoparticle-encapsulated methylene blue offers new ideas for methylene blue-mediated PDT in cancer [22].
Hypericin Hypericin is lipophilic and has been shown to be very efficient in generating singlet oxygen and superoxide anions. It has been shown that Hypericin-mediated photodynamic therapy (PDT) can treat nasopharyngeal carcinoma (NPC) by inhibiting matrix metalloproteinase-9 (MMP-9) expression [23].
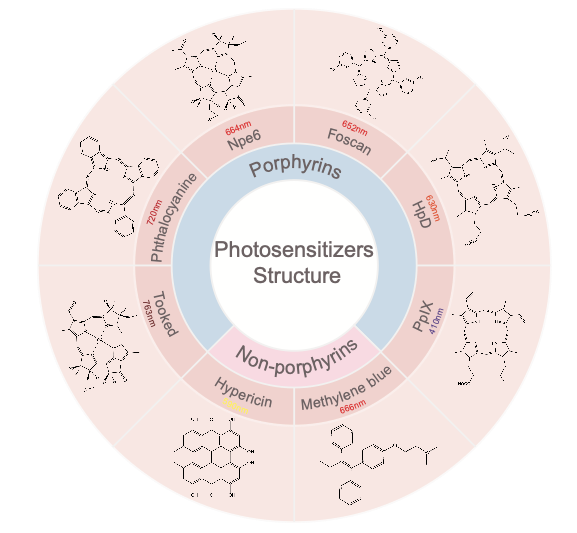
Figure 1. Photosensitizer structures and their therapeutic wavelengths
3. Photodynamic therapy in cancer
3.1. Breast Cancer
Breast cancer occurs in the ductal lining cells of the breast or in the lobules of the breast glandular tissue. Over time, these in situ cancer cells may progress and invade surrounding breast tissue, then spread to nearby lymph nodes or metastasize distantly to other organs in the body. Most patients die from breast cancer due to massive metastasis of the cancer cells. Current treatments for breast cancer usually include surgical excision, radiation therapy and medication.
Wu et al [24] employed nanofactors characterized by high efficiency of ROS generation and low toxicity to treat the 4T1 cell line using 660 nm photoluminescence. After encapsulation, MB-liposomes were observed to have higher in vitro ROS generation capacity than free MB and killed more cancer cells. Machado et al [25] analyzed curcumin (Cur) nanoemulsions as a drug delivery system (DDS+) and used it as a PS in the PDT of MCF-7 cells. The results showed a 10% reduction in the number of live cancer cells and an increase in the number of ROS as seen in the Curcumin is an effective tool for PDT in the treatment of breast cancer.
Triple-negative breast cancer (TNBC), as one of the aggressive breast cancer subtypes, still has no effective treatment to completely cure it. In contrast, Lv et al [26] constructed a multifunctional nanoplatform FA-CD@PP-CpG based on a 4T1 tumor mouse model to synergize with PDT and doxorubicin (DTX) to enhance immunotherapy, which exhibited excellent PDT efficacy and photothermal conversion ability and significantly inhibited tumor growth in vivo under irradiation at 650 nm and 808 nm, respectively. In FA-CD@PP-CpG low-dose loaded DTX could promote the infiltration of cytotoxic T lymphocytes (CTLs), inhibit myeloid-derived suppressor cells (MDSCs), and efficiently polarize them to the M1 phenotype to further enhance the anti-tumor efficacy.
3.2. Lung cancer
Lung cancer is the malignant tumor with the highest mortality rate worldwide and is usually diagnosed at an advanced stage with limited treatment options. Lung cancer is divided into two categories: non-small cell carcinoma (NSCLC) and small cell carcinoma (SCLC).
The common types of NSCLC are squamous cell carcinoma, adenocarcinoma, and large cell carcinoma, which have evolved over the past two decades from the empirical use of cytotoxic drugs to better tolerated and more efficacious regimens by targeting specific molecular subtypes of the disease. Inhibitors of EGFR, ALK, RET, BRAF, ROS1, NTRK, MET, and KRAS are currently used to treat NSCLC harboring the corresponding mutations, but patients almost universally develop resistance during treatment. PDT can restore organ and tissue function in patients with severe ulcers after surgery In a follow-up case, NSCLC patients were irradiated without tumor treatment at 630 nm for 500 s at 200 J/cm. approximately thirty days after PDT illumination, there were no complications from surgical lobectomy, and there were no signs of disease on imaging and clinical examination 90 days after PDT [27].
3.3. Glioma
Gliomas are tumors that originate from the glial cells of the brain and are the most common primary intracranial tumors. Among them, glioblastoma (GBM) accounts for 57.3% of all gliomas [28] and is the most aggressive glioma [29].
The standard treatment for gliomas is based on surgical resection, combined with a combination of radiotherapy and chemotherapy. However, the unique microenvironment of gliomas, such as the presence of the blood-brain barrier (BBB), oxidative stress, hypoxia, and angiogenesis, make the prognosis of conventional therapies poor. In patients with high-grade gliomas, disease recurrence is almost inevitable. Despite advances in current therapeutic strategies, there are no standard treatment options for recurrent gliomas and recurrent GBM (rGBM).
Over the past 40 years or so, PDT has evolved as a promising therapeutic strategy. In contrast to common PDT tumor-mediated modalities such as uptake, induction of cell death after accumulation of PS, disruption of tumor vasculature, and enhancement of the tumor immune response, PDT also inhibits the growth and promotes apoptosis of glioma stem cells (GSC). In addition, most of the developed anti-glioma drugs have difficulty in crossing the blood-brain barrier (BBB) in order to reach the tumor site to kill cancer cells, while PDT induces the generation of singlet oxygen (1O2) leading to an imbalance in endothelial regulation of vasorelaxation resulting in redistribution of calcium, which results in an increase in the permeability of the drug to the blood-brain barrier (BBB) [30].
4. Photodynamic Combination Therapy in Cancer Treatment
4.1. Photodynamic combined immunotherapy
Tumor metastasis has been one of the great challenges in tumor therapy, and promoting the activation of the body’s own immune system will help to reduce the probability of metastasis. In PDT therapy, necrotic or apoptotic tumor cells release associated antigens, which induces acute inflammation and APC infiltration in the tumor, further presenting antigens to T cells and activating them to kill the tumor cells. Yu [31] and others constructed nanoparticle GO ( HPPH)-PEG-HK as photosensitizers and increased the infiltration of cytotoxic CD8+ T-lymphocytes in tumors through activation of dendritic cells, resulting in significant inhibition of primary tumor growth and elevation of antitumor effects in lungs. Gu et al [32], in a retrospective study, evaluated the peripheral blood of 52 patients with stage III-IV colorectal cancer (CRC) who received PDT and the immune cell changes in tumor tissues. The results showed that the local infiltration of various immune cells, such as CD3+ T cells and CD4+ T cells, was significantly increased in tumor tissues of patients with stage IV CRC, and many inflammatory and immune cells infiltrated into the tumor tissues at 48 h after PDT. The overall survival (OS) of the PDT group was significantly longer than that of the non-PDT group.
4.2. Photodynamic synergistic nanomaterial therapy
Nanoparticle-based PDT is a new therapeutic strategy that relies on nanoparticles as carriers or PS. According to the three key elements of PDT, i.e., PS, excitation light, and oxygen, nanomaterials can target the above to enhance PDT efficacy.
Conventional PS is unstable, untargeted, highly hydrophobic, and susceptible to the body’s internal environment, whereas the selection of appropriate nanocarriers is not only effective in improving the biocompatibility of PS, but also in delivering PS to the target cells and reducing the side effects. Li et al [33] designed an efficient nuclear-targeting delivery nanoparticle in which conjugated RGD peptides conferred specific binding and recognition of the nuclear-targeting delivery system to the tumor vasculature and tumor cell membranes to significantly enhance PS specificity and reduce side effects. In addition, nanoparticle loading can facilitate tumor-specific therapy [34].
The excitation light of a single PDT is mainly concentrated in the visible region with limited penetration depth, which does not easily penetrate tumor cells in deeper layers or with chromophores (e.g., melanoma). Indocyanine green (ICG), a multifunctional PS, irradiated at an energy density of 31.2 J/cm2, improves the penetration and efficacy of PDT [35]. Automated PDT (APDT), which circumvents the limited penetration depth of conventional PDT, has also been proposed to synergistically enhance the treatment of deep-seated tumors while releasing oxygen [36].
Hypoxia within the tumor is also a major challenge for PDT. In addition to delivering oxygen to the tumor site, elevating peroxidase concentration to catalyze the generation of oxygen in the tumor microenvironment is also a strategy. Tang et al [37] used MnO2 as a nano-shell to design an H2O2-degradable nanoplatform loaded with PS that could react with H2O2 and H+ in the tumor microenvironment and overcome tumor hypoxia, demonstrating the highest tumor inhibition efficiency. Both in vitro and in vivo studies demonstrated the promotion of oxygen autogenesis for photodynamic therapy.
4.3. Photodynamic synergistic chemotherapy treatment
Combining chemotherapy with PDT has been a major research direction in recent years, as the efficiency of chemotherapy is reduced by severe side effects due to low targeting efficiency and chemoresistance. For the combination of chemotherapy and PDT, some studies have suggested a simple chemical conjugation approach for both agents [38]. However, a key limitation of this strategy is that the drugs do not maintain sufficient bioavailability because they are directly exposed to various enzymes in the bloodstream. Therefore, encapsulation of drugs using nanoparticles is one of the main research directions in conjugated therapy.
He et al [39] used core-shell nanoparticles based on nanoscale coordination polymers (NCPs) carrying a high payload of cisplatin and the photosensitizer pyrolipid, NCP@pyrolipid, for combination chemotherapy and photodynamic therapy (PDT). The results showed superior potency and efficacy compared to monotherapy in low-dose tumor regression (83% reduction in tumor volume) in a cisplatin-resistant human head and neck cancer SQ20B xenograft mouse model.Chu et al [40] developed a pre-drug delivery system that prevents drug leakage during systemic circulation, where the photosensitizer pyropheophorbide-a (Ppa) generated ROS that triggered on-demand release of CPT from local regions and exhibited cytotoxic effects on tumor cells, achieving a comprehensive inhibition of tumor growth.
5. Conclusion
In terms of anti-tumor therapy, PDT, with its unique photoinitiation mechanism and multiple combination therapies, provides more options for patients who cannot repeat surgery or who have seen little success with traditional therapies. In combination therapy, tumor internal hypoxia, PS targeting and cytotoxicity are improved, thus enhancing the anti-tumor effect while maintaining the advantages of PDT. In combination therapy for patients with lung cancer, glioma, breast cancer, etc., PDT kills tumor cells and promotes the cascade reaction of immune cells near the tumor at the same time, promotes drug targeting and drug resistance, and the prognosis of patients with adverse reaction symptoms is not obvious. Currently, clinical evidence for PDT combination therapy still has a long way to go. Since existing research, continued exploration of relevant PS applicable to PDT combination therapy may be expected to prolong the survival time of patients with currently incurable cancers, which has far-reaching significance for the treatment of malignant tumors that need to avoid the risk of surgery as well as to improve the prognosis of patients’ quality of life.
References
[1]. Julita Kulbacka. (2018). “Photodynamic Therapy - Mechanisms, Photosensitizers and Combinations.,” Biomedicine & Pharmacotherapy 106 1098–1107.
[2]. Libo Li. (2018). “Photodynamic Combinational Therapy in Cancer Treatment.,” Journal of B.U.ON. : Official Journal of the Balkan Union of Oncology 23 (3): 561–567.
[3]. Maria BR Pierre. (2021). “Nanocarriers for Photodynamic Therapy Intended to Cutaneous Tumors.,” Current Drug Targets 22 (10): 1090–1107.
[4]. Tongsen Zheng. (2021). “Photodynamic Therapy Induces Human Esophageal Carcinoma Cell Pyroptosis by Targeting the PKM2/Caspase-8/Caspase-3/GSDME Axis.,” Cancer Letters 520 143–159.
[5]. Patience Mthunzi-Kufa. (2021). “A Review of Chemotherapy and Photodynamic Therapy for Lung Cancer Treatment.,” Anti-Cancer Agents in Medicinal Chemistry 21 (2): 149–161.
[6]. Dorota Bartusik-Aebisher. (2022). “Photodynamic Therapy in Breast Cancer Treatment.,” Journal of Applied Biomedicine 20 (3): 98–105.
[7]. Kenneth K Wang. (2020). “Photodynamic Therapy for Gastrointestinal Cancer.,” Photochemistry and Photobiology 96 (3): 517–523.
[8]. Alfreda Padzik-Graczyk. (2013). “ [Photodynamic Method of Cancer Diagnosis and Therapy--Mechanisms and Applications].,” Postepy Biochemii 59 (1): 53–63.
[9]. Zhaochu Yang. (2021). “Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions.,” Pharmaceutics 13 (9):.
[10]. Michael R Hamblin. (2016). “New Photosensitizers for Photodynamic Therapy.,” The Biochemical Journal 473 (4): 347–364.
[11]. José AS Cavaleiro. (2018). “Cancer, Photodynamic Therapy and Porphyrin-Type Derivatives.,” Anais Da Academia Brasileira de Ciencias 90 (1 Suppl 2): 993–1026.
[12]. Yoram Salomon. (2003). “Photodynamic Therapy with Pd-Bacteriopheophorbide (TOOKAD): Successful in Vivo Treatment of Human Prostatic Small Cell Carcinoma Xenografts.,” International Journal of Cancer 104 (6): 782–789.
[13]. Kevin M Smith. (2023). “Amino Acid Derivatives of Chlorin-e(6)-A Review.,” Molecules (Basel, Switzerland) 28 (8):.
[14]. Arata Tomiyama. (2020). “Enhanced Malignant Phenotypes of Glioblastoma Cells Surviving NPe6-Mediated Photodynamic Therapy Are Regulated via ERK1/2 Activation.,” Cancers 12 (12):.
[15]. Thomas H Foster. (2005). “Photophysical Parameters, Photosensitizer Retention and Tissue Optical Properties Completely Account for the Higher Photodynamic Efficacy of Meso-Tetra-Hydroxyphenyl-Chlorin vs Photofrin.,” Photochemistry and Photobiology 81 (4): 849–859.
[16]. Graham Putnam. (2004). “mTHPC-Mediated Photodynamic Therapy for Early Oral Squamous Cell Carcinoma.,” International Journal of Cancer 111 (1): 138–146.
[17]. Mark Wainwright. (2008). “Photodynamic Therapy: The Development of New Photosensitisers.,” Anti-Cancer Agents in Medicinal Chemistry 8 (3): 280–291.
[18]. Juyoung Yoon. (2022). “A Nanostructured Phthalocyanine/Albumin Supramolecular Assembly for Fluorescence Turn-On Imaging and Photodynamic Immunotherapy.,” ACS Nano 16 (2): 3045–3058.
[19]. RW Boyle. (2008). “Photodynamic Therapy: Novel Third-Generation Photosensitizers One Step Closer?: Commentary,” British Journal of Pharmacology 154 (1): 1–3.
[20]. Robert E Simpson. (2021). “Reversible Tuning of Mie Resonances in the Visible Spectrum,” ACS Nano 15 (12): 19722–19732.
[21]. Xue Duan. (2014). “A Monomeric Photosensitizer for Targeted Cancer Therapy.,” Chemical Communications (Cambridge, England) 50 (95): 14983–14986.
[22]. Zhonggui He. (2020). “Recent Progress of Hypoxia-Modulated Multifunctional Nanomedicines to Enhance Photodynamic Therapy: Opportunities, Challenges, and Future Development.,” Acta Pharmaceutica Sinica. B 10 (8): 1382–1396.
[23]. BH Bay. (2007). “Hypericin Photoactivation Triggers Down-Regulation of Matrix Metalloproteinase-9 Expression in Well-Differentiated Human Nasopharyngeal Cancer Cells.,” Cellular and Molecular Life Sciences : CMLS 64 (7–8): 979–988.
[24]. Jiashing Yu. (2018). “Methylene-Blue-Encapsulated Liposomes as Photodynamic Therapy Nano Agents for Breast Cancer Cells.,” Nanomaterials 9 (1): 14.
[25]. Marília Freitas Calmon. (2019). “Effect of Curcumin-Nanoemulsion Associated with Photodynamic Therapy in Breast Adenocarcinoma Cell Line.,” Bioorganic & Medicinal Chemistry 27 (9): 1882–1890.
[26]. Chunyan Dong. (2019). “Tumor-Targeted Drug and CpG Delivery System for Phototherapy and Docetaxel-Enhanced Immunotherapy with Polarization toward M1-Type Macrophages on Triple Negative Breast Cancers.,” Advanced Materials 31 (52): 1904997.
[27]. Sandeep Bansal. (2022). “Photodynamic Therapy for Peripheral Lung Cancer.,” Photodiagnosis and Photodynamic Therapy 38 102825.
[28]. Ahmedin Jemal. (2020). “Cancer Statistics, 2020.,” CA: A Cancer Journal for Clinicians 70 (1): 7–30.
[29]. Russell J Buono. (2023). “RNA Sequencing in Hypoxia-Adapted T98G Glioblastoma Cells Provides Supportive Evidence for IRE1 as a Potential Therapeutic Target.,” Genes 14 (4):.
[30]. Edik Rafailov. (2017). “Photodynamic Opening of Blood-Brain Barrier.,” Biomedical Optics Express 8 (11): 5040–5048.
[31]. Zhaofei Liu. (2017). “Inhibiting Metastasis and Preventing Tumor Relapse by Triggering Host Immunity with Tumor-Targeted Photodynamic Therapy Using Photosensitizer-Loaded Functional Nanographenes.,” ACS Nano 11 (10): 10147–10158.
[32]. Hao Chen. (2022). “Photodynamic Therapy Improves the Clinical Efficacy of Advanced Colorectal Cancer and Recruits Immune Cells into the Tumor Immune Microenvironment.,” Frontiers in Immunology 13 1050421.
[33]. Jianlin Shi. (2014). “Intranuclear Photosensitizer Delivery and Photosensitization for Enhanced Photodynamic Therapy with Ultralow Irradiance,” Advanced Functional Materials 24 (46): 7318–7327.
[34]. PJ Schuler. (2017). “Influence of Photodynamic Therapy on Peripheral Immune Cell Populations and Cytokine Concentrations in Head and Neck Cancer.,” Photodiagnosis and Photodynamic Therapy 19 194–201.
[35]. Abbas Bahador. (2017). “The Effect of Indocyanine Green Loaded on a Novel Nano-Graphene Oxide for High Performance of Photodynamic Therapy against Enterococcus Faecalis.,” Photodiagnosis and Photodynamic Therapy 20 148–153.
[36]. Zhixiang Lu. (2023). “Internal Light Sources-Mediated Photodynamic Therapy Nanoplatforms: Hope for the Resolution of the Traditional Penetration Problem.,” Advanced Healthcare Materials e2301326.
[37]. Xiaochen Dong. (2019). “Hydrangea-Structured Tumor Microenvironment Responsive Degradable Nanoplatform for Hypoxic Tumor Multimodal Imaging and Therapy.,” Biomaterials 205 1–10.
[38]. Sergey M Deyev. (2009). “Targeting Cancer Cells by Using an Antireceptor Antibody-Photosensitizer Fusion Protein.,” Proceedings of the National Academy of Sciences of the United States of America 106 (23): 9221–9225.
[39]. Wenbin Lin. (2015). “Self-Assembled Core-Shell Nanoparticles for Combined Chemotherapy and Photodynamic Therapy of Resistant Head and Neck Cancers.,” ACS Nano 9 (1): 991–1003.
[40]. Zhiyong Qian. (2020). “Polymeric Nanoparticles with ROS-Responsive Prodrug and Platinum Nanozyme for Enhanced Chemophotodynamic Therapy of Colon Cancer.,” Advanced Science (Weinheim, Baden-Wurttemberg, Germany) 7 (20): 2001853.
Cite this article
He,Y. (2024). Research progress of photodynamic therapy in the anti-tumor field. Theoretical and Natural Science,57,119-126.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Julita Kulbacka. (2018). “Photodynamic Therapy - Mechanisms, Photosensitizers and Combinations.,” Biomedicine & Pharmacotherapy 106 1098–1107.
[2]. Libo Li. (2018). “Photodynamic Combinational Therapy in Cancer Treatment.,” Journal of B.U.ON. : Official Journal of the Balkan Union of Oncology 23 (3): 561–567.
[3]. Maria BR Pierre. (2021). “Nanocarriers for Photodynamic Therapy Intended to Cutaneous Tumors.,” Current Drug Targets 22 (10): 1090–1107.
[4]. Tongsen Zheng. (2021). “Photodynamic Therapy Induces Human Esophageal Carcinoma Cell Pyroptosis by Targeting the PKM2/Caspase-8/Caspase-3/GSDME Axis.,” Cancer Letters 520 143–159.
[5]. Patience Mthunzi-Kufa. (2021). “A Review of Chemotherapy and Photodynamic Therapy for Lung Cancer Treatment.,” Anti-Cancer Agents in Medicinal Chemistry 21 (2): 149–161.
[6]. Dorota Bartusik-Aebisher. (2022). “Photodynamic Therapy in Breast Cancer Treatment.,” Journal of Applied Biomedicine 20 (3): 98–105.
[7]. Kenneth K Wang. (2020). “Photodynamic Therapy for Gastrointestinal Cancer.,” Photochemistry and Photobiology 96 (3): 517–523.
[8]. Alfreda Padzik-Graczyk. (2013). “ [Photodynamic Method of Cancer Diagnosis and Therapy--Mechanisms and Applications].,” Postepy Biochemii 59 (1): 53–63.
[9]. Zhaochu Yang. (2021). “Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions.,” Pharmaceutics 13 (9):.
[10]. Michael R Hamblin. (2016). “New Photosensitizers for Photodynamic Therapy.,” The Biochemical Journal 473 (4): 347–364.
[11]. José AS Cavaleiro. (2018). “Cancer, Photodynamic Therapy and Porphyrin-Type Derivatives.,” Anais Da Academia Brasileira de Ciencias 90 (1 Suppl 2): 993–1026.
[12]. Yoram Salomon. (2003). “Photodynamic Therapy with Pd-Bacteriopheophorbide (TOOKAD): Successful in Vivo Treatment of Human Prostatic Small Cell Carcinoma Xenografts.,” International Journal of Cancer 104 (6): 782–789.
[13]. Kevin M Smith. (2023). “Amino Acid Derivatives of Chlorin-e(6)-A Review.,” Molecules (Basel, Switzerland) 28 (8):.
[14]. Arata Tomiyama. (2020). “Enhanced Malignant Phenotypes of Glioblastoma Cells Surviving NPe6-Mediated Photodynamic Therapy Are Regulated via ERK1/2 Activation.,” Cancers 12 (12):.
[15]. Thomas H Foster. (2005). “Photophysical Parameters, Photosensitizer Retention and Tissue Optical Properties Completely Account for the Higher Photodynamic Efficacy of Meso-Tetra-Hydroxyphenyl-Chlorin vs Photofrin.,” Photochemistry and Photobiology 81 (4): 849–859.
[16]. Graham Putnam. (2004). “mTHPC-Mediated Photodynamic Therapy for Early Oral Squamous Cell Carcinoma.,” International Journal of Cancer 111 (1): 138–146.
[17]. Mark Wainwright. (2008). “Photodynamic Therapy: The Development of New Photosensitisers.,” Anti-Cancer Agents in Medicinal Chemistry 8 (3): 280–291.
[18]. Juyoung Yoon. (2022). “A Nanostructured Phthalocyanine/Albumin Supramolecular Assembly for Fluorescence Turn-On Imaging and Photodynamic Immunotherapy.,” ACS Nano 16 (2): 3045–3058.
[19]. RW Boyle. (2008). “Photodynamic Therapy: Novel Third-Generation Photosensitizers One Step Closer?: Commentary,” British Journal of Pharmacology 154 (1): 1–3.
[20]. Robert E Simpson. (2021). “Reversible Tuning of Mie Resonances in the Visible Spectrum,” ACS Nano 15 (12): 19722–19732.
[21]. Xue Duan. (2014). “A Monomeric Photosensitizer for Targeted Cancer Therapy.,” Chemical Communications (Cambridge, England) 50 (95): 14983–14986.
[22]. Zhonggui He. (2020). “Recent Progress of Hypoxia-Modulated Multifunctional Nanomedicines to Enhance Photodynamic Therapy: Opportunities, Challenges, and Future Development.,” Acta Pharmaceutica Sinica. B 10 (8): 1382–1396.
[23]. BH Bay. (2007). “Hypericin Photoactivation Triggers Down-Regulation of Matrix Metalloproteinase-9 Expression in Well-Differentiated Human Nasopharyngeal Cancer Cells.,” Cellular and Molecular Life Sciences : CMLS 64 (7–8): 979–988.
[24]. Jiashing Yu. (2018). “Methylene-Blue-Encapsulated Liposomes as Photodynamic Therapy Nano Agents for Breast Cancer Cells.,” Nanomaterials 9 (1): 14.
[25]. Marília Freitas Calmon. (2019). “Effect of Curcumin-Nanoemulsion Associated with Photodynamic Therapy in Breast Adenocarcinoma Cell Line.,” Bioorganic & Medicinal Chemistry 27 (9): 1882–1890.
[26]. Chunyan Dong. (2019). “Tumor-Targeted Drug and CpG Delivery System for Phototherapy and Docetaxel-Enhanced Immunotherapy with Polarization toward M1-Type Macrophages on Triple Negative Breast Cancers.,” Advanced Materials 31 (52): 1904997.
[27]. Sandeep Bansal. (2022). “Photodynamic Therapy for Peripheral Lung Cancer.,” Photodiagnosis and Photodynamic Therapy 38 102825.
[28]. Ahmedin Jemal. (2020). “Cancer Statistics, 2020.,” CA: A Cancer Journal for Clinicians 70 (1): 7–30.
[29]. Russell J Buono. (2023). “RNA Sequencing in Hypoxia-Adapted T98G Glioblastoma Cells Provides Supportive Evidence for IRE1 as a Potential Therapeutic Target.,” Genes 14 (4):.
[30]. Edik Rafailov. (2017). “Photodynamic Opening of Blood-Brain Barrier.,” Biomedical Optics Express 8 (11): 5040–5048.
[31]. Zhaofei Liu. (2017). “Inhibiting Metastasis and Preventing Tumor Relapse by Triggering Host Immunity with Tumor-Targeted Photodynamic Therapy Using Photosensitizer-Loaded Functional Nanographenes.,” ACS Nano 11 (10): 10147–10158.
[32]. Hao Chen. (2022). “Photodynamic Therapy Improves the Clinical Efficacy of Advanced Colorectal Cancer and Recruits Immune Cells into the Tumor Immune Microenvironment.,” Frontiers in Immunology 13 1050421.
[33]. Jianlin Shi. (2014). “Intranuclear Photosensitizer Delivery and Photosensitization for Enhanced Photodynamic Therapy with Ultralow Irradiance,” Advanced Functional Materials 24 (46): 7318–7327.
[34]. PJ Schuler. (2017). “Influence of Photodynamic Therapy on Peripheral Immune Cell Populations and Cytokine Concentrations in Head and Neck Cancer.,” Photodiagnosis and Photodynamic Therapy 19 194–201.
[35]. Abbas Bahador. (2017). “The Effect of Indocyanine Green Loaded on a Novel Nano-Graphene Oxide for High Performance of Photodynamic Therapy against Enterococcus Faecalis.,” Photodiagnosis and Photodynamic Therapy 20 148–153.
[36]. Zhixiang Lu. (2023). “Internal Light Sources-Mediated Photodynamic Therapy Nanoplatforms: Hope for the Resolution of the Traditional Penetration Problem.,” Advanced Healthcare Materials e2301326.
[37]. Xiaochen Dong. (2019). “Hydrangea-Structured Tumor Microenvironment Responsive Degradable Nanoplatform for Hypoxic Tumor Multimodal Imaging and Therapy.,” Biomaterials 205 1–10.
[38]. Sergey M Deyev. (2009). “Targeting Cancer Cells by Using an Antireceptor Antibody-Photosensitizer Fusion Protein.,” Proceedings of the National Academy of Sciences of the United States of America 106 (23): 9221–9225.
[39]. Wenbin Lin. (2015). “Self-Assembled Core-Shell Nanoparticles for Combined Chemotherapy and Photodynamic Therapy of Resistant Head and Neck Cancers.,” ACS Nano 9 (1): 991–1003.
[40]. Zhiyong Qian. (2020). “Polymeric Nanoparticles with ROS-Responsive Prodrug and Platinum Nanozyme for Enhanced Chemophotodynamic Therapy of Colon Cancer.,” Advanced Science (Weinheim, Baden-Wurttemberg, Germany) 7 (20): 2001853.









