1. Introduction
The ability of woody plants to regenerate from tissue cultures offers profound implications for ecological restoration, genetic improvement, and sustainable forestry practices. The exploration into the regenerative capacities of such plants, particularly through in vitro tissue culture methods, has unveiled potential pathways for advancing both agricultural and environmental applications. Salix species, among other woody plants, exhibit notable regeneration capabilities, making them ideal candidates for studies focused on genetic transformation systems necessary for enhancing traits like stress tolerance and growth efficiency.
1.1. Research Context and Importance of Woody Plant Regeneration
The regeneration of woody plants encompasses a wide array of processes, from simple wound healing to the complex development of new organs or even whole organisms. Early practices of plant propagation have historically leveraged the natural regenerative abilities of plants. With advancements postulated by Haverlant in 1992, the field of tissue culture has dramatically evolved, particularly with the discovery of auxin and cytokinin, which have significantly influenced the fate of plant development in culture conditions [1]. This progression in tissue culture technology has been particularly instrumental in fostering studies on woody plants like Populus and Salix, where efforts have been made to introduce traits such as herbicide resistance and improved stress responses through genetic transformation.
1.2. Current Advances in Woody Plant Regeneration Techniques
The application of tissue culture techniques in woody plants serves multiple purposes, from rapid propagation of valuable timber species to conservation of endangered species. Various studies have successfully established regeneration systems across a diverse array of woody plants, enhancing our capacity to manipulate and understand their genetic makeup. For instance, significant milestones have been achieved with Populus in terms of herbicide resistance and with Salix matsudana for its genetic transformation systems using somatic embryogenesis.
1.3. Specific Focus on Salix Species
The genus Salix, known for its resilience and adaptability to harsh environments, presents a unique system for studying plant regeneration. Recent research has delved into the regeneration capacities of various Salix species, demonstrating the critical roles of genotype, explant choice, and hormonal regulation in successful tissue culture [2]. These studies underscore the variability in regeneration potential among species and highlight the tailored approaches needed for effective culture systems.
1.4. Challenges and Prospective Solutions
Despite the advancements in regeneration techniques, the process remains challenging due to genetic and environmental factors that significantly affect the outcomes. Issues such as explant browning, low induction rates, and the complex interaction between genetic traits and culture conditions need targeted solutions to enhance regeneration efficiency. Furthermore, the development of robust genetic transformation systems is essential for imparting desirable traits to improve stress resistance and growth characteristics.
2. Experimental Materials and Methods
This section outlines the detailed experimental approaches, including the preparation and sterilization of materials, preparation of media with various plant hormones, and the procedures followed for the aseptic cultivation of Salix linearistipularis explants. The methodologies aim to ensure reproducibility and accuracy in the regeneration experiments conducted [3].
2.1. Materials
Plant Materials: Explants used were internodes devoid of buds, and terminal and lateral buds from Salix linearistipularis plants, which were grown under in vitro aseptic conditions for approximately 40 days prior to experimentation.
Laboratory Equipment: The experiments utilized standard laboratory equipment including an aseptic clean bench, a plant constant temperature culture box, centrifuge, pipettes, electronic balance, cultural bottles and flats, tweezers, and sterilized surgical knives.
2.2. Reagents and Media Preparation
Table 1 provides a comprehensive overview of the chemicals and reagents utilized in the experimental procedures for plant tissue culture and regeneration of Salix linearistipularis. This table includes essential details such as the company providing each reagent, the specific product codes, quantities, and the recommended storage conditions. These reagents play critical roles in preparing the various growth media and hormonal solutions necessary for the experiments [4]. Each reagent’s storage conditions are specified to ensure their stability and effectiveness during use.
Table 1. List of Reagents
Reagent | Company | Specification | Storage Condition |
MS Medium | Sigma | M5519 100g | Room Temperature |
Agar | Biotopped, Japan | 250g | Room Temperature |
WPM | Shanghai First Research | 100g | 4°C |
Sodium Chloride | Shengong Biological Engineering | 250g | Room Temperature |
NAA | Shanghai Zhenzhon Biotechnology | 50g | Room Temperature |
6-BA | Solarbio | 25g | Room Temperature |
KT | Beijing Huayue Ocean Life | 250mg | 4°C |
TDZ | Solarbio | 100mg | 4°C |
IBA | Solarbio | 5g | 4°C |
2.3. Media Preparation
Different media were prepared for the growth and differentiation of plant explants. All media were autoclaved at 120°C for 20 minutes and stored in a 70°C oven until use, as shown in Table 2.
Table 2. Media Preparation
MS Medium Preparation | Dissolved 4.94g MS and 30g sugar in 1L distilled water, adjusted pH to 5.6-5.8, added 8g agar. |
WPM Medium Preparation | Mixed 2.41g WPM and 25g sugar in 1L distilled water, adjusted pH, added 5.8g agar. |
Hormone Solutions | Solutions of NAA, 6-BA, KT, TDZ, and IBA were prepared in ethanol or NaOH as appropriate, sterilized through filtration, and added to the media under aseptic conditions to avoid hormone degradation. |
3. Experimental Methods
3.1. Aseptic Treatment of Plant Material
Plant explants were first washed with detergent, rinsed with tap water, and then subjected to several rinses with distilled water on an aseptic clean bench. Surface sterilization was achieved using 5% sodium hypochlorite followed by repeated rinses in sterile distilled water. Explants were placed in culture tubes containing different types of sterile media [5]. Each type of explant was exposed to varying concentrations of hormonal treatments to assess their effects on callus induction and plant regeneration. Table 3 outlines the specific experimental setups used to assess the regeneration capabilities of Salix linearistipularis explants under various hormonal treatments.
Table 3. Experimental Treatments and Conditions
Treatment ID | Explant Type | Hormones (mg/L) | Base Medium | Observation Parameters |
T1 | Terminal Bud | IBA 0.1 | MS | Plant length, freshness |
T2 | Lateral Bud | NAA 0.1, 6-BA 0.1 | WPM | Shoot segment length |
3.2. Data Collection and Statistical Analysis
The regeneration outcomes were quantitatively measured by calculating the callus induction rate, callus proliferation rate, and adventitious bud induction rate using the following formulas:
\( Callus Induction Rate (\%)= (\frac{Number of explants with callus }{ Total number of explants})× 100 \ \ \ (1) \)
\( Callus Proliferation Rate (\%)= (\frac{Number of explants with significant callus growth}{ Total number of explants} )× 100 \ \ \ (2) \)
\( Adventitious Bud Induction Rate (\%)= (\frac{Number of explants with buds}{Total number of explants} )× 100 \ \ \ (3) \)
The data collected from the experiments were analyzed statistically to identify significant differences in regeneration capabilities across different treatments, thus allowing for an understanding of the optimal conditions for the regeneration of Salix linearistipularis [6].
4. Experimental Results
This section presents the results obtained from the experimental propagation of Salix linearistipularis using different types and concentrations of plant hormones. The effects of these treatments on callus induction and the proliferation rates of terminal and lateral buds were examined comprehensively.
4.1. Plant Propagation Results
4.1.1. Terminal Buds Propagation. The propagation effectiveness of terminal buds was analyzed under four different culture media: MS, WPM, MS supplemented with 0.1 mg/L IBA, and WPM supplemented with 0.1 mg/L IBA. Observations made 35 days post-cultivation indicated significant differences in plant growth metrics such as total plant length, internode length, growth time, and overall plant vitality, as shown in Table 4.
Table 4. Growth Outcomes for Terminal Buds Across Different Media
Media Type | Total Plant Length (cm) | Internode Length (cm) | Growth Time (days) | Plant Freshness Rating |
MS | 18.5 | 3.5 | 35 | Excellent |
WPM | 17.8 | 3.2 | 35 | Very Good |
MS + 0.1 mg/L IBA | 20.2 | 3.8 | 35 | Excellent |
WPM + 0.1 mg/L IBA | 19.7 | 3.7 | 35 | Excellent |
As illustrated in Figure 1, panels B and D (not shown here), the addition of IBA to the media significantly enhanced the growth outcomes compared to the basic MS and WPM media [7]. Conversely, lateral buds did not exhibit satisfactory regeneration under any of the media tested, indicating their unsuitability for use as primary explants in regeneration experiments under the given conditions.

Figure 1. Plant propagation in different conditions
4.1.2. Lateral Buds Propagation. Despite initial poor performance, modifications in hormone concentrations improved the regeneration outcomes for lateral buds. Specifically, MS medium supplemented with a low concentration cocktail of 6-BA, IBA, and TDZ was tested. Figure 2 demonstrates that after 30-35 days, lateral buds cultivated in this modified medium exhibited growth comparable to that of terminal buds treated with IBA, fulfilling experimental requirements.
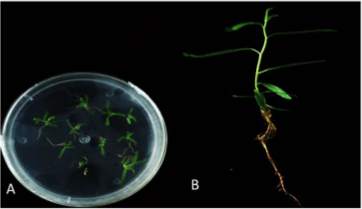
Figure 2. Plant propagation through the lateral buds
4.2. Callus Induction and Proliferation
The impact of various hormonal combinations on callus induction and proliferation was thoroughly assessed. The efficacy of these combinations was quantified by the initiation time, proliferation rate, and physical characteristics of the induced callus [8].
4.2.1. Effects of NAA and 6-BA Combinations. The combination of NAA and 6-BA significantly influenced callus induction and proliferation, with optimal results observed at higher concentrations of 6-BA, as shown in Table 5.
Table 5. Induction and Proliferation Rates at Varied Concentrations of NAA and 6-BA
NAA (mg/L) | 6-BA (mg/L) | Induction Rate (%) | Proliferation Rate (%) |
0.1 | 0.1 | 70 | 60 |
0.1 | 1.0 | 100 | 100 |
0.5 | 1.0 | 95 | 90 |
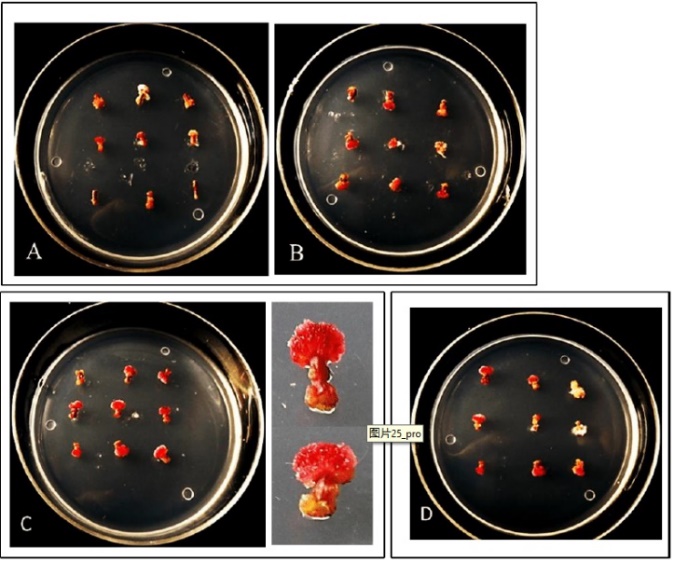
Figure 3. Callus induction and proliferation of the combination s of NAA (0.1 mg/L) and 6 BA(0.1-1→mg /L) A: NAA 0.1mg/L, 6B-A 0.7mg/L; B: NAA 0.1 mg/L, 6-BA 0.8mg/L; C: NAA 0.1mg/L, 6-BA 0.9mg/L; D: NAA 0.1mg/L, 6-BA 1mg/L
Figure 3 reveals that the combination of 0.1 mg/L NAA with 1 mg/L 6-BA resulted in 100% induction and proliferation rates, with callus exhibiting superior freshness and compactness.
5. Conclusion
The study of woody plant regeneration through tissue culture techniques, particularly focusing on Salix species, offers valuable insights into advancing ecological restoration, genetic enhancement, and sustainable forestry practices. By exploring the regenerative capacities of woody plants and employing in vitro tissue culture methods, researchers have identified potential pathways for enhancing agricultural productivity and environmental conservation. The importance of tissue culture in advancing regeneration studies cannot be overstated. Historical practices of plant propagation have evolved significantly, especially with the advent of tissue culture technology, which has been instrumental in manipulating plant development through the use of hormones like auxin and cytokinin. This technological progression has paved the way for genetic transformation systems aimed at improving traits such as stress tolerance and growth efficiency in woody plants like Populus and Salix.
Current advancements in regeneration techniques have enabled rapid propagation of valuable timber species and conservation of endangered plants. Significant milestones have been achieved in plants like Populus and Salix, with efforts focused on introducing traits like herbicide resistance and enhanced stress responses through genetic transformation. Salix species, renowned for their resilience and adaptability to harsh environments, provide an excellent system for studying plant regeneration. Recent research has elucidated the critical roles of genotype, explant choice, and hormonal regulation in successful tissue culture of Salix species. However, challenges persist, including issues like explant browning, low induction rates, and the intricate interplay between genetic traits and culture conditions. Despite these challenges, experimental studies have demonstrated promising results in propagating Salix linearistipularis. Terminal buds treated with IBA exhibited superior growth outcomes compared to untreated buds, while lateral buds showed improved regeneration potential with modified hormone concentrations.
References
[1]. Birnbaum, K. D. and Sanchez, A. (2008). Slicing across kingdoms: regeneration in plants and animals. Cell 132, 697-710.
[2]. Hartmann, H. T., Kester, D. E., Davies, F. T. and Geneve, R. (2010). Hartmann & Kester’s plant propagation: principles and practices, 8th edn. Englewood Cliffs, NJ, USA: Prentice-Hall.
[3]. Skoog, F. and Miller, C. O. (1957). Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 54, 118-130.
[4]. Szytula A and Leciejewicz J 1989 Handbook on the Physics and Chemistry of Rare Earths vol 12, ed K A Gschneidner Jr and L Erwin (Amsterdam: Elsevier) p 133
[5]. Momoko, I., Yoichi O., Akira, I. and Keiko S. (2016). Plant regeneration: cellular origins and molecular mechanisms. The Company of Biologists Ltd Development 143, 1442-1451
[6]. Azad, M. A. K. Yokota . Ohkubo , Andoh , Yahara , S. and Yoshizawa , N. ( In vitro regeneration of the medicinal woody plant Phellodendron amurense Rupr through excised leaves . Plant Cell, Tissue and Organ Culture 80,43 50
[7]. Md , I . Naseem A . and Mohammad A . (2011). The role of cytokinins on in vitro shoot production in Salix tetrasperma Roxb .., a tree of ecological importance . Trees 25 , 577 584
[8]. Meena, K., Cheruvathur, John, B. and Dennis, T. T. (2012) Pulvinus: an ideal explant for plant regeneration in Caesalpinia bonduc (L.) Roxb., an important ethnomedicinal woody climber. Acta Physiol Plant 34, 693–699
Cite this article
Cai,M. (2024). Advancing woody plant regeneration: Insights from salix species tissue culture studies. Theoretical and Natural Science,49,60-65.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Birnbaum, K. D. and Sanchez, A. (2008). Slicing across kingdoms: regeneration in plants and animals. Cell 132, 697-710.
[2]. Hartmann, H. T., Kester, D. E., Davies, F. T. and Geneve, R. (2010). Hartmann & Kester’s plant propagation: principles and practices, 8th edn. Englewood Cliffs, NJ, USA: Prentice-Hall.
[3]. Skoog, F. and Miller, C. O. (1957). Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 54, 118-130.
[4]. Szytula A and Leciejewicz J 1989 Handbook on the Physics and Chemistry of Rare Earths vol 12, ed K A Gschneidner Jr and L Erwin (Amsterdam: Elsevier) p 133
[5]. Momoko, I., Yoichi O., Akira, I. and Keiko S. (2016). Plant regeneration: cellular origins and molecular mechanisms. The Company of Biologists Ltd Development 143, 1442-1451
[6]. Azad, M. A. K. Yokota . Ohkubo , Andoh , Yahara , S. and Yoshizawa , N. ( In vitro regeneration of the medicinal woody plant Phellodendron amurense Rupr through excised leaves . Plant Cell, Tissue and Organ Culture 80,43 50
[7]. Md , I . Naseem A . and Mohammad A . (2011). The role of cytokinins on in vitro shoot production in Salix tetrasperma Roxb .., a tree of ecological importance . Trees 25 , 577 584
[8]. Meena, K., Cheruvathur, John, B. and Dennis, T. T. (2012) Pulvinus: an ideal explant for plant regeneration in Caesalpinia bonduc (L.) Roxb., an important ethnomedicinal woody climber. Acta Physiol Plant 34, 693–699









