1. Introduction
BHT, originating from the ancient Chinese medicine classic " Treatise on Cold Damage ", is one of the famous heat clearing agents in traditional Chinese medicine. This formula is composed of four drugs: gypsum, Rhizoma Anemarrhenae, Radix Glycyrrhizae, japonica rice and gypsum, and is a representative formula for treating Yangming Qi Fen Re Sheng syndrome.
In modern medical research, the antipyretic and anti-inflammatory effects of BHT have gradually received attention, and its potential mechanisms have become a hot research topic to prove the rationality of the compatibility of traditional Chinese medicine classic formulas and guide their clinical applications more scientifically. In clinical practice, fever and inflammatory reactions are common in bacterial infections. At present, Western medicine often uses antibiotics such as penicillin to treat bacterial infections, which can cause varying degrees of damage to the digestive and nervous systems, and even damage liver and kidney function. In situations where some antibiotics are ineffective, traditional Chinese medicine provides another treatment option.
In Zhang Zhongjing's " Treatise on Cold Damage", it is mentioned that "Bai Hu Tang is indicated for Shang Han condition presented by slip pulse and the coldness of the limbs due to intermediate heat." This indicates that BHT has significant effects in clearing heat, promoting diuresis, and detoxifying. Existing research has shown that the various effective ingredients contained in BHT, including calcium ions, saponins from Anemarrhena, mangiferin, and glycyrrhizic saponins, can act on multiple biological pathways and targets, playing multiple roles in the treatment process.
In clinical practice, BHT is often used to treat infectious diseases, such as lobar pneumonia, epidemic encephalitis B, epidemic hemorrhagic fever, gingivitis, etc. It has obvious antipyretic effect on fever models caused by different heat sources, and plays an anti-inflammatory role by regulating the level of inflammatory factors through multiple channels. Existing research mostly focuses on the overall effect of BHT mixture, but there is little exploration of the specific effects of certain components and they are relatively scattered.
The purpose of this article is to provide a comprehensive analysis of existing research findings, to explore the possible mechanisms of BHT in antipyresis and anti-inflammation, and to analyze how certain effective components in BHT exert their effects by influencing the generation and release of inflammatory mediators, modulating immune responses, and inhibiting inflammatory signaling pathways. This article analyzes the problems and challenges in modern research, proposes future research directions and suggestions, in order to provide reference for the in-depth research and clinical application of BHT.
2. Analysis of active ingredients
2.1. Effective ingredients of gypsum
The main active ingredient of gypsum is calcium ions produced by CaSO4 · 2H2O. Calcium ions play various important physiological roles in the human body, and their pharmacological effects are also quite extensive, such as affecting cell signaling, regulating immune cell function, and regulating enzyme activity.
2.2. Active ingredients of rhizoma anemarrhenae
Anemarrhena saponin is the main component in the rhizomes of the lily family plant Anemarrhena. There are more than 40 types of saponin components in Anemarrhena, including total saponin extract (Anemarrhena total saponin) and the main monomeric saponin components in Anemarrhena. According to literature reports, Anemarrhena asphodeloides has pharmacological activities such as anti-tumor, anti senile dementia, improving learning and memory ability, anticoagulation, antithrombotic, hypoglycemic, hypolipidemic, antidepressant, and hypotensive [1].
Mangiferin, also known as Chinonin, is a carbon ketone glycoside of tetrahydroxypyranone, belonging to the biphenylpyrone flavonoid class. Its relative molecular weight is 422.33, and its chemical structural formula is shown in Figure 1. In addition to the traditional Chinese medicine Anemarrhena, it is mainly extracted from the leaves, stems, fruits, and bark of plants in the Rhus and Gentianaceae families, and has pharmacological effects such as antioxidant, antibacterial, anti-tumor, and anti-inflammatory effects [2].
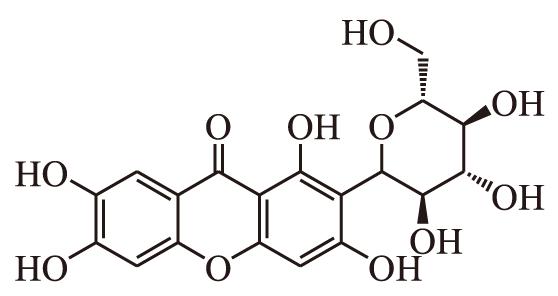
Figure 1. The chemical structure of mangiferin [3].
2.3. Effective components of Radix Glycyrrhizae
The primary effective constituents of Radix Glycyrrhizae, also known as licorice root, encompass triterpene saponins, flavonoids, isoflavonoids, polysaccharides, and other compounds.
Triterpene saponins from Radix Glycyrrhizae, such as glycyrrhizic acid and glycyrrhetinic acid, are prominent. Glycyrrhizic acid is the principal active constituent of Radix Glycyrrhizae, composed of one molecule of glycyrrhetinic acid and two molecules of glucuronic acid, with two stereoisomers, 18α and 18β. Contemporary pharmacological research has demonstrated that Radix Glycyrrhizae and its extracts exhibit a spectrum of pharmacological activities, including antibacterial, antiviral, anti-inflammatory, antioxidant, choleretic, cardiotonic, sedative, and analgesic properties.
Flavonoids from Radix Glycyrrhizae, including liquiritigenin and isoliquiritigenin, contribute to the plant's therapeutic profile. These flavonoids possess a range of pharmacological effects, such as antioxidant, anti-inflammatory, antibacterial, hepatoprotective, hormone-like activities, hypoglycemic, lipid-lowering, anticancer, and antidepressant actions. Liquiritigenin, one of the more abundant flavonoid components in Radix Glycyrrhizae, is the aglycone of liquiritin and acts as an inhibitor of Akt protein kinase. It is also recognized as a highly selective estrogen receptor agonist, conferring cytoprotective benefits. Isoliquiritigenin, another flavonoid abundant in Radix Glycyrrhizae and a common natural pigment, exhibits multiple activities, including anti-inflammatory, anticancer, antihistamine, antioxidant, antiplatelet aggregation, anticancer, antiallergic, antiviral, and estrogen-like effects.
2.4. Japonica Rice
Japonica rice is not a key ingredient in BHT. Its role is primarily reflected in the following observations: after the addition of Japonica rice to the BHT decoction, there is an increase in the content of calcium (Ca) elements and changes in the content of Al, Cu, Fe, Mn, Pb, and Zn elements. The BHT decoction with Japonica rice has a more prolonged effect and a greater reduction in body temperature compared to the decoction without Japonica rice. Additionally, the core pharmacological targets of the BHT decoction with Japonica rice are more numerous than those of the decoction without Japonica rice [4].
3. Figures
3.1. Calcium ions
The central regulator of body temperature is located in the hypothalamus, which maintains homeostasis of body temperature by monitoring and responding to changes in internal and external temperatures, and by regulating the processes of heat production and heat dissipation. Calcium ions modulate the thermoregulatory set point and the febrile response by affecting the electrophysiological properties of hypothalamic neurons and the release of neurotransmitters. In the hypothalamic thermoregulatory neurons, changes in the concentration of calcium ions can influence the excitability of the neurons. Calcium ions alter the firing patterns and frequencies of neurons by activating or inhibiting specific ion channels and receptors, such as L-type voltage-dependent calcium channels (VDCCs) and N-type calcium channels [5]. This change in electrophysiological properties directly affects the hypothalamus's response to changes in body temperature, thereby regulating the activity of the thermoregulatory center.
Calcium ions are also involved in the regulation of the release of hormones and neurotransmitters related to thermoregulation. They are essential signaling molecules for the release of catecholamines such as adrenaline and noradrenaline, which increase heat production and reduce heat dissipation by acting on peripheral tissues, thus affecting body temperature [6].
Furthermore, calcium channel antagonists, such as nifedipine, have been shown to have an antipyretic effect. Calcium channel antagonists reduce the excitability of hypothalamic neurons by blocking the entry of calcium ions into cells, thereby decreasing the activity of the thermoregulatory center and achieving the goal of reducing fever [7].
3.2. Mangiferin
Mangiferin, glycyrrhetinic acid, and ammonium glycyrrhetinic acid are attached or embedded in the nanoparticle aggregates (Nas) of BHT. N-BHT is easily taken up by cells and targets the brain and lungs. N-BHT is more effective in reducing fever than BHT [8].
Mangiferin can exert its anti-inflammatory effect by regulating the MAPK signaling pathway in white blood cells. In the LPS induced inflammation model, mangiferin can inhibit the sustained high expression of ERK and JNK in white blood cells. LPS can trigger an inflammatory response by activating Toll like receptor 4 (TLR4) on immune cells, leading to the activation and sustained high expression of MAPK members such as ERK and JNK, thereby promoting the production and release of inflammatory factors. Mangiferin reduces the activity of MAPK members, reduces the generation of inflammatory factors, and alleviates the inflammatory response. Mangiferin can also downregulate the phosphorylation level of ERK protein. Phosphorylated ERK enters the nucleus to promote the transcription of inflammation related genes, while mangiferin blocks this process by reducing ERK phosphorylation, thereby inhibiting the expression of inflammatory genes [9].
Mangiferin can inhibit CD4+T lymphocytes during inflammatory response in a dose-dependent manner, and reduce the gene and protein expression levels of IL-2 and IL-4 in CD4+T lymphocytes. IL-2 and IL-4 are pro-inflammatory cytokines mainly produced by activated CD4+T lymphocytes, which can activate more CD4+T lymphocytes, promote B cell proliferation and differentiation, enhance NK cell killing activity, activate monocytes and macrophages. IL-2 and IL-4 can synergistically stimulate mast cell proliferation with IL-3, inducing a large amount of IgG and IgE production [9].
3.3. Anemarrhena asphodeloides saponins and polysaccharides
Anemarrhena asphodeloides saponins can significantly inhibit the production of inflammatory cytokines induced by lipopolysaccharide (LPS) in macrophages by regulating the mitogen-activated protein kinase (MAPK) signaling pathway. They suppress the overexpression of macrophage inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), IL-1β, and nitric oxide (NO). The p38MAPK and JNK signaling pathways are two important branches of the MAPK pathway family, playing crucial roles in various physiological and pathological processes, including cell cycle, proliferation, apoptosis, and cellular stress. Anemarrhena asphodeloides saponins influence this pathway to thereby inhibit inflammatory responses[10].
Additionally, the DNCB-induced delayed-type hypersensitivity (DTH) mouse model has confirmed that the Anemarrhena asphodeloides polysaccharides (TPA) component can significantly reduce ear swelling. TPA can inhibit T-cell proliferation, modulate the balance between Th1 and Th2 cells, suppress cellular immune responses, and alleviate ear inflammation and immunological damage. TPA is also capable of inhibiting the inflammatory response of monocytes/macrophages induced by lipopolysaccharide (LPS), suppressing the release of tumor TNF-α and NO, thereby demonstrating anti-inflammatory effects [11].
3.4. Glycyrrhetinic acid and hypoglycyrrhetinic acid
Glycyrrhetinic acid and glycyrrhetinic acid can inhibit some enzymes that inactivate hormones during glucocorticoid metabolism, exerting anti-inflammatory effects similar to glucocorticoids, but do not have hormonal side effects. Glycyrrhetinic acid and its derivatives can inhibit 11 β- The activity of hydroxysteroid dehydrogenase. eleven β- HSD enzymes can convert inactive cortisone into active form of hydrocortisone. By inhibiting 11 β-HSD enzymes, glycyrrhetinic acid, and hypoglycyrrhetinic acid reduced the levels of active glucocorticoids, thereby simulating the anti-inflammatory effects of glucocorticoids [12].
Glycyrrhizic acid and glycyrrhetinic acid can directly inhibit the production of inflammatory cytokines, such as suppressing the transcriptional transfer of information from the NF-κB p105 to the NF-κB1 factor during the inflammatory expression process induced by LPS [12]. Glycyrrhizic acid can reduce the expression of inflammatory factors, such as TNF-α and IL-1β, by inhibiting the activation of the NF-κB pathway. Additionally, glycyrrhizic acid can suppress the nuclear translocation of NF-κB by inhibiting the activity of the IκB kinase complex within the NF-κB pathway, preventing the phosphorylation and degradation of IκBα, which in turn reduces the expression of inflammatory genes [12].
Glycyrrhizic acid can act as a natural inhibitor of High Mobility Group Box 1 (HMGB1). HMGB1 is a significant inflammatory mediator that can be released from inside the cell to the extracellular environment, where it activates immune cells and triggers an inflammatory response by binding to its receptors, such as Toll-like receptors (TLRs). Glycyrrhizic acid inhibits the activity of inflammatory factors by binding to HMGB1 and blocking its interaction with its receptors [13].
Glycyrrhizic acid can modulate the balance between Th1 and Th2 cells. Th1 and Th2 cells are two distinct subsets of helper T cells. Th1 cells are primarily involved in cell-mediated immune responses and secrete cytokines such as interferon-gamma (IFN-γ), while Th2 cells participate in humoral immunity and secrete cytokines like interleukin-4 (IL-4) and interleukin-5 (IL-5). Glycyrrhizic acid can influence the activity and differentiation of these two types of cells. Glycyrrhetinic acid can enhance the activity of Th1 cells, promoting the production of IFN-γ, while at the same time suppressing the activity of Th2 cells and reducing the production of cytokines such as IL-4 [14].
Glycyrrhizic acid also possesses anti-complementary activity, capable of selectively inhibiting the activation pathways of the complement system to reduce the occurrence of inflammatory responses. This inhibitory effect may be achieved by influencing the synthesis of complement components or by inhibiting key enzymes involved in the activation process of the complement system [14].
Glycyrrhetinic acid can also suppress the production of inflammatory mediators such as prostaglandins and leukotrienes by inhibiting the activity of enzymes related to arachidonic acid metabolism. For instance, glycyrrhetinic acid is capable of inhibiting the activity of phospholipase A2 (PLA2). PLA2 is an enzyme that catalyzes the breakdown of phospholipids in the cell membrane, releasing arachidonic acid (AA), which then participates in the biosynthesis of inflammatory mediators like prostaglandins and leukotrienes. By inhibiting PLA2, glycyrrhetinic acid reduces the generation of precursor substances for inflammatory mediators, thereby exerting its anti-inflammatory effects [12].
4. Conclusion
This article systematically explores the antipyretic and anti-inflammatory mechanisms of BHT. Through analysis of the existing research data, the following conclusions can be drawn: BHT significantly inhibits the production of inflammatory factors through its active components, such as mangiferin and glycyrrhizic acid, including the reduction of levels of TNF-α, IL-1β, and IL-6, thereby playing a role in fever reduction and anti-inflammation. In addition, the NAs in BHT have shown better antipyretic effects and are easily taken up by cells, especially their targeting to the lungs and brain, providing a new strategy for the treatment of diseases related to high fever. Although this study provides a scientific basis for the anti-inflammatory and antipyretic effects of BHT, there are certain limitations. Current research is mostly focused on in vitro experiments and animal models, with relatively fewer clinical studies on humans. Moreover, there is still a lack of in-depth understanding of how the various herbal components in BHT work synergistically and the specific mechanisms of action within the human body. Future research can delve into the following aspects: first, more clinical trial data need to be collected and analyzed to verify the efficacy and safety of BHT in humans; second, modern pharmacological and molecular biological techniques should be utilized to further clarify the synergistic mechanisms of action among the components in BHT; finally, considering the complexity of traditional Chinese medicine formulas, new research methods such as systems biology and network pharmacology should be developed to fully understand the mode of action of BHT.
References
[1]. Liu Y 2018 Research progress on the pharmacological activities and mechanisms of action of Anemarrhena asphodeloides saponins J Pharm Pract 36 01 24-29
[2]. Zhang L J Huang C & Fan S J 2021 Mangiferin and organ fibrosis: A mini review BioFactors 2021 1 1-10
[3]. Shang Y Kong W Jiang S et al 2023 Mangiferin improves inflammation in benign prostatic hyperplasia by acting on dual targets COX-2 and 5-LOX J Shenyang Pharm Univ 40 03 309-315+360
[4]. Tian H 2022 The influence of japonica rice on the composition and effects of Baihu Tang [Doctoral dissertation Beijing University of Chinese Medicine]
[5]. Lancaster E & Nicoll R A 2014 Calcium signaling and synaptic plasticity Neuron 63 5 903-917
[6]. Ramanadham M & Tripathi P N 2018 Calcium signaling mechanisms in thermogenesis J Cell Physiol 233 3 7425-7435
[7]. Sharma R & Thakur M 2016 Calcium channel blockers in the treatment of fever J Clin Diagn Res 10 9 U01-U04
[8]. Lü S Su H Sun S et al 2018 Isolation and characterization of nanometer aggregates from a Bai-Hu-Tang decoction and their antipyretic effect Sci Rep 8 12209
[9]. Wei Z Yan L Deng J et al 2013 The influence of mangiferin on the MAPK pathway and serum cytokines in chronic inflammatory rats induced by lipopolysaccharide Chin Herb Med 44 01 52-58
[10]. Liu Z Sui H Yan E et al 2013 The effect of Anemarrhena asphodeloides saponins on the release of inflammatory mediators induced by lipopolysaccharide in macrophages Chin J Public Health 29 03 384-386
[11]. Lei X Dong W Bi X et al 2015 Anti-inflammatory and immune-regulatory activities of various chemical fractions of Anemarrhena asphodeloides Chin Med Mater 38 09 1904-1907
[12]. DONG F Y & WANG J J 2014 Anti-inflammatory mechanism of glycyrrhetinic acid and its derivatives J Dalian Med Univ 36 2 198-201
[13]. Huang J Xu J Fan J et al 2024 Research progress on glycyrrhizic acid targeting high mobility group box 1 to inhibit inflammatory-related diseases Chin J New Drugs 33 04 345-350
[14]. Liu L Ren C & Zhao H 2010 Research progress on the immune-regulatory effect of glycyrrhizic acid J Chin Exp Prescr 6 5
Cite this article
Zhang,X. (2024). Exploring the antipyretic and anti-inflammatory mechanisms of Bai-Hu-Tang. Theoretical and Natural Science,58,9-14.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Liu Y 2018 Research progress on the pharmacological activities and mechanisms of action of Anemarrhena asphodeloides saponins J Pharm Pract 36 01 24-29
[2]. Zhang L J Huang C & Fan S J 2021 Mangiferin and organ fibrosis: A mini review BioFactors 2021 1 1-10
[3]. Shang Y Kong W Jiang S et al 2023 Mangiferin improves inflammation in benign prostatic hyperplasia by acting on dual targets COX-2 and 5-LOX J Shenyang Pharm Univ 40 03 309-315+360
[4]. Tian H 2022 The influence of japonica rice on the composition and effects of Baihu Tang [Doctoral dissertation Beijing University of Chinese Medicine]
[5]. Lancaster E & Nicoll R A 2014 Calcium signaling and synaptic plasticity Neuron 63 5 903-917
[6]. Ramanadham M & Tripathi P N 2018 Calcium signaling mechanisms in thermogenesis J Cell Physiol 233 3 7425-7435
[7]. Sharma R & Thakur M 2016 Calcium channel blockers in the treatment of fever J Clin Diagn Res 10 9 U01-U04
[8]. Lü S Su H Sun S et al 2018 Isolation and characterization of nanometer aggregates from a Bai-Hu-Tang decoction and their antipyretic effect Sci Rep 8 12209
[9]. Wei Z Yan L Deng J et al 2013 The influence of mangiferin on the MAPK pathway and serum cytokines in chronic inflammatory rats induced by lipopolysaccharide Chin Herb Med 44 01 52-58
[10]. Liu Z Sui H Yan E et al 2013 The effect of Anemarrhena asphodeloides saponins on the release of inflammatory mediators induced by lipopolysaccharide in macrophages Chin J Public Health 29 03 384-386
[11]. Lei X Dong W Bi X et al 2015 Anti-inflammatory and immune-regulatory activities of various chemical fractions of Anemarrhena asphodeloides Chin Med Mater 38 09 1904-1907
[12]. DONG F Y & WANG J J 2014 Anti-inflammatory mechanism of glycyrrhetinic acid and its derivatives J Dalian Med Univ 36 2 198-201
[13]. Huang J Xu J Fan J et al 2024 Research progress on glycyrrhizic acid targeting high mobility group box 1 to inhibit inflammatory-related diseases Chin J New Drugs 33 04 345-350
[14]. Liu L Ren C & Zhao H 2010 Research progress on the immune-regulatory effect of glycyrrhizic acid J Chin Exp Prescr 6 5









